Abstract
While the enhanced permeability and retention effect may promote the preferential accumulation of nanoparticles into well-vascularized primary tumors, it is ineffective in the case of metastases hidden within a large population of normal cells. Due to their small size, high dispersion to organs, and low vascularization, metastatic tumors are less accessible to targeted nanoparticles. To tackle these challenges, we designed a nanoparticle for vascular targeting based on an αvβ3 integrin-targeted nanochain particle composed of four iron oxide nanospheres chemically linked in a linear assembly. The chain-shaped nanoparticles enabled enhanced ‘sensing’ of the tumor-associated remodeling of the vascular bed offering increased likelihood of specific recognition of metastatic tumors. Compared to spherical nanoparticles, the chain-shaped nanoparticles resulted in superior targeting of αvβ3 integrin due to geometrically enhanced multivalent docking. We performed multimodal in vivo imaging (Fluorescence Molecular Tomography and Magnetic Resonance Imaging) in a non-invasive and quantitative manner, which showed that the nanoparticles targeted metastases in the liver and lungs with high specificity in a highly aggressive breast tumor model in mice.
Keywords: Iron oxide nanoparticle, nanochain, metastasis, integrin targeting, magnetic resonance imaging
While conventional small molecule therapeutics are distributed within cancer and healthy tissues in a non-specific manner, nanoparticle delivery systems have been developed to exploit a feature of the tumor microenvironment, the so-called ‘Enhanced Permeability and Retention’ (EPR) effect.1 Due to the tumor’s leaky vasculature, the intratumoral accumulation of nanoparticles is high relative to normal tissues.2–5 While the EPR strategy may be effective in large, well-vascularized tumors, it is very ineffective in metastatic disease, which presents small clusters of malignant cells within variable tissue types.6 Notably, the 5-year survival rate of breast cancer patients sharply decreases from 98% in cases with localized primary lesions to 23% in cases of distant metastases.7 The vast majority of cancer deaths are due to metastatic disease, hence it is essential to design nanotechnology-based agents that can successfully target metastatic tumors.
Targeting a metastatic lesion within a large population of normal cells presents a unique challenge. Metastases present biobarriers due to their smaller size, higher dispersion to organs, and lower vascularization than primary tumors, making them less accessible to molecular or nanoparticle agents. However, metastatic lesions can upregulate specific cell-surface molecules and secreted factors that differ from the rest of its host organ. If the appropriate chemical specificity is selected, targeted nanoparticles could provide a unique opportunity to home imaging and therapeutic compounds to metastases. Several conceptual and experimental theories of metastatic cancer have been recently explored that may be exploited for nanoparticle targeting.8, 9 For example, β3 integrins have shown to play a key role in migration, invasion and metastasis.10–16 It has been shown that the metastatic site transitions from selectin-dependent tumor cell rolling on the endothelium to firm attachment that is mediated primarily by αvβ3 integrins.10, 12 Thus, αvβ3 integrin is a very attractive target.17 While various studies have used integrin-binding peptides to target nanoparticles to tumors,16–24 the preclinical development of these systems has focused predominantly on tumors at the primary site. Taking under consideration the microenvironment of metastatic tumors, a targeted nanoparticle capable of ‘sensing’ the metastasis-associated remodeling of vascular bed may offer an increased likelihood of specific recognition of tumors. In this work, we report the design of a nanoparticle targeting αvβ3 integrin for non-invasive imaging of metastasis (Figure 1a). Specifically, we selected the cyclic tripeptide arginine–glycine–aspartic acid (cRGD) peptide as the ligand for vascular targeting of the nanoparticle to metastases.19, 23, 24 We exploited the nanochain technology to fabricate a chain-shaped nanoparticle composed of four iron oxide (IO) nanospheres chemically linked into a linear assembly (Figure 1b).25, 26 The high aspect ratio and flexibility of the nanoparticle substantially increased its chances to successfully seek metastatic lesions due to geometrically enhanced multivalent docking27 to the vasculature of metastases. Due to this high avidity and increased magnetic relaxivity of the nanochain particles26, we were able to detect metastatic lesions in an aggressive breast tumor model in mice using small animal fluorescence molecular tomography (FMT) and magnetic resonance imaging (MRI). This approach should also be useful for selective targeting of therapeutic agents to metastases.
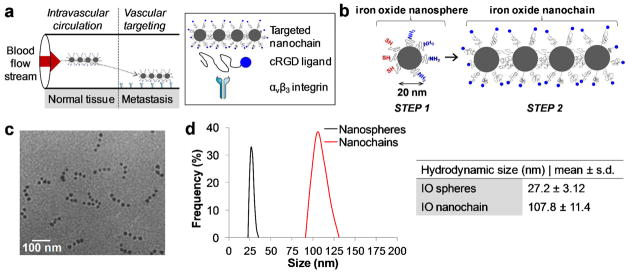
Figure 1.
Characterization of the RGD-NC nanoparticles. (a) Illustration of the models for the successful delivery of RGD-NC nanoparticles to metastasis via vascular targeting. (b) Diagram of the RGD-NC nanoparticle and its constituent components. (c) TEM image of RGD-NC nanoparticles predominantly composed of four IO spheres. (d) Size distribution of the parent IO nanospheres and RGD-NC nanoparticles obtained by DLS measurements.
RESULTS
Fabrication and characterization of the integrin-targeted nanoparticle
Fabrication of the integrin-targeted nanoparticle (termed RGD-NC) was based on the nanochain technology,26 which is a two-step approach using solid-phase chemistry. In the first step, amine-functionalized IO nanospheres were attached on a solid support via a crosslinker containing a disulfide bridge. Liberation of the nanosphere using thiolytic cleavage created thiols on the portion of the particle’s surface that interacted with the solid support resulting in a particle with two faces, one displaying only amines and the other only thiols. Therefore, we were able to topologically control the conversion of amines on the surface of the IO nanospheres into thiols, resulting in a particle with asymmetric surface chemistry. In the second step, employing solid-phase chemistry and step-by-step addition of particles, the two unique faces on the same IO nanosphere served as fittings to assemble them into linear nanochains (Figure 1b). The nanochains were analyzed via visual inspection of multiple TEM images. As shown in Figure 1c, the nanochains were synthesized in a highly controlled manner. Most of the nanochains are linear and consist of 4 IO spheres with the overall geometrical dimensions of the particle being about 100 × 20 nm (length x width). To evaluate the robustness of the nanochain synthesis, the number of IO nanospheres per nanochain was measured in TEM images. While 6% of the total particles in the suspension were the parent (unbound) IO spheres, the majority of the particles (72%) comprised of nanochains with 4 IO spheres (12 and 10% were nanochains with 3 or 5 IO spheres, respectively). As shown in Figure 1d, the hydrodynamic size of the particle and its constituent IO spheres, as measured by dynamic light scattering (DLS), verified the TEM images. It should be noted that DLS measured the effective hydrodynamic diameter based on the diffusion of the particles. Since the hydrodynamic diameter measured by DLS does not correspond to the geometrical size of non-spherical particles, we relied on visual analysis of TEM images to measure the exact dimensions of the nanochain. Detailed characterization of the nanochain particles is reported in a previous publication.26
The cyclo (Arg-Gly-Asp-D-Phe-Cys) or c(RGDfC) was conjugated onto the distal end of the PEG-NH2 on the particle’s surface. While Figure 1c shows the nanochain after modification with the RGD peptide, a TEM image of the nanochain prior to modification is shown in Figure S1 in Supporting Information. In addition to conjugation of the peptide, the nanochain particles were labeled with an NIR fluorophore (VivoTag 680) to be detectable by fluorescence imaging. To evaluate the effect of the geometry on the magnetization, we compared the r2 relaxivity of the RGD-NC particle to that of its parent IO nanospheres by measuring the transverse (R2) relaxation rates at 1.4 Tesla. The r2 value of the RGD-NC particle was 121 s−1 mM−1, which was 2.1-fold higher than that of its constituent IO spheres. Detection of metastasis via receptor-mediated targeting depends on the generation of signal from each nanoparticle. Thus, we calculated the T2 relaxivity on a per nanoparticle basis, which was 8.4 times higher for the RGD-NC particle compared to its constituent IO nanospheres.
Targeting of the RGD-NC nanoparticles to integrin-expressing endothelial cells was evaluated in vitro under static and flow conditions. Bovine aortic endothelial cells (BAEC) were treated with TNF-α to induce expression of αvβ3 integrins28 and then incubated with an excess of the RGD-NC nanoparticles for different periods of time. As shown in Figure S2a in Supporting Information, the time course of the nanoparticle uptake by the cells showed that the binding of the nanoparticles occurs rapidly during the first 30 min of incubation. In a similar manner, we evaluated the cellular uptake by 4T1 cells indicating that the integrin-targeting RGD-NC nanoparticles were also able to target the cancer cells (Figure S2b). This is significant, because the metastatic 4T1 cells colonize the endothelium as we show later in the histological evaluation.
Successful vascular targeting requires that a nanoparticle can escape the blood flow and drift towards the blood vessel walls (e.g. high margination), followed by strong attachment to the targeting site offsetting the blood flow forces that tend to detach the particle (e.g. high avidity). Since both margination and avidity of nanoparticles in circulation strongly depends on the geometry of the nanoparticle,27, 29–32 we measured the margination rates and avidity of the nanochains in microvasculature constructs under flow conditions using our previously established in vitro method.32 The experiments were conducted in a microfluidic flow network setup (Figure S2c in Supporting Information), because channel dimensions and infusion rates can be accurately controlled removing the complexity of in vivo studies. Firstly, TEM images of the nanochain suspension in cell culture media were obtained, after the nanoparticles were flowed in the microchannel for 20 min at 50 μL/min, indicating that the particles maintain their structural integrity under flow conditions (Figure S2c). To separate margination from targeting avidity, we initially evaluated the margination of non-targeted nanochains. To avoid undesirable specific binding events, the channel was coated with fibronectin, which captures marginating particles in a broad non-specific manner.32 At a flow rate of 50 μL/min, which is in the range of expected blood flow in tumor microcirculation,33 the nanochain exhibited 2.3-fold higher margination than the IO sphere (Figure S2d). Targeting avidity of the RGD-NC particle was also assessed under flow using the microfluidic device coated with TNF-α-treated BAEC cells. RGD-NC nanoparticles and RGD-targeted nanospheres displayed a biphasic behavior comprising of an initial rapid attachment phase followed by a slower attachment rate (Figure S2e). Importantly, after 5 and 20 min, the RGD-NC nanoparticles achieved 9.5 and 2.9-fold higher attachment compared to their spherical counterparts. We should note that the ligand density on the surface of nanospheres or nanochains was the same being about 25 RGD peptides per sphere. Thus, the total number of RGD peptides on a nanochain was about 100.
Targeting the primary tumor
We used the orthotopic 4T1 mammary tumor model in mice to assess the potential utility of RGD-NC nanoparticles for detection of primary tumors and metastases. The 4T1 cell line is one of the few breast cancer models that efficiently metastasizes to sites and organs similar to that observed in the human disease.34, 35 Previous studies34 have shown that growth of cells at the primary site displays a biphasic behavior: 1) the primary tumor rapidly grows in the first 2 weeks after inoculation of tumor cells in the mammary fat pad; 2) the tumor shrinks in the next 2 weeks due to infiltration of leukocytes and extensive necrosis; 3) during the 5th week, the tumor grows again with metastases occurring primarily in the liver, spleen and lungs.
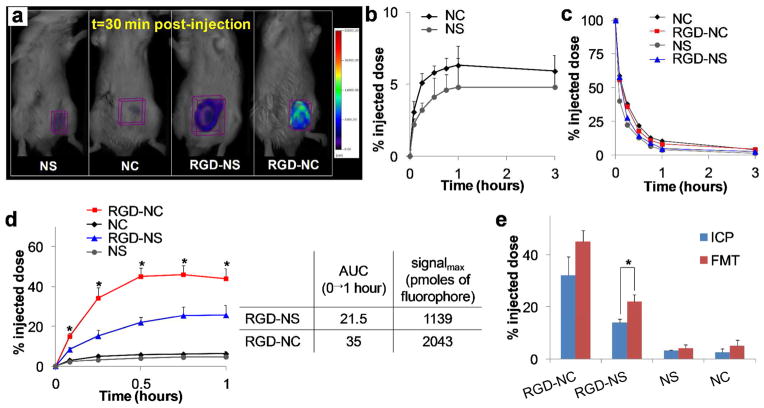
Initially, we compared the RGD-NC particles to RGD-targeted IO nanospheres (RGD-NS) and their non-targeted variants in their ability to target the primary tumor (early-stage: week 2 after tumor inoculation). All formulations were administered at a dose containing an equal number of particles per kg of body weight (i.e. 1.3 × 1014 particles/kg b.w. corresponding to 0.21 and 0.87 mg Fe/kg b.w. for the IO nanospheres and nanochains, respectively). Using Fluorescence Molecular Tomography (FMT) imaging, we non-invasively and quantitatively monitored the time-dependent intratumoral accumulation of the various particles (equal number of NIR fluorophores/particle). Figure 2a shows representative FMT images that were taken 30 min after systemic administration of the formulations. Due to their enhanced multivalent docking, the RGD-NC nanoparticles substantially outperformed the targeted nanospheres. As expected, the non-targeted nanochains exhibited slow accumulation into the primary tumor primarily due to the EPR effect. As shown in Figure 2b, the intratumoral accumulation of the non-targeted nanochains was about 5.9% of the injected dose at t=3 hours post-injection. We should emphasize that the nanochains and nanospheres were not fully covered with the polyethylene glycol (PEG) coating. This is evident by the blood residence time of the nanoparticles shown in Figure 2c (blood t1/2 ~20 min; measured in the heart), since the circulation time of nanoparticles depends on the degree of PEG shielding.36, 37 Our objective was to detect signal from the RGD-NC particles targeting integrins on the tumor-associated vascular bed with no interference from signal in the vascular site. If the particles are bound to the tumor vasculature, the time point of maximum signal from the tumor site should coincide with low concentration of the nanoparticles in the bloodstream. Figure 2d shows the time course of the fluorescent signal in the tumor for each formulation. Indeed, the RGD-NC-injected animals displayed maximum signal in the tumor in the 30–60 min time window, while the nanoparticles were almost depleted from the bloodstream. Most importantly, at t=45 min after injection, the tumor accumulation of RGD-NC was 8-fold higher than their non-targeted variant. At that time point, vascular targeting via the RGD peptide resulted in more than 40% of the administered nanochains being localized in the primary tumor. Quantitative measures of the intratumoral deposition of each particle (i.e. area under the curve and maximum signal denoted as AUC0→1h and signalmax, respectively) are shown in the table of Figure 2d. In general, these data are consistent with the in vitro targeting experiments showing that the RGD-NC particles achieved significantly higher vascular targeting at an earlier time point. At later time points, low levels of agent remained in circulation 24 hours after administration. Mostly the agent was found in the liver and spleen with less that 5% of the injected dose being in the heart, kidney.
Figure 2.
Evaluation of the ability of the RGD-NC nanoparticles to target primary 4T1 tumors (early-stage tumor model: week 2 after tumor inoculation). (a) FMT images show the accumulation of RGD-targeted and non-targeted IO spheres and nanochains in primary tumors at 30 min post-injection (dose: equal number of particles per kg of body weight). The nanoparticles of each formulation exhibited the same fluorescence signal per particle. (b) Quantification of the time-course of accumulation of the non-targeted nanospheres and nanochains in the tumor due to the EPR effect. (c) Time-course of the amount of nanoparticles in the heart as a measure of the blood residence time of each formulation. (d) Comparison of the intratumoral accumulation of targeted nanochains and nanospheres and their non-targeted variants in the first 1 hour after administration. It should be noted the range of x- and y-axis are different between Figures 2b and c. While the RGD-targeted IO spheres exhibited higher tumor accumulation than the non-targeted formulations, they were substantially outperformed by the RGD-NC nanoparticles (data presented as mean ± standard deviation). (e) The primary tumors of animals injected with NS, NC, RGD-NS or RGD-NC were perfused, excised, and weighted 30 min after administration. After digestion of the tissues, the iron concentration was measured using inductively coupled plasma optical emission spectroscopy (ICP-OES). Control animals were used to correct for background levels of endogenous iron. In the FMT and ICP measurements, data points marked with asterisks are statistically significant relative to all groups (n = 6 animals per formulation; * P < 0.05).
To validate the in vivo FMT-based measurements, the iron concentration of the primary tumors of animals injected with the formulations was directly measured ex vivo using ICP-OES (inductively coupled plasma optical emission spectroscopy). Animals injected with saline were used for correction of the background levels of iron in the tumor tissue. Fig. 2e shows the intratumoral iron content 30 min after injection of targeted and non-targeted nanochains and nanospheres, verifying the patterns observed with FMT imaging. While the FMT measurements slightly overestimated the intratumoral concentration of the nanoparticles compared to the ICP measurement, the only statistically different condition was the case of RGD-NS. Notably, ICP confirmed that more than 35% of the administered RGD-NC particle accumulated in the tumor within 30 min after administration.
Targeting metastases
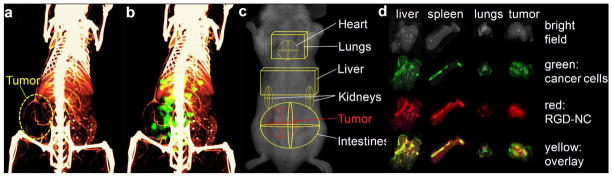
To evaluate the ability of the RGD-NC particles to target metastasis, we used mice with a late stage 4T1 tumor (week 5 after tumor inoculation). We performed whole body angiography at 99 μm resolution using a micro-CT system (Siemens Inveon) and a liposomal imaging agent encapsulating a high cargo of an iodinated contrast agent.38–40 Consistent with previous reports,34 Figure 3a indicates that the primary tumor of this animal model at a late-stage presented a necrotic core with little internal vascularization and a vascularized periphery. The same animal was systemically injected with RGD-NC nanoparticles tagged with an NIR fluorophore and imaged with the FMT system. 3D-rendered volumes of the micro-CT angiogram and the FMT image (45 min post-injection) were co-registered using a script-based software (in MATLAB) and the interactive visualization software Amira (Figure 3b). The accumulation of the RGD-NC particles in the primary tumor site was primarily observed in the location of blood vessels, which is consistent with the expression of integrins due to tumor-associated angiogenesis. Importantly, significant fluorescence signal was detected in other organs away from the primary tumor site. Based on previously published work,41 regions of interest (ROIs) were selected in the FMT image to indicate the location of major organs (Figure 3c), showing that the RGD-NC nanoparticles accumulated in other organs besides the primary tumor (e.g. liver and lungs). To confirm the colocalization of RGD-NC particles and metastatic tumors, organs were imaged ex vivo using a CRi Maestro fluorescence imaging system, since the 4T1 cell line was engineered to stably express green fluorescence protein (GFP). We should note that the fluorescence signals from GFP and the nanoparticle’s NIR tag do not overlap. Figure 3d confirms the presence of metastatic tumors in the liver, spleen and lungs (other organs are not shown, since no signal from GFP was detected). Grossly, the metastatic tumors appeared as white nodules in bright field imaging, compared to the darker liver parenchyma. More importantly, the signals from metastatic cancer cells (green) and RGD-NC particles (red) overlaid significantly (yellow) indicating the localization of the nanoparticles in metastatic lesions.
Figure 3.
Imaging of metastases. (a) Micromorphological imaging of normal and tumor vasculature at 99 Jm resolution of a metastatic 4T1 tumor (week 5) using a Siemens Inveon micro-CT and a liposome-based iodinated contrast agent. (b) Co-registration of the micro-CT image with the FMT image of the same animal injected with the RGD-NC nanoparticles. (c) The ROIs indicate the location of the tumor and different organs as obtained from previously published work.41 (d) Using a CRi Maestro fluorescence imaging system, ex vivo imaging of organs indicates the colocalization of RGD-NC particles and 4T1 metastatic cells expressing GFP.
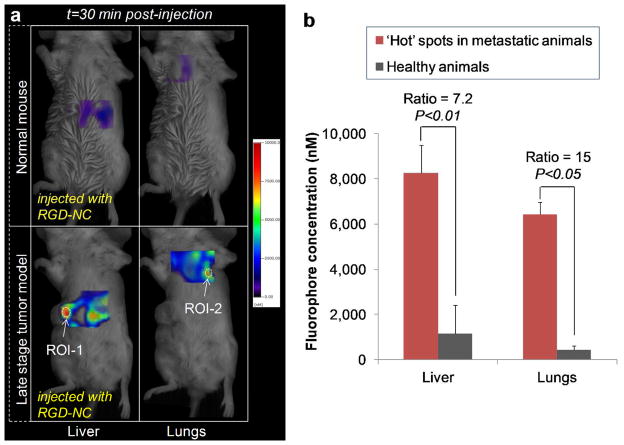
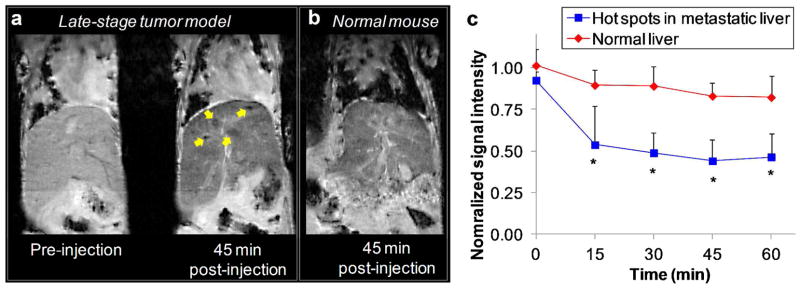
The efficacy of the RGD-NC nanoparticles to target metastatic tumors was quantitatively evaluated in a group of mice harboring metastatic 4T1 tumors (n=6) using FMT imaging. Figure 4a shows representative images of a normal mouse (top row) and a mouse with metastases (bottom row) imaged with the FMT system at t=30 min after injection of RGD-NC. The relatively low signal in the lungs of normal animals (n=6) suggested the presence of the agent primarily in the bloodstream. Since RGD-NC nanoparticles are primarily cleared by liver Kupffer cells (and splenic macrophages), the liver of the same animals exhibited relatively appreciable signal compared to the lungs. On the other hand, FMT imaging of mice with late stage 4T1 tumors showed significant accumulation of RGD-NC primarily in regions of the liver and lungs. Using the designated ROIs for each organ (as shown in Figure 3c), we measured the concentration of RGD-NC in locations of those organs displaying significantly enhanced signal (i.e. hot spots). In each ‘metastatic’ animal, we identified 1–3 hot spots in the liver and lungs designated as ROI-1 and ROI-2 in Figure 4a, respectively. The quantitative analysis shown in Figure 4b revealed a significant concentration of the agent in these hot spots. More importantly, these hot spots displayed a 15 and 7.2-fold increase of signal compared to the background signal in healthy liver and lungs.
Figure 4.
Evaluation of the ability of the RGD-NC nanoparticles to target metastatic 4T1 tumors (late-stage tumor model: week 5 after tumor inoculation). (a) Representative FMT images show the accumulation of the RGD-NC particles in the liver and lungs of healthy and metastasis-bearing mice at 30 min post-injection. In the animal with metastases, hot spots with a significantly elevated concentration of the particles are indicated in the liver and spleen as ROI-1 and ROI-2, respectively. (b) Quantification of the fluorescence signal obtained from the FMT images of a group of healthy mice and a group of metastatic mice 30 min after injection of RGD-NC particles (data presented as mean ± standard deviation). The signal of the hot spots in the lungs and liver of the metastatic group was compared to the average signal of these organs in the healthy group (n = 6 animals per group).
Imaging of metastasis using MRI
To detect metastases using a clinically relevant imaging modality, we performed imaging with MRI. Figure 5 shows representative coronal T2-weighted images of healthy mice (n=3) and metastatic 4T1 mice (n=2) obtained using a 9.4 Tesla MRI before and after administration of the RGD-NC nanoparticles (at a dose of 1.74 mg Fe/kg b.w.). This dose is substantially lower than typical dose of IO nanoparticles used in MR imaging studies (e.g. 10 mg Fe/kg).42 MR images were acquired a few minutes prior to injection of the agent and 15, 30, 45 and 60 min after injection. The scanning parameters in the pre- and post-injection images were identical. Figure 5a compares the pre-injection and 45-min post-injection images of the liver in a metastatic animal. The uptake of the agent by the macrophages in the liver generated an appreciable negative contrast. However, targeting of the RGD-NC nanoparticles to metastatic lesions achieved a significantly higher negative contrast (yellow arrows in Figure 5a) that ‘overshadowed’ the background contrast in the liver. As described in the next section, histological evaluation of the liver confirms the accumulation of the agent in metastatic tumors. Figure 5b shows a 45-min post-injection image of a healthy liver demonstrating that the uptake of the agent in the liver generated homogeneous contrast with no ‘hot’ spots. To quantitatively evaluate the ability of the RGD-NC nanoparticles to target metastasis, the absolute MR signal intensity in the metastatic lesions and the healthy liver was measured using manually drawn regions of interest (ROI). Figure 5c shows that the time-course of the signal intensity in the hot spots or the entire healthy liver (normalized to the signal of an adjacent muscle; scale: 0–1). Lower values indicate greater contrast in T2 images, and a normalized intensity value of 1 corresponds to no contrast compared to the pre-contrast image. The pre-injection values for both the normal liver and the ‘hot’ spots in the metastatic liver were fairly similar and close to 1. As expected, due to clearance of the particles by the liver, injection of RGD-NC resulted in contrast enhancement in the healthy liver with an intensity value of 0.83. However, the metastatic lesions exhibited a normalized signal intensity value of ~0.44 in the post-injection images, indicating significantly higher contrast compared to the post-injection background signal of the healthy or uninvolved regions of the metastatic liver. It is important to note that this contrast may be further improved by optimization of the MRI imaging parameters or by quantifying the T2* relaxation values directly.
Figure 5.
Representative in vivo MR images of the liver in normal and ‘metastatic’ mice using a 9.4 Tesla MRI. (a) Coronal T2-weighted images of the liver of a metastatic mouse before and 45 min after injection of the RGD-NC nanoparticles. In the 45-min post-injection image, the yellow arrows show micrometastases of about 0.5 mm in size with increased contrast enhancement. (b) Coronal T2-weighted images of the liver of a normal mouse 45 min after injection of the RGD-NC nanoparticles. (c) The time-course of the MR signal intensity in the liver ‘hot’ spots was quantitatively evaluated. The absolute MR signal intensity in the metastatic lesions and the healthy liver was measured in manually drawn ROIs. The signal intensity in the hot spots or the entire healthy liver was normalized to the signal of an adjacent muscle (scale: 0–1). Since lower values indicate greater contrast in T2 images, normalized intensity values of 0 and 1 correspond to maximum and minimum contrast, respectively, compared to the pre-contrast intensity values (data presented as mean ± standard deviation; n=3; each metastatic animal exhibited 2–4 hot spots; *P<0.05).
Histological evaluation
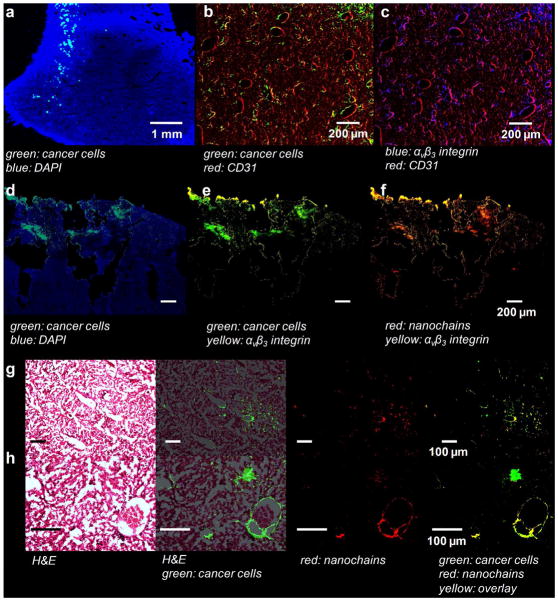
After the last in vivo imaging session, tissues were collected and histological analysis was performed to confirm the localization of the RGD-NC nanoparticles in metastatic tumors. We histologically examined the animals used in the in vivo imaging studies verifying the presence of RGD-NC nanoparticles in the majority of metastases. We evaluated the location of the metastatic cancer cells with respect to blood vessels and the associated expression of αvβ3 integrin. Using fluorescence microscopy, we verified that metastatic tumors were present in the liver and lungs of all the animals at a late stage (week 5 after tumor inoculation). Images of entire histological sections of the organs were obtained at a low magnification (5x) using the automated tiling function of the microscope. A representative image of the left lobe of liver is shown in Figure 6a displaying the presence of clusters of metastatic cells (green) dispersed in the liver parenchyma. Imaging at higher magnification showed that the metastatic cancer cells were localized primarily on the endothelial walls (Figure 6b). Furthermore, these were exactly the locations that exhibited ‘remodeling’ as indicated by the overexpression of αvβ3 integrins (Figure 6c). We should note that negligible integrin expression was observed in the normal parenchyma of liver (images not shown). The expression of integrins of metastatic cancer cells on the endothelium should favor vascular targeting of the RGD-NC nanoparticles to metastatic tumors. Indeed, fluorescence microscopy showed that RGD-NC particles were predominantly distributed around those same blood vessels colonized by 4T1 cells. Furthermore, the nanoparticles colocalized with the integrin expression of 4T1 cancer cells (Fig, 6d–f). In addition to fluorescence microscopy showing the colocalization of cancer cells and RGD-NC particles, bright field microscopy was performed on the same histological sections after standard hematoxylin-eosin staining (Figure 6g–h).
Figure 6.
Histological evaluation of the microdistribution of the RGD-NC nanoparticles in the liver of animal with metastatic tumors. (a) Fluorescence image of a histological section of the left lobe of the liver (5x magnification; blue: nuclear stain (DAPI); green: 4T1 cancer cells (GFP)). Images of entire histological sections of the organs were obtained using the automated tiling function of the microscope. (b–c) The location of metastatic cancer cells is shown with respect to endothelial cells and expression of αvβ3 integrin in the same histological section (10x magnification; green: 4T1 cancer cells; red: endothelial cells; blue: αvβ3 integrin). (d–f) The RGD-NC particles accumulated in locations of 4T1 cells that expressed avβ3 integrin (10x magnification; blue: DAPI; green: 4T1 cancer cells; yellow: αvβ3 integrin; red: RGD-NC). (g–h) Fluorescence and bright field microscopy was performed on histological sections stained with hematoxylin-eosin showing the colocalization of RGD-NC and cancer cells and their relative anatomical location in the liver (5x and 10x magnification in g and h; green: 4T1 cancer cells; red: RGD-NC; yellow: overlay).
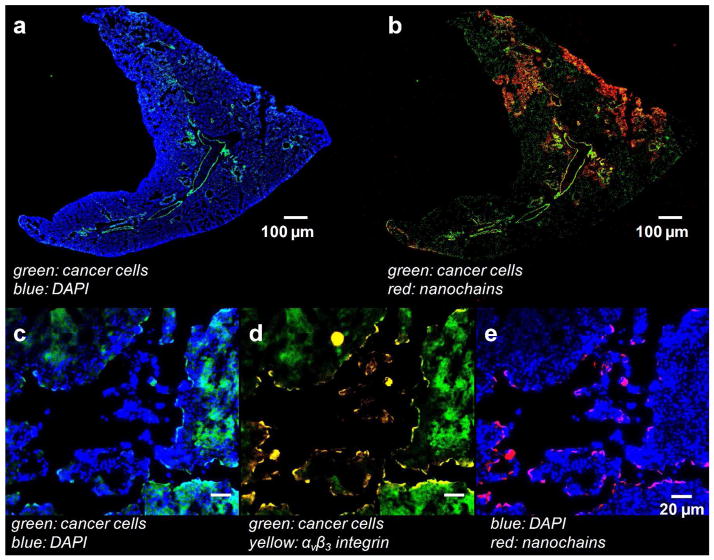
We also performed histological analysis on the lungs of the same animals. Figure 7a (Supporting Information) shows that micrometastases were present in the lungs at week 5 after tumor inoculation. Similarly to the liver, the RGD-NC particles accumulated in those locations that were colonized by metastatic cancer cells (Figure 7b). Notably, Figures 7c–e indicate that the location of RGD-NC coincided with overexpression of αvβ3 integrins on the metastasized cancer cells.
Figure 7.
Histological evaluation of the microdistribution of the RGD-NC nanoparticles in the lungs of animal with metastatic tumors. (a–b) The colocalization of fluorescently-tagged RGD-NC particles and metastatic cancer cells is shown in the same histological section (5x magnification; green: 4T1 cancer cells; red: RGD-NC; blue: DAPI). (c–e) The location of RGD-NC particles is shown with respect to metastatic cancer cells and expression of αvβ3 integrin in the same histological section (40x magnification; blue: DAPI; green: 4T1 cancer cells; yellow: αvβ3 integrin red: RGD-NC).
DISCUSSION
By defining the topology of two different functional groups on the surface of the parent IO nanospheres, we were able to assemble four nanospheres in a linear orientation. Furthermore, the robustness of the nanochain synthesis25, 26 enabled fabricating the chain-shaped nanoparticle with a high degree of uniformity. The effect of shape43, 44 and clustering of iron oxide cores45, 46 significantly increased the T2 relaxivity per particle compared to their spherical counterparts, which is highly beneficial in imaging of vascular targets.
Another advantage of the nanochain technology is the control of the shape and overall dimensions of the nanoparticle. The in vitro flow and in vivo FMT imaging studies revealed that the oblate shape of the RGD-NC nanoparticles resulted in superior attachment of the nanochains to the target site compared to spherical nanoparticles. The shape of nanoparticles has been shown to play a critical role in their margination towards the vessel walls in microcirculation.30, 31, 47 In contrast to spherical particles, oblate-shaped particles are subjected to torques resulting in tumbling and rotation.29, 30 These complex dynamics of non-spherical particles cause translational as well as rotational motions. In a previous study,32 we showed that a rod-shaped nanoparticle displayed ~8 times higher margination rate than a comparable nanosphere. Thus, by defining the geometry of the RGD-NC nanoparticle with an aspect ratio of about 5,27, 48 the particles had an increased ability to sense the overexpression of specific receptors (i.e. β3 integrins) of the tumor-associated endothelium, which was our ‘docking site’ on metastatic tumors.
In addition to the high probability of the RGD-NC particles to interact with the vascular bed of tumors, the shape of the RGD-NC particles enhanced their adhesive strength for vascular targeting. In general, while nanoparticles provide enhanced targeting avidity due to multivalent interactions, the geometry of the nanochains further enhanced the ligand-receptor interactions increasing the likelihood of specific recognition of tumors. Successful vascular targeting requires that the adhesive strength of the nanoparticle offsets the hemodynamic forces applied on the particle tending to detach the particle from the endothelium. In comparison to spherical nanoparticles, oblate-shaped nanoparticles, such as the RGD-NC nanoparticle, form a greater number of ligand-receptor occurrences per particle while the blood flow-related forces are lower.27
Even with their high avidity, one would expect a relatively small number of RGD-NC particles to accumulate at sites of metastasis due to the small size, dispersion and low degree of neovascularization of metastatic lesions. While tumors larger than 100 mm3 may favor the EPR effect resulting in deposition of high amounts of nanoparticles in primary tumors, this is not the case for metastatic tumors.49 For example, liver is one of the most common organs for metastasis of breast cancer.50, 51 Typically, normal liver displays a higher vascular density than liver metastases.52, 53 Post mortem analysis of histological liver sections from women with metastatic breast cancer revealed that metastatic tumors presented a lower number of vessels than the liver parenchyma.50 In conjunction with the absence of EPR-driven accumulation of nanoparticles into metastases, it is apparent that successful vascular targeting becomes essential.
Thus, the biological mechanisms of metastasis have to be carefully considered to design metastasis-targeted nanomaterials. During cancer progression, tumor cells show changes in their plasticity, including the epithelial to mesenchymal transition (EMT), which is strongly associated with cell invasion and migration. Numerous factors have been identified to induce EMT including substrate binding integrins, resulting in a mesenchymal-like cell that is able to move freely by forming focal adhesions.54–56 Following intravasation, integrin expression on the circulating cancer cells plays central role in the formation of metastases at a distal site. Extensive experimental evidence also shows that tumor cells interact with platelets supporting tumor metastasis. β3 integrin-mediated binding to activated platelets protects tumor cells from the immune system and promotes their arrest at the endothelium, supporting the establishment of metastatic secondary lesions.10, 11 While initial adhesion onto endothelium involves selectins, CD44, and other glycoconjugates, the metastatic site transitions from selectin-dependent tumor cell rolling on the endothelium to firm attachment that is mediated by integrins.10, 12 Not surprisingly, analysis of the adhesiveness of blood circulating cancer cells revealed that cell attachment to the vasculature requires a key integrin, αvβ3.13–15 Therefore, peptides targeting αvβ3 integrin are exceptionally capable ligands to direct the nanochains to locations of metastasis.
Besides primary and metastatic tumors, the αvβ3 integrin is expressed in the extracellular matrix, since this integrin mediates the adhesion of normal cells to a large number of extracellular matrix proteins, including fibronectin, fibrinogen, collagen, and laminin. However, due to its size, a nanochain cannot extravasate through normal endothelium into normal tissues. More importantly, our strategy depends on vascular targeting with the targeting site being the endothelium of metastasis-associated blood vessels. While αvβ3 integrin is minimally expressed on normal resting blood vessels, it is significantly upregulated in the lumen of newly formed blood vessels within primary tumors or blood vessels that have been colonized by metastatic cancer cells.25, 57, 58 Thus, besides αvβ3 integrin in blood vessels during wounds healing,59 vascular targeting of the RGD-NC particle provides specific binding to metastatic sites.
Indeed, histological evaluation of metastatic tumors showed that clusters of metastatic cancer cells were residing on the endothelium of blood vessels, which coincided with an overexpression of integrins. More importantly, the RGD-NC particles colocalized in exactly these endothelial walls associated with the metastatic cancer cells. Even though the RGD-NC particles exhibited high avidity for integrin-expressing cells, it is very challenging to discriminate the vascular targeting-based signal from the intravascular signal of the agent circulating in the bloodstream of a tumor vessel associated with a metastatic tumor.39 While prolonged blood circulation of nanoparticles may be desirable in therapeutic scenarios, detection of metastasis will be ‘masked’, since the signal from high levels of circulating nanochains in the bloodstream will ‘interfere’ with the signal from successfully targeted nanochains on the tumor-associated vascular bed. The ideal scenario of a clinically relevant imaging protocol for detection of metastases would require a few hours using an imaging agent with suitable time windows for its targeting and clearance from the bloodstream. Such imaging agent should sufficiently circulate to effectively target metastatic lesions in a short time frame (i.e. within hours after administration), at which time point its concentration in the blood is low, resulting in the greatest contrast enhancement. Thus, the blood circulation (Figure 2c) and tumor targeting profiles of the RGD-NC nanoparticles (Figure 2d) satisfy the design criterion for a nanoparticle-based imaging agent.
In particular, imaging metastasis in the liver with targeted nanoparticles presents challenges due to the fact that nanoparticles are cleared by Kupffer cells in the liver, which creates background signal and potential false positive diagnoses. However, the distribution of cleared nanoparticles is relatively homogeneous in the liver resulting in homogeneous contrast enhancement without hot spots. Furthermore, the dose of the RGD-NC agent used in this study is relatively low resulting in low and homogeneous contrast enhancement in the liver due to clearance of the agent. Notably, the metastatic sites appeared as hot spots with significantly higher contrast enhancement than the background signal from the rest of the liver tissue. However, further studies are required to accurately measure the diagnostic sensitivity, specificity and positive predictive value of this technology.
To date, imaging applications of nanoparticles have focused predominantly on targeting tumors at the primary site (e.g. integrins, folate receptor, EGFR, HER2 etc).16–24, 60–65 Even though attention of the field has recently shifted towards metastasis, there are still very few examples of diagnostics for metastases,6 including contrast agents for radioimaging of molecular markers (e.g. targeting αvβ3 integrins66, 67 or CXCR-468), visualization aids for surgery, and HER2-targeting IO nanoparticles.69 While the detection accuracy of CT, MRI and fluorodeoxyglucose positron emission tomography (FDG-PET) for relatively large metastatic tumors (>1 cm in size) has significantly improved in the recent past,70–72 current imaging methods rarely detect the early spread of metastatic tumor cells,73 which prohibits early and most likely effective interventions. Treatment decisions of metastatic disease (e.g. surgical resection, radiation, thermal and radio-frequency ablation) heavily depends on imaging and diagnostic tools to detect the precise location and extent of disease spread.74 Micrometastases (0.2–2 mm in size) can rarely be detected via changes in perfusion using radionuclide or Doppler perfusion techniques.75, 76 In this work, we show that nanoparticles offer unique opportunities to deal with the complexity of metastatic cancer by exploiting properties that appear at the nano-scale.
CONCLUSION
In conclusion, we demonstrated that the geometry of a chain-shaped nanoparticle promoted targeting of metastatic tumors due to multivalent docking onto integrins of the vascular bed of metastasis. Using multimodal in vivo imaging (i.e. FMT and MRI), we were able to image metastatic tumors in the liver and lungs in a highly aggressive breast tumor model. As the biological mechanisms of metastasis continue to unravel, we expect that more surface markers of metastatic lesions will be identified that can be employed by nanoparticle delivery systems for targeting metastatic disease.
METHODS
Synthesis and characterization of the nanochain particles
The nanochains were synthesized following our previously published method.26 Briefly, solid-phase chemistry was used to partially modify the surface functionality of IO nanospheres. CLEAR resin (Peptides International Inc, Louisville, KY) functionalized with amines was modified with a homobifunctional cleavable cross-linker reactive towards amines (DTSSP). Amine-functionalized IO nanospheres were introduced, allowed to bind to the solid support and then cleaved off using a reducing agent (TCEP). The same type of resin was used and the modified spheres with surface asymmetry were introduced in a step-by-step manner. After recovering the chain via a reducing agent, the suspension was further cleaned using dialysis. The nanoparticles were characterized in terms of their size (DLS), structure (TEM), and magnetic relaxivity (Bruker minispec relaxometer). The cyclo (Arg-Gly-Asp-D-Phe-Cys) or c(RGDfC) was conjugated onto PEG(3400) via maleimide chemistry. In addition to conjugation of the cRGDe peptide, the nanochain particles were tagged with an NIR fluorophore (Vivotag 680) to be detectable by FMT imaging or fluorescence spectroscopy or microscopy. Details of the synthesis and characterization of the nanoparticles are shown in the Supporting Information.
Mouse tumor model
All animal procedures were conducted under a protocol approved by the CWRU IACUC. We used an orthotopic 4T1 breast tumor model in mice.77 The 4T1 cell line was engineered to stable express green fluorescent protein (GFP) to allow tracking and quantification of the cells in vivo and histologically. Briefly, we inoculated 0.5 × 106 4T1 cells orthotopically in a no. 9 mammary fat pad of female BALB/c mice that was surgically exposed while the mice were anesthetized. The animals were used in the in vivo studies at week 2 (only primary tumor) or week 5 (primary and metastatic tumors). Based on our prior experience, we chose these time points, since they represent different stages of angiogenesis, necrosis, invasion, and metastasis and are informative and relevant to the human disease.
Fluorescence Molecular Tomography
We performed fluorescence imaging on the 4T1 mammary model in mice (at week 2 or 5) using the FMT 2500 Quantitative Tomography In Vivo Imaging System (Perkin Elmer). Phantoms for each nanoparticle formulation were used to calibrate the FMT to take quantitative deposition measurements. We then intravenously injected each of the four formulations at a dose of 1.3 × 1014 particles per kg b.w. The animals were imaged before and after IV injection of the formulations at multiple time points (15, 30, 45 min and 3, 6, 24 h). After the last imaging session, tumor and organs (kidneys, lungs, brain, liver, spleen and intestine) were retrieved and weighed. To verify the findings of the in vivo imaging and confirm the presence of metastases in the organs, we imaged the organs ex vivo using the FMT and a CRi Maestro fluorescence imaging system. The organs were then processed for histological analysis.
Angiography using contrast-enhanced micro-CT imaging
Contrast-enhanced angiography was performed using a Siemens Inveon micro-CT system (isotropic 99 Jm resolution, 80 kVp, 500 μA) and a long-circulating liposomal imaging agent encapsulating a high cargo of an iodinated contrast agent.39 Following IV injection of the agent at a dose of 2.6 g iodine/kg b.w., the animals were imaged with the micro-CT system. Subsequently the animals were IV injected with RGD-NC followed by imaging with the FMT system. The two images were co-registered using a semi-automatic 3D segmentation-based registration approach that was implemented in script-based software (in MATLAB) and interactive visualization software Amira (Visage Imaging Inc).78 Fiducial markers placed around the tumor mass were visible in both the micro-CT and the FMT images. Using a region growing algorithm with seed points defined by the user, we segmented the fiducial markers from both volumes.79 Then, we used Amira’s AffineTransform tool to register the floating volume landmarks to the reference volume landmarks.
MRI imaging
MRI images were acquired on a 9.4 T Bruker MRI system. A volume coil (3.5-cm inner diameter) was employed. High resolution images were obtained before and 15, 30, 45, 60 and 120 min after IV injection of the RGD-NC nanoparticles (at a dose of 7.5 mg Fe/kg b.w.) using a T2-weighted RARE sequence with the following parameters: TR/TE=1000/45 msec, matrix=128 × 128, FOV=5 × 5 cm, and 1 average. This resulted in an in-plane spatial resolution of 390 Jm and a slice thickness of 1 mm.
Histological evaluation
After the last imaging acquisition with FMT or MRI, tissues were collected from the mice for histological studies. The animals were anesthetized with an IP injection of ketamine/xylazine and transcardially perfused with heparinized PBS followed by 4% paraformaldehyde in PBS. Tumors and organs were explanted and post-fixed overnight in 4% paraformaldehyde in PBS. The tissues were soaked in 30% sucrose (w/v) in PBS at 4 °C for cryosectioning. Serial sections of 12 μm thickness were collected using a cryostat (Leica CM 300). To visualize the tumor microvasculature, the tissue slices were immunohistochemically stained for the endothelial antigen CD31 (BD Biosciences, Pharmingen). The tissues were also stained with the nuclear stain DAPI. Standard hematoxylin-eosin staining was also performed. The tissue sections were imaged at 5, 10 or 40x on the Zeiss Axio Observer Z1 motorized FL inverted microscope. To obtain an image of the entire tissue section, a montage of each section was made using the automated tiling function of the microscope.
Statistical analysis
Means were determined for each variable in this study and the resulting values from each experiment were subjected to one-way analysis of variance with post hoc Bonferroni test. A P value of less than 0.01 was used to confirm significant differences. Normality of each data set was confirmed using the Anderson-Darling test.
Supplementary Material
Acknowledgments
This work was partially supported by a grant from the American Cancer Society IRG-91-022-18 (EK) and a pilot grant from the Case Comprehensive Cancer Center P30 CA043703 (EK). R.T. was supported by a fellowship from the NIH Interdisciplinary Biomedical Imaging Training Program (5T32EB007509). Z.B. was supported by a fellowship from the NIH Medical Student Research Training grant (T35HL082544). We thank Dr. James Basilion for discussions, and Erik Schmidt, Christopher Shoup and A. Lee Rivera for help with the particle fabrication and the in vitro flow experiments. We also thank Kristen Lozada, Joseph Meyers, Gopal Ramamurthy, and Tenia Harris for help with the animal studies.
Footnotes
Supporting Information Available
Additional figures and experimental details. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor Vascular Permeability and the EPR Effect in Macromolecular Therapeutics: A Review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 2.Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, Hawkins M, O’Shaughnessy J. Phase III Trial of Nanoparticle Albumin-Bound Paclitaxel Compared with Polyethylated Castor Oil-Based Paclitaxel in Women with Breast Cancer. J Clin Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 3.Lasic DD. Doxorubicin in Sterically Stabilized Liposomes. Nature. 1996;380:561–562. doi: 10.1038/380561a0. [DOI] [PubMed] [Google Scholar]
- 4.Lasic DD, Papahadjopoulos D. Liposomes Revisited. Science. 1995;267:1275–1276. doi: 10.1126/science.7871422. [DOI] [PubMed] [Google Scholar]
- 5.Safra T. Cardiac Safety of Liposomal Anthracyclines. Oncologist. 2003;8 (Suppl 2):17–24. doi: 10.1634/theoncologist.8-suppl_2-17. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder A, Heller DA, Winslow MM, Dahlman JE, Pratt GW, Langer R, Jacks T, Anderson DG. Treating Metastatic Cancer with Nanotechnology. Nat Rev Cancer. 2012;12:39–50. doi: 10.1038/nrc3180. [DOI] [PubMed] [Google Scholar]
- 7.Cancer Facts and Figures 2011. American Cancer Society; 2011. [Google Scholar]
- 8.van Zijl F, Krupitza G, Mikulits W. Initial Steps of Metastasis: Cell Invasion and Endothelial Transmigration. Mutat Res. 2011;728:23–34. doi: 10.1016/j.mrrev.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coghlin C, Murray GI. Current and Emerging Concepts in Tumour Metastasis. J Pathol. 2010;222:1–15. doi: 10.1002/path.2727. [DOI] [PubMed] [Google Scholar]
- 10.Gay LJ, Felding-Habermann B. Contribution of Platelets to Tumour Metastasis. Nat Rev Cancer. 2011;11:123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felding-Habermann B, Habermann R, Saldivar E, Ruggeri ZM. Role of Beta3 Integrins in Melanoma Cell Adhesion to Activated Platelets under Flow. J Biol Chem. 1996;271:5892–5900. doi: 10.1074/jbc.271.10.5892. [DOI] [PubMed] [Google Scholar]
- 12.McCarty OJ, Mousa SA, Bray PF, Konstantopoulos K. Immobilized Platelets Support Human Colon Carcinoma Cell Tethering, Rolling, and Firm Adhesion under Dynamic Flow Conditions. Blood. 2000;96:1789–1797. [PubMed] [Google Scholar]
- 13.Arnaout MA, Mahalingam B, Xiong JP. Integrin Structure, Allostery, and Bidirectional Signaling. Annu Rev Cell Dev Biol. 2005;21:381–410. doi: 10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed] [Google Scholar]
- 14.Felding-Habermann B, O’Toole TE, Smith JW, Fransvea E, Ruggeri ZM, Ginsberg MH, Hughes PE, Pampori N, Shattil SJ, Saven A, et al. Integrin Activation Controls Metastasis in Human Breast Cancer. Proc Natl Acad Sci U S A. 2001;98:1853–1858. doi: 10.1073/pnas.98.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorger M, Krueger JS, O’Neal M, Staflin K, Felding-Habermann B. Activation of Tumor Cell Integrin Alphavbeta3 Controls Angiogenesis and Metastatic Growth in the Brain. Proc Natl Acad Sci U S A. 2009;106:10666–10671. doi: 10.1073/pnas.0903035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desgrosellier JS, Cheresh DA. Integrins in Cancer: Biological Implications and Therapeutic Opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen K, Chen X. Integrin Targeted Delivery of Chemotherapeutics. Theranostics. 2011;1:189–200. doi: 10.7150/thno/v01p0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang G, Zhou Z, Srinivasan R, Penn MS, Kottke-Marchant K, Marchant RE, Gupta AS. Affinity Manipulation of Surface-Conjugated Rgd Peptide to Modulate Binding of Liposomes to Activated Platelets. Biomaterials. 2008;29:1676–1685. doi: 10.1016/j.biomaterials.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang C, Jugold M, Woenne EC, Lammers T, Morgenstern B, Mueller MM, Zentgraf H, Bock M, Eisenhut M, Semmler W, et al. Specific Targeting of Tumor Angiogenesis by RGD-Conjugated Ultrasmall Superparamagnetic Iron Oxide Particles Using a Clinical 1. 5-T Magnetic Resonance Scanner. Cancer Res. 2007;67:1555–1562. doi: 10.1158/0008-5472.CAN-06-1668. [DOI] [PubMed] [Google Scholar]
- 20.Ke T, Jeong EK, Wang X, Feng Y, Parker DL, Lu ZR. RGD Targeted Poly(L-Glutamic Acid)-Cystamine-(Gd-Do3a) Conjugate for Detecting Angiogenesis Biomarker Alpha(V) Beta3 Integrin with Mrt, Mapping. Int J Nanomedicine. 2007;2:191–199. [PMC free article] [PubMed] [Google Scholar]
- 21.Danhier F, Vroman B, Lecouturier N, Crokart N, Pourcelle V, Freichels H, Jerome C, Marchand-Brynaert J, Feron O, Preat V. Targeting of Tumor Endothelium by RGD-Grafted PLGA-Nanoparticles Loaded with Paclitaxel. J Control Release. 2009;140:166–173. doi: 10.1016/j.jconrel.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Montet X, Funovics M, Montet-Abou K, Weissleder R, Josephson L. Multivalent Effects of RGD Peptides Obtained by Nanoparticle Display. J Med Chem. 2006;49:6087–6093. doi: 10.1021/jm060515m. [DOI] [PubMed] [Google Scholar]
- 23.Liu XQ, Song WJ, Sun TM, Zhang PZ, Wang J. Targeted Delivery of Antisense Inhibitor of Mirna for Antiangiogenesis Therapy Using cRGD-Functionalized Nanoparticles. Mol Pharm. 2011;8:250–259. doi: 10.1021/mp100315q. [DOI] [PubMed] [Google Scholar]
- 24.Murphy EA, Majeti BK, Barnes LA, Makale M, Weis SM, Lutu-Fuga K, Wrasidlo W, Cheresh DA. Nanoparticle-Mediated Drug Delivery to Tumor Vasculature Suppresses Metastasis. Proc Natl Acad Sci U S A. 2008;105:9343–9348. doi: 10.1073/pnas.0803728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peiris PM, Bauer L, Toy R, Tran E, Pansky J, Doolittle E, Schmidt E, Hayden E, Mayer A, Keri RA, et al. Enhanced Delivery of Chemotherapy to Tumors Using a Multicomponent Nanochain with Radio-Frequency-Tunable Drug Release. ACS Nano. 2012;6:4157–4168. doi: 10.1021/nn300652p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peiris PM, Schmidt E, Calabrese M, Karathanasis E. Assembly of Linear Nano-Chains from Iron Oxide Nanospheres with Asymmetric Surface Chemistry. PLoS One. 2011;6:e15927. doi: 10.1371/journal.pone.0015927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Decuzzi P, Ferrari M. The Adhesive Strength of Non-Spherical Particles Mediated by Specific Interactions. Biomaterials. 2006;27:5307–5314. doi: 10.1016/j.biomaterials.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 28.Gao B, Saba TM, Tsan MF. Role of Alpha(V)Beta(3)-Integrin in TNF-Alpha-Induced Endothelial Cell Migration. Am J Physiol Cell Physiol. 2002;283:C1196–1205. doi: 10.1152/ajpcell.00064.2002. [DOI] [PubMed] [Google Scholar]
- 29.Gavze E, Shapiro M. Motion of Inertial Spheroidal Particles in a Shear Flow near a Solid Wall with Special Application to Aerosol Transport in Microgravity. Journal of Fluid Mechanics. 1998;371:59–79. [Google Scholar]
- 30.Lee SY, Ferrari M, Decuzzi P. Shaping Nano-/Micro-Particles for Enhanced Vascular Interaction in Laminar Flows. Nanotechnology. 2009;20:495101. doi: 10.1088/0957-4484/20/49/495101. (11pp) [DOI] [PubMed] [Google Scholar]
- 31.Gentile F, Chiappini C, Fine D, Bhavane RC, Peluccio MS, Cheng MM, Liu X, Ferrari M, Decuzzi P. The Effect of Shape on the Margination Dynamics of Non-Neutrally Buoyant Particles in Two-Dimensional Shear Flows. J Biomech. 2008;41:2312–2318. doi: 10.1016/j.jbiomech.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 32.Toy R, Hayden E, Shoup C, Baskaran H, Karathanasis E. The Effects of Particle Size, Density and Shape on Margination of Nanoparticles in Microcirculation. Nanotechnology. 2011;22:115101. doi: 10.1088/0957-4484/22/11/115101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ganong WF. Review of Medical Physiology. 21. Lange Medical Books/McGraw-Hill, Medical Publishing Division; New York: 2003. [Google Scholar]
- 34.Tao K, Fang M, Alroy J, Sahagian GG. Imagable 4t1 Model for the Study of Late Stage Breast Cancer. BMC Cancer. 2008;8:228. doi: 10.1186/1471-2407-8-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dykxhoorn DM, Wu Y, Xie H, Yu F, Lal A, Petrocca F, Martinvalet D, Song E, Lim B, Lieberman J. Mir-200 Enhances Mouse Breast Cancer Cell Colonization to Form Distant Metastases. PLoS One. 2009;4:e7181. doi: 10.1371/journal.pone.0007181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNeeley KM, Karathanasis E, Annapragada AV, Bellamkonda RV. Masking and Triggered Unmasking of Targeting Ligands on Nanocarriers to Improve Drug Delivery to Brain Tumors. Biomaterials. 2009;30:3986–3995. doi: 10.1016/j.biomaterials.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 37.McNeeley KM, Annapragada A, Bellamkonda RV. Decreased Circulation Time Offsets Increased Efficacy of Pegylated Nanocarriers Targeting Folate Receptors of Glioma. Nanotechnology. 2007;18:385101. [Google Scholar]
- 38.Karathanasis E, Chan L, Balusu SR, D’Orsi CJ, Annapragada AV, Sechopoulos I, Bellamkonda RV. Multifunctional Nanocarriers for Mammographic Quantification of Tumor Dosing and Prognosis of Breast Cancer Therapy. Biomaterials. 2008;29:4815–4822. doi: 10.1016/j.biomaterials.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 39.Karathanasis E, Suryanarayanan S, Balusu SR, McNeeley K, Sechopoulos I, Karellas A, Annapragada AV, Bellamkonda RV. Imaging Nanoprobe for Prediction of Outcome of Nanoparticle Chemotherapy by Using Mammography. Radiology. 2009;250:398–406. doi: 10.1148/radiol.2502080801. [DOI] [PubMed] [Google Scholar]
- 40.Karathanasis E, Chan L, Karumbaiah L, McNeeley K, D’Orsi CJ, Annapragada AV, Sechopoulos I, Bellamkonda RV. Tumor Vascular Permeability to a Nanoprobe Correlates to Tumor-Specific Expression Levels of Angiogenic Markers. PLoS ONE. 2009;4:e5843. doi: 10.1371/journal.pone.0005843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vasquez KO, Casavant C, Peterson JD. Quantitative Whole Body Biodistribution of Fluorescent-Labeled Agents by Non-Invasive Tomographic Imaging. PLoS One. 2011;6:e20594. doi: 10.1371/journal.pone.0020594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crayton SH, Tsourkas A. Ph-Titratable Superparamagnetic Iron Oxide for Improved Nanoparticle Accumulation in Acidic Tumor Microenvironments. ACS Nano. 2011;5:9592–9601. doi: 10.1021/nn202863x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park JH, von Maltzahn G, Zhang LL, Schwartz MP, Ruoslahti E, Bhatia SN, Sailor MJ. Magnetic Iron Oxide Nanoworms for Tumor Targeting and Imaging. Advanced Materials. 2008;20:1630–1635. doi: 10.1002/adma.200800004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gossuin Y, Disch S, Vuong QL, Gillis P, Hermann RP, Park JH, Sailor MJ. Nmr Relaxation and Magnetic Properties of Superparamagnetic Nanoworms. Contrast Media & Molecular Imaging. 2010 doi: 10.1002/cmmi.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu J, Ma S, Sun J, Xia C, Liu C, Wang Z, Zhao X, Gao F, Gong Q, Song B, et al. Manganese Ferrite Nanoparticle Micellar Nanocomposites as MRI Contrast Agent for Liver Imaging. Biomaterials. 2009;30:2919–2928. doi: 10.1016/j.biomaterials.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Ai H, Flask C, Weinberg B, Shuai X, Pagel MD, Farrel D, Duerk J, Gao J. Magnetite-Loaded Polymeric Micelles as Ultrasensitive Magnetic-Resonance Probes. Advanced Materials. 2005;17:1949–1952. [Google Scholar]
- 47.Chauhan VP, Popovic Z, Chen O, Cui J, Fukumura D, Bawendi MG, Jain RK. Fluorescent Nanorods and Nanospheres for Real-Time In Vivo Probing of Nanoparticle Shape-Dependent Tumor Penetration. Angew Chem Int Ed Engl. 2011;50:11417–11420. doi: 10.1002/anie.201104449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Decuzzi P, Causa F, Ferrari M, Netti PA. The Effective Dispersion of Nanovectors within the Tumor Microvasculature. Ann Biomed Eng. 2006;34:633–641. doi: 10.1007/s10439-005-9072-6. [DOI] [PubMed] [Google Scholar]
- 49.Adiseshaiah PP, Hall JB, McNeil SE. Nanomaterial Standards for Efficacy and Toxicity Assessment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:99–112. doi: 10.1002/wnan.66. [DOI] [PubMed] [Google Scholar]
- 50.Nacev A, Kim SH, Rodriguez-Canales J, Tangrea MA, Shapiro B, Emmert-Buck MR. A Dynamic Magnetic Shift Method to Increase Nanoparticle Concentration in Cancer Metastases: A Feasibility Study Using Simulations on Autopsy Specimens. Int J Nanomedicine. 2011;6:2907–2923. doi: 10.2147/IJN.S23724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pagani O, Senkus E, Wood W, Colleoni M, Cufer T, Kyriakides S, Costa A, Winer EP, Cardoso F. International Guidelines for Management of Metastatic Breast Cancer: Can Metastatic Breast Cancer Be Cured? J Natl Cancer Inst. 2010;102:456–463. doi: 10.1093/jnci/djq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic Behavior of Breast Cancer Subtypes. J Clin Oncol. 2010;28:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 53.Terayama N, Terada T, Nakanuma Y. An Immunohistochemical Study of Tumour Vessels in Metastatic Liver Cancers and the Surrounding Liver Tissue. Histopathology. 1996;29:37–43. doi: 10.1046/j.1365-2559.1996.d01-484.x. [DOI] [PubMed] [Google Scholar]
- 54.Friedl P, Gilmour D. Collective Cell Migration in Morphogenesis, Regeneration and Cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 55.Bidard FC, Pierga JY, Vincent-Salomon A, Poupon MF. A “Class Action” Against the Microenvironment: Do Cancer Cells Cooperate in Metastasis? Cancer Metastasis Rev. 2008;27:5–10. doi: 10.1007/s10555-007-9103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kirfel G, Rigort A, Borm B, Herzog V. Cell Migration: Mechanisms of Rear Detachment and the Formation of Migration Tracks. Eur J Cell Biol. 2004;83:717–724. doi: 10.1078/0171-9335-00421. [DOI] [PubMed] [Google Scholar]
- 57.Brooks PC, Clark RA, Cheresh DA. Requirement of Vascular Integrin Alpha V Beta 3 for Angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 58.Brooks PC, Stromblad S, Klemke R, Visscher D, Sarkar FH, Cheresh DA. Antiintegrin Alpha V Beta 3 Blocks Human Breast Cancer Growth and Angiogenesis in Human Skin. J Clin Invest. 1995;96:1815–1822. doi: 10.1172/JCI118227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clark RA, Tonnesen MG, Gailit J, Cheresh DA. Transient Functional Expression of Alphavbeta 3 on Vascular Cells During Wound Repair. Am J Pathol. 1996;148:1407–1421. [PMC free article] [PubMed] [Google Scholar]
- 60.Huang X, Peng X, Wang Y, Shin DM, El-Sayed MA, Nie S. A Reexamination of Active and Passive Tumor Targeting by Using Rod-Shaped Gold Nanocrystals and Covalently Conjugated Peptide Ligands. ACS Nano. 2010;4:5887–5896. doi: 10.1021/nn102055s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng Y, Meyers JD, Agnes RS, Doane TL, Kenney ME, Broome AM, Burda C, Basilion JP. Addressing Brain Tumors with Targeted Gold Nanoparticles: A New Gold Standard for Hydrophobic Drug Delivery? Small. 2011;7:2301–2306. doi: 10.1002/smll.201100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gabizon A, Horowitz AT, Goren D, Tzemach D, Shmeeda H, Zalipsky S. In Vivo Fate of Folate-Targeted Polyethylene-Glycol Liposomes in Tumor-Bearing Mice. Clinical Cancer Research. 2003;9:6551–6559. [PubMed] [Google Scholar]
- 63.Gabizon A, Shmeeda H, Horowitz AT, Zalipsky S. Tumor Cell Targeting of Liposome-Entrapped Drugs with Phospholipid-Anchored Folic Acid-PEG Conjugates. Adv Drug Deliv Rev. 2004;56:1177–1192. doi: 10.1016/j.addr.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 64.Park JW, Hong K, Kirpotin DB, Colbern G, Shalaby R, Baselga J, Shao Y, Nielsen UB, Marks JD, Moore D, et al. Anti-HER2 Immunoliposomes: Enhanced Efficacy Attributable to Targeted Delivery. Clin Cancer Res. 2002;8:1172–1181. [PubMed] [Google Scholar]
- 65.Park JW, Kirpotin DB, Hong K, Shalaby R, Shao Y, Nielsen UB, Marks JD, Papahadjopoulos D, Benz CC. Tumor Targeting Using Anti-HER2 Immunoliposomes. J Control Release. 2001;74:95–113. doi: 10.1016/s0168-3659(01)00315-7. [DOI] [PubMed] [Google Scholar]
- 66.Haubner R, Wester HJ, Weber WA, Mang C, Ziegler SI, Goodman SL, Senekowitsch-Schmidtke R, Kessler H, Schwaiger M. Noninvasive Imaging of Alpha(V)Beta3 Integrin Expression Using 18f-Labeled RGD-Containing Glycopeptide and Positron Emission Tomography. Cancer Res. 2001;61:1781–1785. [PubMed] [Google Scholar]
- 67.Haubner R, Wester HJ, Burkhart F, Senekowitsch-Schmidtke R, Weber W, Goodman SL, Kessler H, Schwaiger M. Glycosylated Rgd-Containing Peptides: Tracer for Tumor Targeting and Angiogenesis Imaging with Improved Biokinetics. J Nucl Med. 2001;42:326–336. [PubMed] [Google Scholar]
- 68.Nimmagadda S, Pullambhatla M, Stone K, Green G, Bhujwalla ZM, Pomper MG. Molecular Imaging of Cxcr4 Receptor Expression in Human Cancer Xenografts with [64Cu]AMD3100 Positron Emission Tomography. Cancer Res. 2010;70:3935–3944. doi: 10.1158/0008-5472.CAN-09-4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kievit FM, Stephen ZR, Veiseh O, Arami H, Wang T, Lai VP, Park JO, Ellenbogen RG, Disis ML, Zhang M. Targeting of Primary Breast Cancers and Metastases in a Transgenic Mouse Model Using Rationally Designed Multifunctional SPIONs. ACS Nano. 2012;6:2591–2601. doi: 10.1021/nn205070h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kinkel K, Lu Y, Both M, Warren RS, Thoeni RF. Detection of Hepatic Metastases from Cancers of the Gastrointestinal Tract by Using Noninvasive Imaging Methods (US, CT, MR Imaging, PET): A Meta-Analysis. Radiology. 2002;224:748–756. doi: 10.1148/radiol.2243011362. [DOI] [PubMed] [Google Scholar]
- 71.Bipat S, van Leeuwen MS, Comans EF, Pijl ME, Bossuyt PM, Zwinderman AH, Stoker J. Colorectal Liver Metastases: CT, MR Imaging, and PET for Diagnosis--Meta-Analysis. Radiology. 2005;237:123–131. doi: 10.1148/radiol.2371042060. [DOI] [PubMed] [Google Scholar]
- 72.Niekel MC, Bipat S, Stoker J. Diagnostic Imaging of Colorectal Liver Metastases with CT, MR Imaging, FDG PET, and/or FDG PET/CT: A Meta-Analysis of Prospective Studies Including Patients Who Have Not Previously Undergone Treatment. Radiology. 2011;257:674–684. doi: 10.1148/radiol.10100729. [DOI] [PubMed] [Google Scholar]
- 73.Pantel K, Alix-Panabieres C, Riethdorf S. Cancer Micrometastases. Nat Rev Clin Oncol. 2009;6:339–351. doi: 10.1038/nrclinonc.2009.44. [DOI] [PubMed] [Google Scholar]
- 74.Robinson PJ. The Early Detection of Liver Metastases. Cancer Imaging. 2002;2:1–3. [Google Scholar]
- 75.Huvos AG, Hutter RV, Berg JW. Significance of Axillary Macrometastases and Micrometastases in Mammary Cancer. Ann Surg. 1971;173:44–46. doi: 10.1097/00000658-197101000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rampaul RS, Miremadi A, Pinder SE, Lee A, Ellis IO. Pathological Validation and Significance of Micrometastasis in Sentinel Nodes in Primary Breast Cancer. Breast Cancer Res. 2001;3:113–116. doi: 10.1186/bcr282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yori JL, Seachrist DD, Johnson E, Lozada KL, Abdul-Karim FW, Chodosh LA, Schiemann WP, Keri RA. Kruppel-Like Factor 4 Inhibits Tumorigenic Progression and Metastasis in a Mouse Model of Breast Cancer. Neoplasia. 2011;13:601–610. doi: 10.1593/neo.11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maintz JBA, Viergever MA. A Survey of Medical Image Registration. In. Medical Image Analysis. 1998;2:1–36. doi: 10.1016/s1361-8415(01)80026-8. [DOI] [PubMed] [Google Scholar]
- 79.Gonzalez RC, Woods RE. Digital Image Processing. 2. Prentice Hall; New Jersey: 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.