Abstract
Background
Elevated heart rate represents an independent risk factor for cardiovascular outcome in patients with heart disease. In the sinoatrial node, rate increase is mediated by β1 adrenoceptor mediated activation of the Gαs pathway. We hypothesized that genetic inactivation of the stimulatory Gαs protein in the sinoatrial node would provide sinus rate control and would prevent inappropriate heart rate acceleration during β-adrenergic activation.
Methods and Results
Domestic pigs (n=10) were evenly assigned to receive either Ad-small interfering RNA (siRNA)-Gαs gene therapy to inactivate Gαs or adenovirus encoding for green fluorescent protein (Ad-GFP) as control. Adenoviruses were applied through virus injection into the sinoatrial node followed by epicardial electroporation, and heart rates were evaluated for 7 days. Genetic inhibition of Gαs protein significantly reduced mean heart rates on day 7 by 16.5% compared with control animals (110±8.8 vs 131±9.4 beats per minute; P<0.01). On β-adrenergic stimulation with isoproterenol, we observed a tendency toward diminished rate response in the Ad-siRNA-Gαs group (Ad-siRNA-Gαs, +79.3%; Ad-GFP, +61.7%; n=3 animals per group; P= 0.294). Adverse effects of gene transfer on left ventricular ejection fraction (LVEF) were not detected following treatment (LVEFAd-siRNA-Gαs, 66%; LVEFAd-GFP, 60%).
Conclusions
In this preclinical proof-of-concept study targeted Ad-siRNA-Gαs gene therapy reduced heart rates during normal sinus rhythm compared with Ad-GFP treatment and prevented inappropriate rate increase after β-adrenergic stimulation. Gene therapy may provide an additional therapeutic option for heart rate reduction in cardiac disease. (J Am Heart Assoc. 2012;1:jah3-e000372 doi: 10.1161/JAHA.111.000372)
Keywords: electrophysiology, gene therapy, heart failure, heart rate, sinoatrial node
Introduction
Elevated heart rate is increasingly recognized as a modifiable risk factor in patients with heart disease. Two recent randomized trials (morbidity–mortality evaluation of the If inhibitor ivabradine in patients with coronary disease and left ventricular dysfunction, BEAUTIFUL; systolic heart failure treatment with the If inhibitor ivabradine trial, SHIFT) identified a resting heart rate >70 beats per minute (bpm) as risk factor for cardiac outcome in patients with coronary artery disease and heart failure.1–5 Standard pharmacological management of patients with cardiovascular disease includes β1-selective blockers, exerting beneficial effects on morbidity and mortality that are mediated in part through heart rate-lowering properties. In a subset of patients, however, the heart rate remains elevated during β blocker treatment. In addition, adverse effects on intracardiac electrical conduction or on myocardial contractility may limit the use of β blockers. A specific inhibitor of the cardiac pacemaker current (If) in the sinoatrial node (SAN), ivabradine, has been developed to provide heart rate reduction without affecting electrical conduction, cardiac contractility, or blood pressure. If is activated by membrane potential hyperpolarization and regulated by direct binding of cAMP in response to β-adrenergic stimulation.6,7 Pharmacological heart rate reduction with ivabradine improved cardiovascular outcome in BEAUTIFUL and SHIFT trial subgroups, confirming the significance of heart rate in cardiac disease.1,3,4
We sought to identify novel treatment modalities for heart rate reduction to further improve management and clinical outcome of heart failure patients. Targeted biological modification of cardiac electrophysiology may circumvent the disadvantage of non-specificity inherent to drug therapy. In particular, gene therapy has previously proven effective in preclinical proof-of-concept studies targeting atrial fibrillation.8–13 At the molecular level, β-adrenergic activation and subsequent increase of intracellular cAMP is mediated by Gαs protein activation. We therefore hypothesized that genetic inactivation of the stimulatory Gαs protein in the SA node would provide rate control during normal sinus rhythm and would prevent undesired heart rate acceleration during β-adrenergic activation. To test this hypothesis in a pilot study, an adenovirus encoding for a respective silencing RNA (Ad-siRNA-Gαs) was directly injected into sinoatrial nodes of domestic pigs, and heart rate was evaluated daily following gene transfer. Suppression of Gαs subunits in the SAN significantly lowered heart rates compared with control animals treated with adenovirus encoding for green fluorescent protein (Ad-GFP). There were no adverse effects on systolic ventricular function. In addition, inappropriate rate increase was not observed after β-adrenergic stimulation with isoproterenol compared with Ad-GFP controls.
Methods
Adenoviruses
An adenovirus encoding for siRNA-Gαs (Ad-siRNA-Gαs; SIRION, Martinsried, Germany) was used to suppress the stimulatory Gαs protein. In the control group, a recombinant adenovirus encoding for green fluorescent protein (Ad-GFP; Qbiogen, Irvine, CA, USA), a reporter gene not affecting cardiac electrophysiology,14,15 was applied. Virus concentration was determined using a mouse antihexon antibody and horseradish peroxidase-conjugated goat anti-mouse secondary antibodies (Adeno-X Rapid Titer Kit, Clontech, Mountain View, CA, USA).
HL-1 Cell Culture and In Vitro Gene Transfer
HL-1 cells, a cardiac muscle cell line derived from the AT-1 mouse atrial myocyte tumor lineage, were provided by Dr. William Claycomb (New Orleans, LA, USA).16 HL-1 cells were cultured and maintained as described previously.13,16,17 Gene transfer was performed when cells were 70% confluent by adding 0.2 mL solution containing Ad-GFP or Ad-siRNA-Gαs (7.9×108 plaque forming units) to 15 mL HL-1 culture media per 75 mL cell culture flask. Cells were harvested 48 hours after adenovirus application.
Animals and In Vivo Gene Delivery
This study was approved by the Local Animal Care and Use Committee and has been carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health (NIH publication No. 86-23, revised 1985). The European Commission Directive 86/609/EEC and the current version of the German Law on the Protection of Animals was followed.
Domestic swine weighing 30 to 35 kg were investigated in this study. A single prophylactic dose of penicillin (200 mg; aniMedica, Senden-Bösensell, Germany) was administered before surgery. Pigs were sedated with ketamine (100 mg/kg; Roche, Grenzach-Wyhlen, Germany), anesthetized with propofol (1 mL of a 1% solution; Astra Zaneca, Wedel, Germany), and ventilated with isoflurane (1% to 2%; Baxter, Unterschleißheim, Germany) in a 1:2 ratio of O2 and N2O2. Ventilation, oxygenation, cardiac electrical activity, and body temperature were monitored and interdigital reflexes were tested to determine adequacy of anesthesia. Median thoracotomy was performed and the pericardium was opened to expose the heart under sterile conditions. Animals were randomized to receive either Ad-siRNA-Gαs or Ad-GFP treatment. Then 1.5 mL solution containing Ad-siRNA-Gαs (2×109 plaque forming units) or Ad-GFP was injected in aliquots of 0.1 mL into the high right atrial wall, carefully avoiding injections into the atrial cavity. Injection of adenoviruses was followed by electroporation as reported previously.10,12,13 Five square wave applications were performed at the site targeted by gene therapy (20 V/100 ms; ECM 830, BTX Harvard Apparatus, Holliston, MA, USA). The electric field causes transient pores to form in the cells of the atrial tissue, improving adenovirus uptake into cells and resulting in ∼50% GFP transgene expression in pigs.10 After gene transfer and approximation of the pericardium the thorax was closed, and the animals received buprenorphine (0.324 mg; Essex Pharma, Munich, Germany) for 1 to 3 days after surgery. Heart rates reflect mean values obtained from two 6-lead ECG recordings performed daily during feeding. Animals were awake and alert at consistent levels during all ECG measurements during the observation period.
β-Adrenergic Stimulation
Pharmacological studies were carried out in subgroups of n=3 pigs from each group on day 7. Isoproterenol (10 μg/kg; Sigma-Aldrich, Steinheim, Germany) was administered intravenously to sedated animals. Heart rate was continuously recorded using 6-lead ECG during the observation time. Baseline heart rate was recorded for 3 minutes before drug administration and for at least 10 minutes following isoproterenol application.
Echocardiography
Echocardiography was performed on the day of gene transfer and before euthanization. Animals were sedated and anesthetized as described, and all examinations were carried out under similar conditions. A detailed description of echocardiographic analysis has been published previously.13
Western Blot Analysis
After data acquisition on day 7, anesthetized animals were euthanized by intravenous application of KCl (1 M) and the hearts were removed and rinsed with phosphate buffered saline. Cardiac tissue was processed as described.10,12,13 HL-1 cells were solubilized for 1 hour at 4°C in lysis buffer containing 1% Triton X-100 and “Complete” protease inhibitors (Roche Diagnostics, Mannheim, Germany). Protein immunodetection was performed by sodium dodecyl sulfate gel electrophoresis and Western blotting as reported.10,12,13 Polyvinylidene difluoride membranes were developed by sequential exposure to blocking reagent (5% dry milk), primary antibodies directed against β1 adrenoceptor (sc-568; Santa Cruz Biotechnology, Heidelberg, Germany), Gαs protein (sc-823; Santa Cruz Biotechnology), adenylate cyclase VI (sc-25500; Santa Cruz Biotechnology), phosphorylated protein kinase A (ab32390; Abcam, Cambridge, MA, USA), or glyceraldehyde-3-phosphate dehydrogenase (GAPDH; G8140-11, US Biological, Swampscott, MA, USA), and appropriate horseradish peroxidase-conjugated secondary antibodies (Abcam). Signals were developed using the enhanced chemiluminescence assay (GE Healthcare, ECL Western Blotting Reagents, Buckinghamshire, UK) and quantified using ImageJ 1.41 Software (National Institutes of Health, Bethesda, MD, USA). Protein content was normalized to GAPDH for quantification of optical density.
Immunohistochemistry and Fluorescence Microscopy
Indicated tissue sections were processed and transgene efficiency was evaluated as described.13 For immunohistochemistry sections were incubated with polyclonal rabbit anti-Gαs antibodies (sc-823; Santa Cruz Biotechnology). Antigen-antibody complexes were visualized with HRP-conjugated goat anti-rabbit IgG (7074; Cell Signaling, Danvers, MA, USA). Peroxidase activity was detected with diaminobenzidine (DAB) using the SK4100 kit (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer's instructions. Direct (GFP) or indirect fluorescence (Gαs) was assessed using a fluorescence microscope (AX 70; Olympus, Hamburg, Germany). The percentage of cells exhibiting significant fluorescence signals compared with cells stained with 4,6-diamidino-2-phenylindole (Sigma-Aldrich) (direct fluorescence microscopy) or hematoxylin and eosin staining (immunohistochemistry) was quantified by blinded observers through cell counting in 5 (direct fluorescence) or 10 (immunodetection of Gαs protein) randomly selected sections of each image.
Statistics
Data are expressed as mean±SEM of n experiments. Normal distribution of the data was confirmed using the Shapiro-Wilk test (SPSS Statistics, IBM, Ehningen, Germany). We used unpaired or paired Student's t tests (two-tailed tests) to compare the statistical significance of the results where appropriate. P<0.05 was considered statistically significant. Heart rates were compared using two-factor analysis of variance (ANOVA) with treatment and time as factors and repeated measures on one factor (time). One-way ANOVA and Tukey's post hoc tests were then applied to identify paired differences between mean heart rates of treatment groups at different times. Multiple comparisons in Figure 1 were performed using one-way ANOVA. If the hypothesis of equal means could be rejected at the 0.05-level, pair wise comparisons of groups were made and the significance level was adjusted for multiple comparisons using the Bonferroni correction (0.05/n, where n is the number of tests performed). GFP expression in different cardiac regions was compared using repeated measures ANOVA and the Bonferroni post hoc test.
Figure 1.
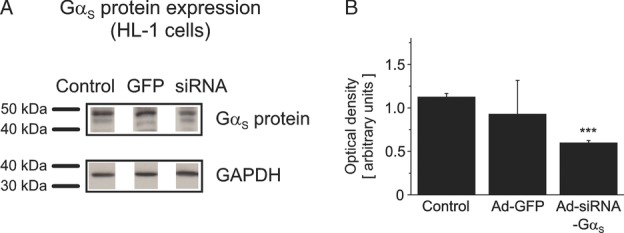
In vitro efficacy of Ad-siRNA-Gαs gene transfer. Gαs protein expression was analyzed using Western blot in HL-1 mouse atrial cardiac myocytes. (A) Gαs protein levels evaluated in untreated control cells and following application of Ad-GFP (GFP) or Ad-siRNA-Gαs (siRNA). (B) Quantification of optical density normalized to GAPDH protein. Gαs expression was suppressed by 51% in HL-1 cells infected with Ad-siRNA-Gαs (n=3) compared with controls (n=3), whereas Ad-GFP did not significantly alter Gαs protein levels (n=3). Data are provided as mean±SEM; ***P<0.001 versus control HL-1 cells. GAPDH indicates glyceraldehyde-3-phosphate-dehydrogenase; GFP, green fluorescent protein.
Results
In Vitro Efficacy of Ad-siRNA-Gαs Gene Transfer
The efficacy of Gαs protein suppression was analyzed in vitro in mouse atrial cardiac myocytes (HL-1 cells). An adenovirus transduction rate of 34% was previously reported under similar experimental conditions.10 Significant reduction of Gαs protein in HL-1 cells was demonstrated by Western blot analysis 48 hours after Ad-siRNA-Gαs treatment (−51.3%; n=3 independent assays; P=0.0005) compared with untreated HL-1 cells (Figure 1A, B). Ad-GFP application did not significantly affect Gαs expression (P=0.643; n=3; Figure 1).
Suppression of Gαs Protein Provides Biological Heart Rate Reduction
Ad-siRNA-Gαs and Ad-GFP transfer was then performed in vivo using an established hybrid approach combining direct adenovirus injection into the sinoatrial node and epicardial electroporation to increase gene expression.10,12,13 Control animals treated with Ad-GFP (n=5) exhibited mean heart rates of 117±5.6 bpm before surgery and 131±9.4 bpm on day 7, respectively (Figure 2A, B). In contrast, pigs that received Ad-siRNA-Gαs displayed mean heart rates of 111±5.6 bpm (day 1) and 110±8.8 bpm (day 7). Genetic inactivation of Gαs protein reduced mean heart rates by 16.5% (day 7) compared with control pigs (n=5; P<0.01) (Figure 2A, B). During the entire follow-up period, the mean reduction of heart rates compared with the Ad-GFP group yielded 13.8±1.3% (range, 7.9% to 16.6%; corresponding to 10.6 to 22.8 bpm) (Figure 2B). Ad-GFP-infected control animals had a 13.8±9.7% (n=5; P=0.278) mean increase in heart rate when comparing day 7 with day 1 (Figure 2C) that was not statistically significant. This effect was not observed in Ad-siRNA-Gαs pigs (+0.0±9.4%; n=5; P=0.882).
Figure 2.
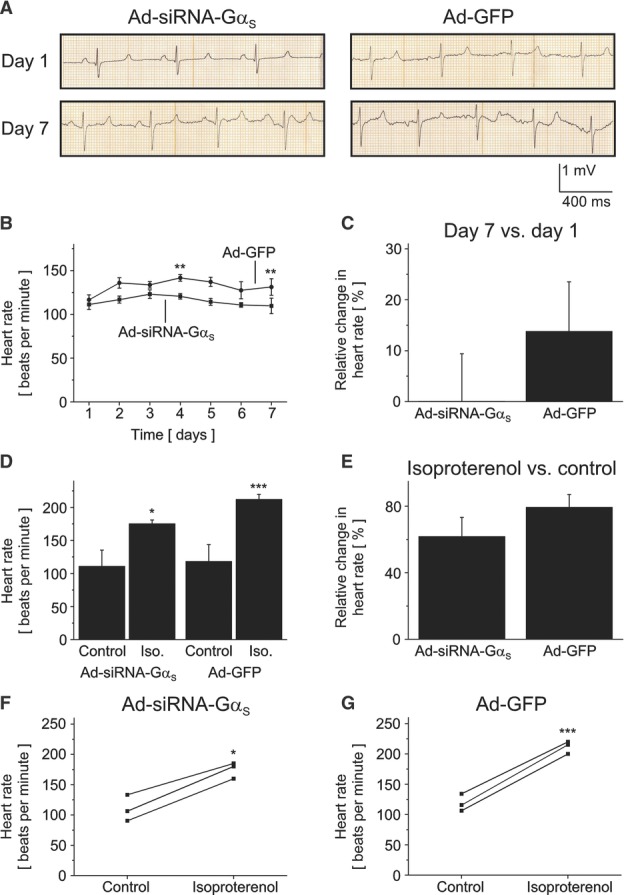
Heart rate reduction following Ad-siRNA-Gαs gene therapy. (A) Representative ECG recordings obtained from pigs before gene therapy (day 1) and after application of Ad-siRNA-Gαs or Ad-GFP (day 7), respectively. (B) Mean heart rates (± SEM) assessed by daily ECG recordings in control animals (n=5) and in pigs treated with Ad-siRNA-Gαs (n=5). Statistical significances among groups were analyzed on days 1, 4, and 7, respectively (**P<0.01). (C) Relative changes in heart rates recorded on day 7 compared with the day of gene transfer (day 1). (D-G) Three animals from each group were subjected to isoproterenol challenge to assess adrenergic response. Administration of isoproterenol on day 7 significantly increased heart rates in Ad-GFP control animals (D, E). Heart rate acceleration by isoproterenol was attenuated in animals infected with Ad-siRNA-Gαs (D, E). (F, G) Comparison of heart rates obtained from individual animals in the Ad-siRNA-Gαs group (F) and in the Ad-GFP group (G) before and after isoproterenol challenge, respectively. Data represent mean values±SEM; *P<0.05, ***P<0.001 compared with respective drug-free control conditions. ECG indicates electrocardiogram; GFP, green fluorescent protein.
Adrenergic Heart Rate Modulation
Activation of the sympathetic nervous system and subsequent heart rate increase enhance myocardial oxygen demand. In patients with heart disease, inappropriate heart rate acceleration after adrenergic stimulation increases the risk for myocardial ischemia and angina pectoris. Three animals from each group were subjected to isoproterenol application on day 7 to simulate activation of the β-adrenergic system. Heart rate was continuously monitored using 6-lead ECG during the observation time. Before drug administration, baseline heart rate was recorded for 3 minutes. Following drug application, heart rate was recorded for at least 10 minutes. For statistical evaluation, we determined peak effects after drug administration. β-adrenergic stimulation with isoproterenol increased heart rates by 79.3±7.7% in Ad-GFP control animals from 118±25.7 bpm to 212±7.6 bpm (n=3; P=0.0008; Figure 2D, E, G). In contrast, isoproterenol administration resulted in a 61.7±11.6% heart rate increase in the Ad-siRNA-Gαs group (111±24.7 bpm vs 175±6.0 bpm; n=3; P=0.011; Figure 2D, E, F). The attenuated isoproterenol response in animals treated with Ad-siRNA-Gαs did not reach statistical significance (P=0.294).
Ad-siRNA-Gαs Gene Therapy Did Not Affect Left Ventricular Function
Adenoviral gene transfer may exert adverse effects on cardiac function. To assess changes in left ventricular function, echocardiographic examinations were performed before gene transfer and after 7 days. Echocardiograms performed on day 1 revealed similar left ventricular ejection fractions (LVEF) among both study groups. Mean LVEF yielded 62.2±2.6% (Ad-siRNA-Gαs) and 61.1±2.3% (Ad-GFP), respectively (n=5 each; P=0.743). On the day of sacrifice, no reduction of LVEF was observed in study animals (LVEFAd-siRNA-Gαs=62.5±2.4%; LVEFAd-GFP=65.0±2.4%; n=5 each), consistent with low levels of transgene expression in left ventricles (see Figure 3). LVEF assessed on day 7 was not significantly different between treatment groups (P=0.479).
Figure 3.
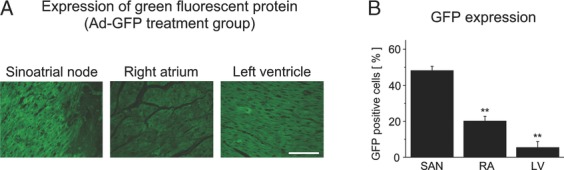
Efficacy and cardiac distribution of transgene expression. (A) Representative microphotographs depicting SAN, RA, and LV after application of Ad-GFP (day 7). GFP reporter gene expression was analyzed via direct fluorescence measurements (scale bar, 100 μm). (B) The relation of GFP positive cells compared with the total number of cardiac cells (in %) is presented for SAN, RA, and LV tissue obtained from 5 animals. Data are given as mean±SEM; **P<0.01 versus sinoatrial node. GFP indicates green fluorescent protein; LV, left ventricle; RV, right atrium; SAN, sinoatrial node.
In Vivo Gene Transfer Efficacy and Transgene Distribution
Cardiac tissue samples were analyzed to evaluate the extent and distribution of electroporation-enhanced gene transfer (n=5).10,12,13 Quantification of GFP reporter gene expression on day 7 following Ad-GFP treatment revealed a mean expression rate of 48.1±2.4% in the targeted SAN area (Figure 3A, B). Green fluorescence signal was detected in right atria (20.1±2.6%; P=0.004) and left ventricles (5.4±3.4%; P=0.001) as well, albeit with significantly reduced efficacy compared with sinoatrial node (Figure 3A, B). Effective suppression of Gαs protein in the sinoatrial node after Ad-siRNA-Gαs application was demonstrated by immunohistochemistry on day 7 (Figure 4A, B). We observed a 70.5% (P<0.0001) decrease of Gαs protein levels in the Ad-siRNA-Gαs group (n=5) compared with control pigs receiving Ad-GFP (n=5), indicating successful target gene knockdown.
Figure 4.
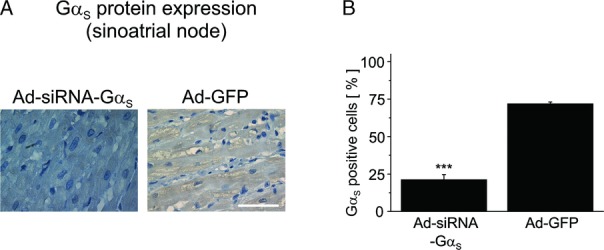
Gαs protein knockdown in the sinoatrial node. Expression of Gαs protein was assessed by immunohistochemistry. (A) Representative microscopic findings after treatment with Ad-siRNA-Gαs and Ad-GFP (scale bar, 50 μm). (B) Quantification of Gαs protein levels in n=5 animals per group. Data are expressed as mean±SEM (***P<0.001 vs Ad-GFP). GFP indicates green fluorescent protein.
Biochemical Remodeling of β-Adrenergic Signal Transduction Proteins
To address the question whether reduced heart rates and Gαs protein suppression were accompanied by secondary expression changes of β-adrenergic signal transduction proteins, Western blot analyses were performed on sinoatrial node tissue obtained from all study animals. We found that protein expression of β1 adrenoceptors (Figure 5A, B), adenylyl cyclase VI (Figure 5C, D), and phosphorylated (activated) protein kinase A (Figure 5E, F) was not affected by gene transfer.
Figure 5.
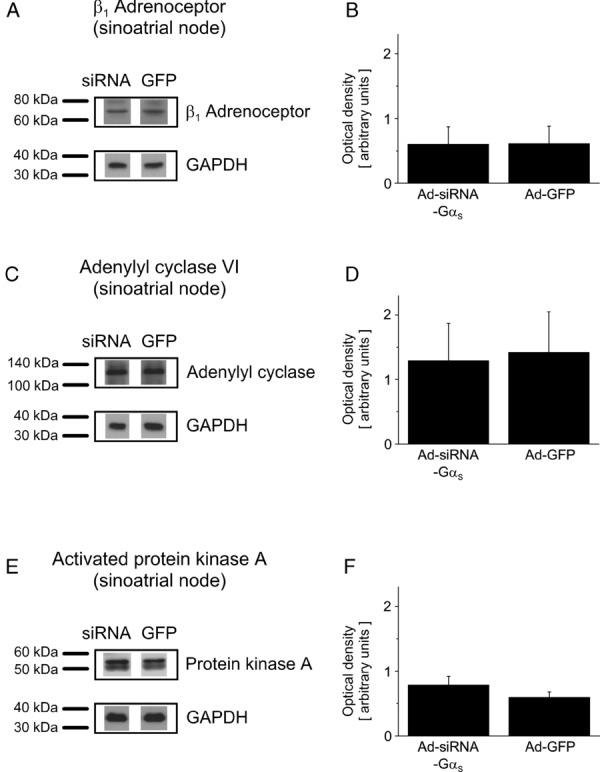
Expression of proteins involved in β-adrenergic signaling after gene therapy. Representative Western blots (A, C, E) and mean optical density (OD) values (B, D, F) are presented for study animals treated with Ad-siRNA-Gαs (siRNA) and Ad-GFP (GFP), respectively (n=5 animals per group). Ad-siRNA-Gαs treatment did not significantly affect expression of β1 adrenoceptors (A, B), adenylyl cyclase VI (C, D), and activated protein kinase A (E, F) in the sinoatrial node. GFP indicates green fluorescent protein.
Discussion
Genetic Heart Rate Control by siRNA-Mediated Gαs Protein Inactivation
Increased resting heart rate has been identified as independent risk factor in cardiac disease, and heart rate-lowering treatment improved cardiovascular outcome in recent trials (BEAUTIFUL, SHIFT).1–5 In a subset of patients small molecule approaches are limited by reduced efficacy and by adverse effects on electrical conduction or cardiac contractility. In search for novel treatment modalities gene therapy may offer increased selectivity compared with current pharmacological therapy. Specifically, genetic modulation of β-adrenergic signal transduction through modification of G protein function in the atrioventricular node or in atrial tissue has proven effective for rate or rhythm control in atrial fibrillation animal models.8,9,18
In the present proof-of-concept large animal pilot study, targeted suppression of the stimulatory Gαs protein in the sinoatrial node prevented heart rate increase observed in Ad-GFP animals during follow-up. Heart rates were lowered by 7.9% to 16.6% in Ad-siRNA-Gαs pigs during normal sinus rhythm compared with control animals (Figure 2). Furthermore, animals receiving Ad-siRNA-Gαs gene therapy exhibited attenuated heart rate increase on β-adrenergic stimulation compared with Ad-GFP controls.
Molecular Mechanisms
Cardiac pacemaker activity is determined by activation of the If current and underlying hyperpolarization-activated channels (HCN) and by intracellular calcium cycling.6,7,19–21 HCN channel opening on membrane hyperpolarization and rhythmic Ca2+ release from ryanodine receptors promote membrane depolarization and initiate the cardiac action potential. Local Ca2+ releases stimulate Na+–Ca2+ exchange currents that accelerate diastolic depolarization in sinoatrial node cells. Triggering of action potentials is controlled by intracellular cAMP levels and protein kinase A activity. These factors increase in response to β-adrenergic stimulation and subsequent activation of stimulatory G protein α subunits, representing a basic physiological mechanism for autonomic heart rate regulation.6,20,21 At the molecular level the chronotropic response to β-adrenergic activation appears to be primarily mediated by modulation of Ca2+ cycling, whereas the basal heart rate depends on Ca2+- and If-dependent mechanisms.20,21 The present study was based on the hypothesis that genetic inactivation of the stimulatory Gαs protein and suppression of β-adrenergic activation in the SAN would provide rate control at baseline and during isoproterenol challenge.
We used an established in vivo gene transfer technique, employing local adenovirus injections in combination with electroporation to improve virus uptake into the cells.10,12,13 This approach resulted in 48.1% GFP reporter gene expression on day 7 in the target region (Figure 3). Furthermore, the targeted Gαs protein was suppressed by 70.5% in the sinoatrial node after Ad-siRNA-Gαs treatment compared with Ad-GFP controls (Figure 4), confirming gene transfer efficacy. The observation corresponds to 51.3% suppression of Gαs protein expression assessed in vitro following Ad-siRNA-Gαs application in HL-1 mouse atrial myocytes (Figure 1). Expression of nontargeted β-adrenergic signal transduction proteins (ie, β1 adrenoceptors, adenylyl cyclase VI, or phosphorylated protein kinase A) was not affected by Ad-siRNA-Gαs gene therapy, ruling out any relevant compensatory remodeling within the targeted pathway (Figure 5).
In summary, we conclude that genetic suppression of Gαs protein activation in the sinoatrial node decreased cardiac pacemaker activity, resulting in lowered sinus rates compared with Ad-GFP controls during follow-up and after β-adrenergic stimulation. Secondary effects of Ad-siRNA-Gαs gene therapy on SAN electrophysiology by biochemical remodeling were not observed. The relative contribution of calcium cycling and of the If current to cardiac pacemaker function is still a matter of ongoing debate. Here, adrenergic modulation of Gαs protein-associated calcium signaling is suggested as predominant target mechanism of the therapeutic approach, because heart rate reduction was observed during follow-up associated with postoperative stress and after isoproterenol application, respectively. This is consistent with recent data obtained from patients with hereditary sinus node dysfunction carrying mutated HCN4 pacemaker channels that are insensitive to the second messenger cAMP.19 These patients exhibited normal rate acceleration during exercise, indicating that Ca2+ cycling rather than HCN4 channels and If current determine heart rate increase during adrenergic activation.
Clinical Implications
The present preclinical study confirms the role of Gαs protein signaling in rate control during normal sinus rhythm. We further demonstrate the efficacy of gene therapy targeting Gαs subunits in the sinoatrial node for rate control in a large animal model. The effect observed with Ad-siRNA-Gαs therapy in pigs (7.9% to 16.6% rate reduction) is similar to pharmacological sinus rate control in humans during treatment with β blockers (13.5% to 16.4% reduction) or ivabradine (7.6% to 13.7% reduction), respectively.1,4,22–24 Note that values for rate reduction are provided percent to allow for ready comparison among species with different basal heart rates. Negative inotropic effects on systolic left ventricular function, a potential limitation of rate-lowering agents such as β blockers, were not observed with localized Ad-siRNA-Gαs treatment. Thus, siRNA-Gαs transfer may provide “exclusive” heart rate reduction similar to ivabradine.25 In contrast to ivabradine, however, Ad-siRNA-Gαs therapy reduced the heart rate primarily during increased adrenergic activation. This mode of action is expected to be particularly beneficial in heart failure that is associated with constant and inappropriate activation of the adrenergic system. Of note, there was no case of sinus arrest, supporting the hypothesis that basal pacemaker activity was not markedly affected by Gαs inactivation.
Heart rate reduction has been shown to improve clinical outcome in patients with coronary artery disease and congestive heart failure by improving coronary perfusion and through reduction of myocardial oxygen demand. Furthermore, beneficial effects of lowered heart rates on atherosclerosis have been reported.26 Recognizing the invasive nature of our gene delivery method, the hybrid gene application technique could currently be performed on heart failure patients during open-chest cardiac surgery required for cardiac revascularization or valve replacement. To further refine gene transfer technology, thoracotomy may be replaced in future studies by interventional, transvenous virus application.
Limitations and Future Directions
This preclinical proof-of-concept study was designed to evaluate feasibility and short-term efficacy of biological sinus rate control using Ad-siRNA-Gαs transfection in pigs. The work shares common limitations of pilot studies in large animal models including small sample size and short follow-up period. The follow-up was limited to 7 days to avoid confounding the results by loss of gene expression that occurs with first-generation adenoviral vectors. Remaining challenges of Ad-siRNA-Gαs gene therapy that need to be overcome include optimized control over spacious gene distribution, proarrhythmic effects, potential tumorigenicity of vehicles and siRNA application, and prevention of local and systemic inflammatory responses. These safety issues need to be carefully addressed in larger groups of animals with extended observation periods before evaluation of antiarrhythmic gene therapy in humans. Although adenoviral vectors were used in this work owing to their ability to induce peak expression within a short time and to their high efficacy in infecting cardiac myocytes, the use of adeno-associated virus or lentivirus as vector would be more appropriate for long-term applications and to study long-term stability, efficacy, and safety of gene therapy.
Conclusion
We demonstrate for the first time effective heart rate reduction by targeted biological modification of Gαs protein signaling in the SAN. In addition, knockdown of the activating component of the β-adrenergic signaling pathway suppressed inadequate catecholaminergic heart rate increase in a large animal model. We suggest that this approach could be used as primary or supplementary treatment option in patients with cardiovascular disease after gene delivery optimization and following evaluation of long-term efficacy, safety, and toxicology.
Acknowledgments
We thank Jennifer Gütermann and Bianca Menrath for excellent technical assistance.
Sources of Funding
This study was supported in part by grants from the University of Heidelberg and the Deutsche Forschungsgemeinschaft (FRONTIERS program to D.T.), from the German Heart Foundation/German Foundation of Heart Research (to D.T.), and from the Max-Planck-Society (TANDEM project to P.A.S.).
Disclosures
None.
References
- 1.Fox K, Ford I, Steg PG, Tendera M, Ferrari RBEAUTIFUL Investigators Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:807-816 [DOI] [PubMed] [Google Scholar]
- 2.Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari RBEAUTIFUL investigators Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet. 2008;372:817-821 [DOI] [PubMed] [Google Scholar]
- 3.Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari R. Relationship between ivabradine treatment and cardiovascular outcomes in patients with stable coronary artery disease and left ventricular systolic dysfunction with limiting angina: a subgroup analysis of the randomized, controlled BEAUTIFUL trial. Eur Heart J. 2009;30:2337-2345 [DOI] [PubMed] [Google Scholar]
- 4.Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi LSHIFT Investigators Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875-885 [DOI] [PubMed] [Google Scholar]
- 5.Böhm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi LThe SHIFT Investigators Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376:886-894 [DOI] [PubMed] [Google Scholar]
- 6.DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351:145-147 [DOI] [PubMed] [Google Scholar]
- 7.DiFrancesco D. The role of the funny current in pacemaker activity. Circ Res. 2010;106:434-446 [DOI] [PubMed] [Google Scholar]
- 8.Donahue JK, Heldman AW, Fraser H, McDonald AD, Miller JM, Rade JJ, Eschenhagen T, Marban E. Focal modification of electrical conduction in the heart by viral gene transfer. Nat Med. 2000;6:1395-1398 [DOI] [PubMed] [Google Scholar]
- 9.Bauer A, McDonald AD, Nasir K, Peller L, Rade JJ, Miller JM, Heldman AW, Donahue JK. Inhibitory G protein overexpression provides physiologically relevant heart rate control in persistent atrial fibrillation. Circulation. 2004;110:3115-3120 [DOI] [PubMed] [Google Scholar]
- 10.Bikou O, Thomas D, Trappe K, Lugenbiel P, Kelemen K, Koch M, Soucek R, Voss F, Becker R, Katus HA, Bauer A. Connexin 43 gene therapy prevents persistent atrial fibrillation in a porcine model. Cardiovasc Res. 2011;92:218-225 [DOI] [PubMed] [Google Scholar]
- 11.Schmidt C, Kisselbach J, Schweizer PA, Katus HA, Thomas D. The pathology and treatment of cardiac arrhythmias: Focus on atrial fibrillation. Vasc Health Risk Manag. 2011;7:193-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soucek R, Thomas D, Kelemen K, Bikou O, Seyler C, Voss F, Becker R, Koenen M, Katus HA, Bauer A. Genetic suppression of atrial fibrillation using a dominant-negative ether-a-go-go-related gene mutant. Heart Rhythm. 2012;9:265-272 [DOI] [PubMed] [Google Scholar]
- 13.Trappe K, Thomas D, Bikou O, Kelemen K, Lugenbiel P, Voss F, Becker R, Katus HA, Bauer A. Suppression of persistent atrial fibrillation by genetic knockdown of caspase 3—a preclinical pilot study. Eur Heart J. 2011doi: 10.1093/eurheartj/ehr269 [DOI] [PubMed] [Google Scholar]
- 14.Doevendans PA, Becker KD, An RH, Kass RS. The utility of fluorescent in vivo reporter genes in molecular cardiology. Biochem Biophys Res Commun. 1996;222:352-358 [DOI] [PubMed] [Google Scholar]
- 15.Hou L, Deo M, Furspan P, Pandit SV, Mironov S, Auerbach DS, Gong Q, Zhou Z, Berenfeld O, Jalife J. A major role for HERG in determining frequency of reentry in neonatal rat ventricular myocyte monolayer. Circ Res. 2010;107:1503-1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA. 1998;95:2979-2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staudacher I, Wang L, Wan X, Obers S, Wenzel W, Tristram F, Koschny R, Staudacher K, Kisselbach J, Koelsch P, Schweizer PA, Katus HA, Ficker E, Thomas D. hERG K+ channel-associated cardiac effects of the antidepressant drug desipramine. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:119-139 [DOI] [PubMed] [Google Scholar]
- 18.Aistrup GL, Cokic I, Ng J, Gordon D, Koduri H, Browne S, Arapi D, Segon Y, Goldstein J, Angulo A, Wasserstrom JA, Goldberger JJ, Kadish AH, Arora R. Targeted nonviral gene-based inhibition of Gαi/o-mediated vagal signaling in the posterior left atrium decreases vagal-induced atrial fibrillation. Heart Rhythm. 2011;8:1722-1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schweizer PA, Duhme N, Thomas D, Becker R, Zehelein J, Draguhn A, Bruehl C, Katus HA, Koenen M. cAMP sensitivity of HCN pacemaker channels determines basal heart rate but is not critical for autonomic rate control. Circ Arrhythm Electrophysiol. 2010;3:542-552 [DOI] [PubMed] [Google Scholar]
- 20.Vinogradova TM, Lakatta EG. Regulation of basal and reserve cardiac pacemaker function by interactions of cAMP-mediated PKA-dependent Ca2+ cycling with surface membrane channels. J Mol Cell Cardiol. 2009;47:456-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lakatta EG, Maltsev VA, Vinogradova TM. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart's pacemaker. Circ Res. 2010;106:659-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, Lubsen J, Lutiger B, Metra M, Remme WJ, Torp-Pedersen C, Scherhag A, Skene ACarvedilol Or Metoprolol European Trial Investigators Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7-13 [DOI] [PubMed] [Google Scholar]
- 23.Figulla HR, Krzeminska-Pakula M, Wrabec K, Chochola J, Kalmbach C, Fridl P. Betaxolol is equivalent to carvedilol in patients with heart failure NYHA II or III: result of a randomized multicenter trial (BETACAR Trial). Int J Cardiol. 2006;113:153-160 [DOI] [PubMed] [Google Scholar]
- 24.Lechat P, Hulot JS, Escolano S, Mallet A, Leizorovicz A, Werhlen-Grandjean M, Pochmalicki G, Dargie H. Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II Trial. Circulation. 2001;103:1428-1433 [DOI] [PubMed] [Google Scholar]
- 25.Lauzier B, Vaillant F, Gelinas R, Bouchard B, Brownsey RW, Thorin E, Tardif JC, Des Rosiers C. Ivabradine reduces heart rate while preserving metabolic fluxes and energy status of healthy normoxic working hearts. Am J Physiol Heart Circ Physiol. 2011;300:H845-H852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beere PA, Glagov S, Zarins CK. Retarding effect of lowered heart rate on coronary atherosclerosis. Science. 1984;226:180-182 [DOI] [PubMed] [Google Scholar]


