Abstract
Pulmonary arterial hypertension is a fatal disease. Intravenous prostanoids are often utilized for long-term management of patients. The therapy requires a significant commitment and change in lifestyle for both the patient and family. Takotsubo cardiomyopathy, transient apical ballooning syndrome, has been reported in association with emotional and physical stress. This case report describes a patient with pulmonary arterial hypertension who developed Takotsubo cardiomyopathy after treatment initiation with intravenous treprostinil. Over time, the syndrome resolved and the patient had return of normal left ventricular function. Takotsubo cardiomyopathy should be recognized as a potential, rare complication of therapy initiation due to the severity of the illness and the emotional stress of the disease.
Keywords: pulmonary hypertension, takotsubo cardiomyopathy, treprostinil
Transient left ventricular apical ballooning syndrome, or Takotsubo cardiomyopathy, is characterized by acute chest pain, electrocardiographic changes, elevated cardiac enzymes, and the absence of significant epicardial coronary artery disease.[1] Though numerous published reports have proposed pathophysiologic mechanisms,[2] no clear mechanism has been identified. Numerous co-morbid conditions and precipitating factors have been reported, including emotional and physical stress, subcutaneous epinephrine administration, chemotherapy, cocaine, and a pheochromocytoma.[3] However, to our knowledge, there has been no report of Takotsubo cardiomyopathy following treatment for pulmonary arterial hypertension (PAH). We describe the case of a woman who developed Takotsubo cardiomyopathy following initiation of intravenous treprostinil for treatment of PAH.
CASE REPORT
AA is a 69-year-old woman with a past medical history of WHO Category I PAH, coronary artery disease, diabetes mellitus, chronic obstructive pulmonary disease, and a history of a partial lung resection due to a blastomycosis infection. The patient presented as a new referral to the outpatient clinic with complaints of increased exertional dyspnea and increasing home oxygen requirements over a period of several months. She was admitted to the hospital for suspected heart failure leading to increased dyspnea. The patient's home medications at the time of hospital admission included the following: aspirin 81 mg daily; atenolol 50 mg daily; amlodipine 10 mg daily; enalapril 5 mg daily; glipizide 5 mg twice daily; tiotropium inhaled 18 mcg daily; and fluticasone/salmeterol inhaled twice daily. She reported a codeine allergy. On social history, she drank one glass of wine nightly, and she had a 40 pack-year history of smoking and had quit 12 years prior to her presentation. She was married and was retired from her occupation of office work. AA's family history was significant as her father had stomach cancer and her brother had coronary artery disease.
On admission, AA was afebrile, her temperature 36.9°C, pulse 69 beats per minute, blood pressure 125/82 mmHg, respiratory rate of 19 breaths per minute, and saturating 98% on 3 liters (L) of oxygen delivered by nasal cannula. Her examination was significant for amblyopia of the right eye. Her cardiac exam was significant for a regular rate and rhythm, audible S1, loud S2, with an accentuated pulmonic, a right-sided holosystolic murmur grade II/VI, and negative for an S3 or S4. She had jugular venous distension to approximately 13 cmH20. Her lung fields revealed good air movement, and were clear to auscultation bilaterally. Her lower extremities were notable for 1+ pitting edema bilaterally up to her ankles.
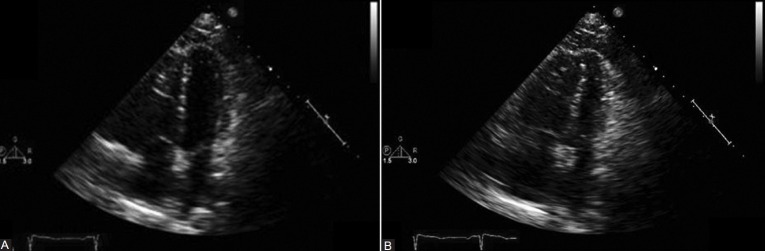
A transthoracic echocardiogram (Figures 1 and 2) on admission was significant for a moderately dilated right ventricle (RV) with moderate reduction of RV performance, mild tricuspid regurgitation, and an estimated RV systolic pressure of 76 mmHg. The left ventricle was noted to be small, with normal wall thickness, and hyperdynamic left ventricular performance. No regional wall motion abnormalities were noted.
Figure 1.
The echo revealed normal ventricular function with no regional wall motion abnormalities. (A) 2D echo upon admission: Apical four-chamber view, end-diastole. (B) 2D echo upon admission: Apical four-chamber view, end-systole.
Figure 2.
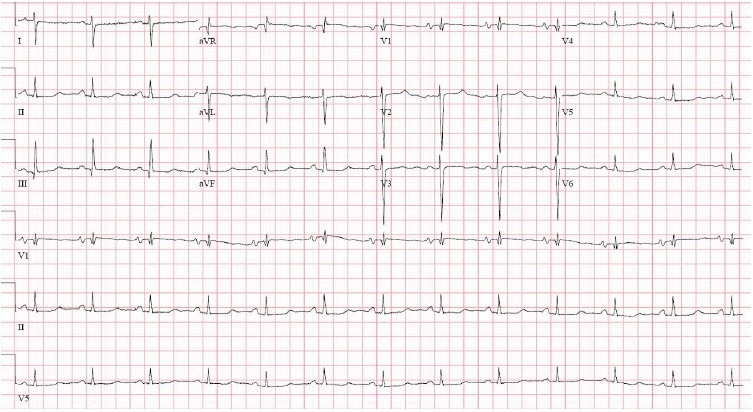
Baseline electrocardiogram after initiation of IV treprostinil. Normal sinus rhythm, right axis deviation, RV hypertrophy, long QT, or U on T.
Based on the initial echocardiogram and upon the patient's clinical examination, a continuous intravenous lasix drip was started for diuresis. Drips of phenylephrine 1 mcg/kg/min and dobutamine 5 mcg/kg/min were also initiated for right ventricular support. Phenylephrine was discontinued shortly after as it did not appear to enhance cardiac output or improve hemodynamics.
Right heart catheterization with intravenous infusion of adenosine was performed 1 day after admission. Baseline pressures (mmHg) were as follows: mean RA 8; RV 77/14; PA 78/38 (mean 57); PVR 25.3 Wood units; and wedge pressure 14. Cardiac output was 1.7 L/min with a cardiac index of 1.1 L/min/m2, and a PA saturation of 64.6%. With intravenous adenosine, pressures (mm Hg) were: mean RA 5, PA 80/37 (mean 52), PVR 23.3 Wood units, and wedge pressure 10. Cardiac output was 1.8 L/min and cardiac index 1.2 L/min/m2, and PA saturation was 67.2%.
The patient had a mild response to testing, not meeting definition of a “responder” and was in cardiogenic shock.[4] Intravenous (IV) treprostinil 4 ng/kg/min daily was initiated and titrated to 10 ng/kg/min daily over the course of the next three days. Within two days, however, the patient developed ST segment changes with dynamic T-wave changes in the clinical setting of acute chest discomfort (Figures 2 and 3) and hypotension.
Figure 3.
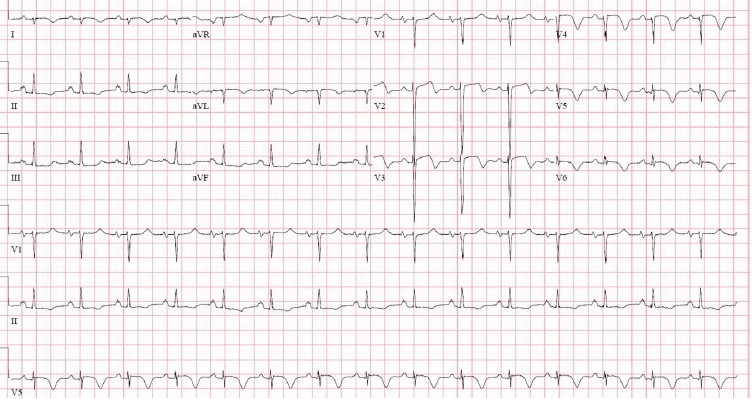
Electrocardiogram during symptomatic chest pain, prior to cardiac catheterization. Normal sinus rhythm, anterolateral ST and T-wave abnormality concerning for ischemia.
Given the patient's discomfort and new ECG findings, AA was taken emergently to the cardiac catheterization laboratory for left heart catheterization and coronary angiography (Figures 4 and 5). She was found to have a lesion in the mid-portion of the first diagonal artery with an 80% occlusion. A 2.25 mm × 8 mm Taxus Atom drug-eluting stent was placed. The remainder of her coronary anatomy, including the left main, left anterior descending, left circumflex, and right coronary arteries were well visualized and patent. The measured left ventricular end-diastolic pressure measured 16 mmHg. Importantly, the patient's cardiac biomarkers, including creatinine kinase (CK), CK-MB, and troponin remained normal on the day of admission during her symptomatic complaints, and following the coronary intervention.
Figure 4.
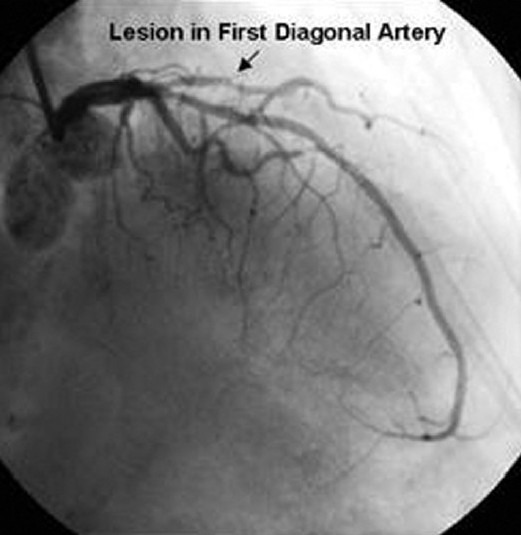
Left heart catheterization pre-percutaneous intervention.
Figure 5.
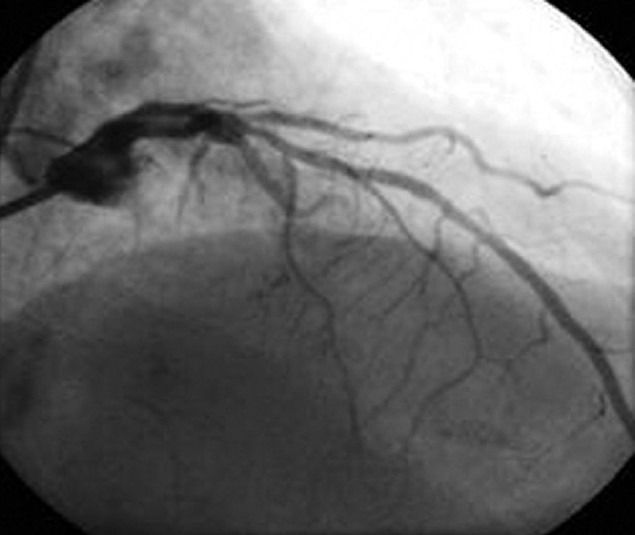
Left heart catheterization post-percutaneous intervention.
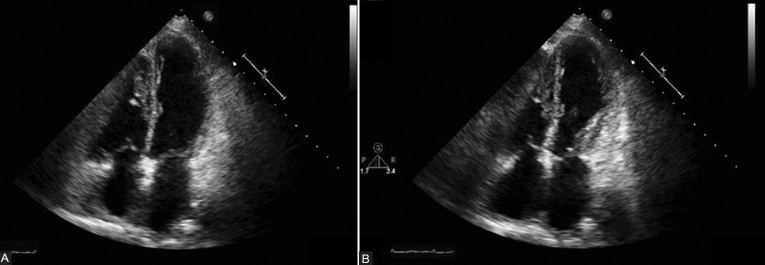
A two-dimensional transthoracic echocardiography was performed after the cardiac catheterization to assess left ventricular function (Figures 6A and B). This revealed a severe reduction in LV performance as well as regional wall motion abnormalities out of proportion to the coronary artery disease previously noted and treated acutely. These findings, septal and apical akinesis, were new compared with prior echo images (Figures 1A and B). Repeat electrocardiogram two days after catheterization revealed improvement of the ST and T-wave changes (Fig. 7).
Figure 6.
Significant left ventricular systolic dysfunction with septal and apical akinesis, notably out of proportion to the coronary artery disease found. (A) 2D echo after IV treprostinil: Apical four-chamber view, end-diastole. (B) 2D echo after IV treprostinil: Apical four-chamber view, end-systole.
Figure 7.
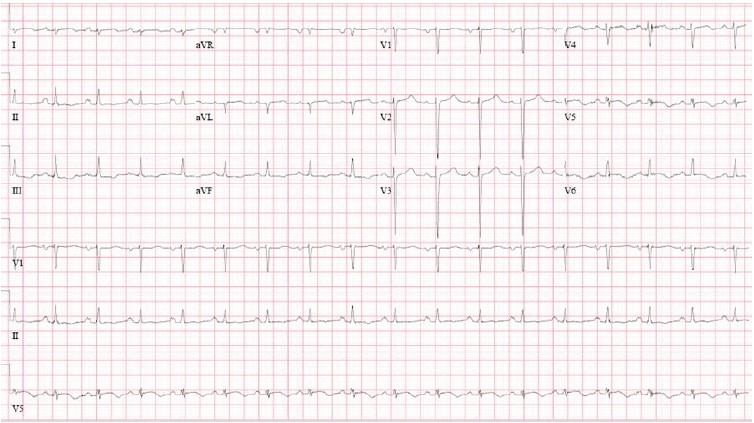
Electrocardiogram 1 day after cardiac catheterization. Sinus tachycardia, rightward axis, nonspecific T-wave abnormality. When compared with prior ECG, marked ST and T-wave abnormality are no longer evident in anterolateral leads.
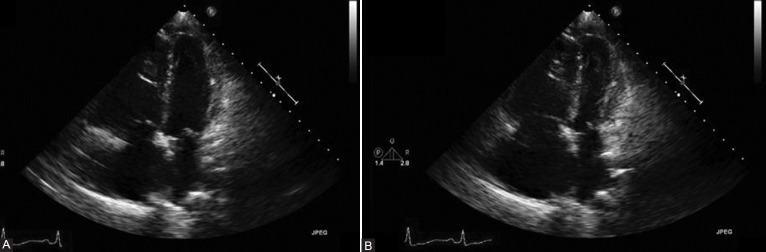
Over the course of the next several days, the patient was weaned off both the intravenous treprostinil and dobutamine, and was started in sildenafil 50 mg PO TID. Given the degree of left ventricular dysfunction seen, carvedilol 12.5 mg PO BID was initiated. She symptomatically improved, and was discharged home. Repeat echocardiography two weeks later (Figures 8A and B) showed resolution of the left ventricular findings and again RV dysfunction consistent to her baseline TTE.
Figure 8.
Normalization of the left ventricular systolic function and resolution of wall motion abnormalities. (A) 2D echo 2 weeks after hospital discharge: apical four-chamber view, end-diastole. (B) 2D echo 2 weeks after hospital discharge: apical four-chamber view, end-systole.
DISCUSSION
The specific mechanistic pathway between elevated sympathetic stimulation and myocardial stunning is unknown. Possible mechanisms include direct myocyte injury and contraction band necrosis related to catecholamine-directed injury, increased sympathetic tone induced by mental stress leading to vasoconstriction and vasospasm, microvascular spasm, and microcirculatory dysfunction.[5] The phenomenon of myocardial stunning causing apical ballooning is commonly described as causing left ventricular dysfunction; however, studies have demonstrated concomitant right ventricular systolic dysfunction.[6] Patients with a compromised right ventricular function at baseline, such as those with PAH, will consequently have bi-ventricular dysfunction,[7] and the patients’ prognosis may be significantly worse with profound cardiogenic shock.
This case represents the first reported incidence of apical ballooning syndrome in a patient with PAH following initiation of IV treprostinil. Treprostinil, a prostacyclin analog used in the advanced treatment of PAH, dilates pulmonary and systemic vessels, and exhibits potent antiproliferative activity in pulmonary artery smooth muscle cells.[8] Treprostinil has been shown to be safe and efficacious, and to improve exercise capacity as assessed by 6-Minute Walk Test and improved dyspnea. Indeed, studies have provided strong evidence supporting prostanoid use as a mainstay of therapy for PAH,[9,10] and this type of therapy is well documented in PAH clinical guidelines.[4]
Although the patient presented in this case was found to have coronary artery disease that was treated at the time of her symptoms and ECG changes, the wall motion abnormalities seen on TTE were out of proportion to the degree of coronary artery disease found and were in a territory not supplied by that vessel. Also the lack of biomarker elevation indicated an insignificant myocardial injury. The subsequent resolution of wall motion abnormalities to normal within two weeks is most consistent with a stress, or Takotsubo's, cardiomyopathy, which is typically transient in nature. The stress inducing the Takotsuobo cardiomyopathy may have been the emotional burden of starting a new medication that requires a significant lifestyle change. Alternatively, the stress may relate to the pharmacology of treprostinil, although this has not been described previously for this drug or from the stress of being in the cardiac care unit with critical illness. The medical team caring for AA opted not to reinitiate therapy with treprostinil given the emotional trauma and based on the patient's and family's wishes. This case report illustrated that a heightened awareness should be present for stress cardiomyopathy when initiating therapies in patients with advanced diseases such as PAH, diseases that require life-altering procedures especially if initiated in the intensive care unit. The authors believe that a thorough psychologic assessment may be warranted prior to initiation of these therapies.
ACKNOWLEDGMENTS
We would like to acknowledge the patient, her family, and the nurses involved in her care.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: A systematic review. Eur Heart J. 2006;27:1523–9. doi: 10.1093/eurheartj/ehl032. [DOI] [PubMed] [Google Scholar]
- 2.Elesber A, Lerman A, Bybee KA, Murphy JG, Barsness G, Singh M, et al. Myocardial perfusion in apical ballooning syndrome correlate of myocardial injury. Am Heart J. 2006;152:469.e9–13. doi: 10.1016/j.ahj.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Bybee KA, Prasad A. Stress-related cardiomyopathy syndromes. Circulation. 2008;118:397–409. doi: 10.1161/CIRCULATIONAHA.106.677625. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Sadamatsu K, Tashiro H, Maehira N, Yamamoto K. Coronary microvascular abnormality in the reversible systolic dysfunction observed after noncardiac disease. Jpn Circ J. 2000;64:789–92. doi: 10.1253/jcj.64.789. [DOI] [PubMed] [Google Scholar]
- 6.Loiske K, Waldenborg M, Frobert O, Rask P, Emilsson K. Left and right ventricular systolic long-axis function and diastolic function in patients with takotsubo cardiomyopathy. Clin Physiol Funct Imaging. 2011;31:203–8. doi: 10.1111/j.1475-097X.2010.01001.x. [DOI] [PubMed] [Google Scholar]
- 7.Citro R, Caso I, Provenza G, Santoro M, Gregorio G, Bossone E. Right ventricular involvement and pulmonary hypertension in an elderly woman with tako-tsubo cardiomyopathy. Chest. 2010;137:973–5. doi: 10.1378/chest.09-0923. [DOI] [PubMed] [Google Scholar]
- 8.Channick RN, Rubin LJ. New and experimental therapies for pulmonary hypertension. Clin Chest Med. 2001;22:539–45. doi: 10.1016/s0272-5231(05)70290-2. [DOI] [PubMed] [Google Scholar]
- 9.Gomberg-Maitland M, Olschewski H. Prostacyclin therapies for the treatment of pulmonary arterial hypertension. Eur Respir J. 2008;31:891–901. doi: 10.1183/09031936.00097107. [DOI] [PubMed] [Google Scholar]
- 10.Simonneau G, Barst RJ, Galie N, Naeije R, Rich S, Blackburn SD, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: A double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165:800–4. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]