Abstract
In many species translocation of sucrose from the mesophyll to the phloem is carrier mediated. A sucrose/H+-symporter cDNA, NtSUT1, was isolated from tobacco (Nicotiana tabacum) and shown to be highly expressed in mature leaves and at low levels in other tissues, including floral organs. To study the in vivo function of NtSUT1, tobacco plants were transformed with a SUT1 antisense construct under control of the cauliflower mosaic virus 35S promoter. Upon maturation, leaves of transformants expressing reduced amounts of SUT1 mRNA curled downward, and strongly affected plants developed chloroses and necroses that led to death. The leaves exhibited impaired ability to export recently fixed 14CO2 and were unable to export transient starch during extended periods of darkness. As a consequence, soluble carbohydrates accumulated and photosynthesis was reduced. Autoradiographs of leaves show a heterogenous pattern of CO2 fixation even after a 24-h chase. The 14C pattern does not change with time, suggesting that movement of photosynthate between mesophyll cells may also be impaired. The affected lines show a reduction in the development of the root system and delayed or impaired flowering. Taken together, the effects observed in a seed plant (tobacco) demonstrate the importance of SUT1 for sucrose loading into the phloem via an apoplastic route and possibly for intermesophyll transport as well.
Photosynthesis in mature leaves produces a surplus of assimilates. Carbohydrates derived from mature leaves are distributed in the plant through the vascular system, mainly in the form of sucrose, to support the growth of heterotrophic tissues such as developing leaves, apices, roots, and reproductive organs. Both active transport by specific carriers across the plasma membrane and symplastic transport via plasmodesmata have been discussed as possible mechanisms for phloem loading (Ap Rees, 1994). Nevertheless, a direct demonstration of the actual role of plasmodesmata in assimilate transport is still missing. Sucrose transport activities have been identified in a number of plant species (for reviews, see Bush, 1993; Frommer et al., 1996) and have been described as sucrose:proton cotransport with a 1:1 stoichiometry (Bush, 1990; Lemoine et al., 1996).
To resolve the question of whether carrier-mediated sucrose transport represents an essential step in phloem loading, the respective genes were identified. A yeast strain was modified so that it could be used as a complementation system to isolate the SUT cDNAs SUT1 from spinach and potato (Solanum tuberosum) (Riesmeier et al., 1992, 1993). Subsequently, homologous genes were isolated from a number of other plant species (Gahrtz et al., 1994; Sauer and Stolz, 1994; Weig and Komor, 1996; Hirose et al., 1997; Kühn et al., 1997; Weber et al., 1997).
The biochemical properties of the transporters when expressed in yeast were similar to those described in protoplasts or in plasma membrane vesicles from a variety of plant species. Detailed electrophysiological analyses in Xenopus oocytes demonstrated that SUT1 and SUC2 function as sucrose:proton co-transporters (Boorer et al., 1996; Zhou et al., 1997). The transporters are highly hydrophobic proteins and belong to a class of metabolite transporters consisting of two sets of six membrane-spanning regions separated by a central cytoplasmic loop (Ward et al., 1997).
SUT1 expression is highest in mature leaves and is subject to regulation by plant hormones (Harms et al., 1994). Immunolocalization studies show that SUT1 is present at high levels in the plasma membrane of sieve elements (Kühn et al., 1997). Antisense repression of the SUT in potato provided evidence for an essential role in phloem loading (Riesmeier et al., 1994; Kühn et al., 1996). However, potato is unusual in that it has been selected for high tuber yields and is almost exclusively propagated asexually via tubers. To investigate the role of SUT1 in a species that has been selected for large leaf area and that propagates exclusively via seeds, a SUT1 cDNA was isolated from tobacco (Nicotiana tabacum) and the expression pattern was characterized. Furthermore, transgenic tobacco plants were created with a reduction in the amount of SUT mRNA due to antisense inhibition. These plants were analyzed with respect to the effects on export capacity, carbohydrate partitioning, photosynthesis, and plant development.
MATERIALS AND METHODS
Recombinant DNA
Recombinant phages (5 × 105) of a cDNA library derived from tobacco (Nicotiana tabacum) leaves (Stratagene) were screened with a 32P-labeled StSUT1 fragment (Riesmeier et al., 1993). Positive clones were obtained and sequenced. For reverse-transcriptase PCR, total RNA was extracted from mature leaves (Riesmeier et al., 1993). Poly(A+) RNA was purified via Dynabeads Oligo (dT)25 (Dynal Inc., Lake Success, NY). Poly(A+) RNA was reverse transcribed using primer T1 (NCC-RAA-YAA-RTC-RTC-CAA-NGG-NCC-NCC). The resulting first-strand cDNA was used as a template for PCR amplification with T2 (GCN-GCN-GGN-GTN-CAR-TTY-GGN-TGG-GCN) and T3 (GAA-AAT-ATA-GAT-GCC-AAA-GCA-AAT-GG) for isolation of the 1.2-kb NtSUT1 fragment. The 5′ end of NtSUT1 was isolated using the 5′-AmpliFINDER RACE Kit (Clontech Laboratories, Palo Alto, CA). The amplified DNA fragments were cloned into pON 184 (pACYC184 derivative with Bluescript polylinker; O. Ninnemann and W.B. Frommer, unpublished data) that had been previously cut with SmaI. The 1.2-kb SmaI/SalI fragment of NtSUT1 was ligated in reverse orientation between the CaMV 35S promoter and the polyadenylation signal of the octopine-synthase gene into the SmaI/SalI restriction site of the binary vector Bin 19 (Bevan, 1984).
Transformation and Analysis of Transgenic Plants
Transfer of the chimeric construct into Agrobacterium tumefaciens GV2260 and transformation of tobacco cv SNN were performed as described previously (Köster-Töpfer et al., 1989). Transgenic plants used for molecular characterization were transferred to soil and analyzed under greenhouse conditions. For northern-blot analysis, RNA was isolated from mature leaves of greenhouse-grown transformants and wild-type plants after 4 to 6 h of light, as described previously (Riesmeier et al., 1993). Northern blots were made under stringent conditions, hybridized in 50% formamide at 42°C, and washed with 2× SSC at 68°C. Transgenic lines were named αNtSUT1-35S. About one-half of the transformants derive from a comparable construct, the only difference being that a triple CaMV 35S enhancer was used (marked with an asterisk). Seeds were collected from selected lines (wild type, αNtSUT1-35S17, αNtSUT1-35S30, and αNtSUT1-35S55*) for further analysis. Offspring were proven to contain the SUT1-antisense construct by a PCR strategy previously described by Hamill et al. (1991) (data not shown).
Physiological Measurements
Plants used for growth analysis, export-rate measurements, photosynthesis, and assessment of the concentration of soluble carbohydrate and starch were grown for 10 to 12 weeks in controlled-environment cabinets. Light intensity was 500 μmol m−2 s−1 with a 14-h photoperiod and a 25°C/20°C day/night temperature cycle. Seeds were germinated on sand in Petri dishes for 12 d before transplantation. Plants were grown in sand and supplied with Rorison's nutrient solution containing 2.2 mm nitrogen (Hewitt, 1966). Photosynthetic rates of the youngest fully expanded leaf were measured by IR gas analysis using a portable analyzer (model LCA3, Analytical Development Co., Ltd., Hoddeston, UK) at growth irradiance.
For analysis of carbohydrates, leaf discs were taken from mature leaves 5 h into the photoperiod and placed in liquid nitrogen, and soluble sugars were extracted in 80% buffered ethanol (5 mm MgCl2, 50 mm Hepes, pH 7.4) at 70°C. Starch was extracted in sodium acetate buffer, pH 4.7, containing α-amylase and amyloglucosidase according to the method of Stitt et al. (1989). Soluble carbohydrates were assayed enzymatically as described by Stitt et al. (1989). Feeding of 14CO2 and partitioning of 14C were performed according to the method of Quick et al. (1989). Plants used for growth analysis were separated into shoot and root and then dried in a forced-air oven at 80°C until a constant dry weight was attained.
To assess the ability of leaves to export carbon, leaves were enclosed in a cuvette containing a Geiger-Müller tube positioned directly under the leaf. The light intensity at the cuvette surface was maintained at the same level as that used for plant growth and the temperature was kept at 25°C. Leaves were left to stabilize for 2 h; at 11:00 am each day, 14CO2 was supplied to each leaf for 5 min according to the method of Hibberd et al. (1996). The rate at which 14C disappeared from each leaf was then recorded on a chart recorder during the photoperiod. The data were fitted to a double exponential curve qt(t) = A1e−λ1t + A2e−λ2t + B, where qt is the total amount of isotope incorporated, t is time, A1 and A2 are the values at 0 time of the two exponentials, λ1 and λ2 are the coefficients of the two exponentials, and B is the value of the asymptote set from the measured proportion of 14C incorporated into starch (see above).
The transfer coefficient for phloem loading was then calculated from k01 = (A1λ1 + A2λ2)/(A1 + A2) according to the method of Rocher and Prioul (1987). While 14C was fed to the leaf and its export was monitored, the rate of net photosynthesis of the leaf was measured by linking the cuvette to an IR gas analyzer (Analytical Development Co., Ltd.). The whole plant was illuminated during the light period with tungsten/halogen lamps identical to those provided in the growth cabinet. Leaves comparable to those used for export were sampled to measure the concentration of sucrose at 11:00 am. Export rates from the leaves were calculated from the measured concentration of sucrose and the transfer coefficient calculated from the 14C export curves. For autoradiography, leaves were supplied with 14CO2 in the same way as for export experiments, but a larger leaf cuvette was used (20 × 30 cm). Leaves were detached from the plant either immediately or 24 h after feeding, and immediately frozen in dry ice followed by exposure to radiographic film for 7 d.
RESULTS
An antisense approach was used to study the physiological role of SUT1 in tobacco. Since tobacco and potato (Solanum tuberosum) are closely related species, and therefore respective genes were likely to be highly homologous, tobacco plants were transformed with the antisense construct used to inhibit phloem loading in potato (Riesmeier et al., 1994). However, none of the 60 screened transformants showed reduced RNA levels or characteristic phenotypic symptoms (W.B. Frommer, unpublished results). To determine whether this was attributable to the different physiology of tobacco plants or to technical problems associated with the lack of sufficient homology between the potato and tobacco genes, the orthologous SUT1 gene was isolated from tobacco.
Isolation of Tobacco SUT Genes
A tobacco leaf cDNA library was screened using the potato SUT1 cDNA as a probe. Eight clones contained inserts of about 450 bp encoding the 3′ terminal sequence of NtSUT1. To obtain larger fragments via reverse-transcriptase PCR, conserved sequences were identified by sequence comparisons of spinach, potato, and Arabidopsis SUT cDNAs, and were then used to generate degenerate oligonucleotides (Riesmeier et al., 1992, 1993; Sauer and Stolz, 1994). First-strand cDNA from mature leaves primed with the degenerate primer T1 was used as a template for two degenerate primers (T2 and T3) to amplify a central, 1245-bp NtSUT1 fragment. The missing 5′ end was obtained after ligation of an anchor onto the first-strand cDNA and subsequent PCR with the anchor primer and a specific primer derived from the central fragment.
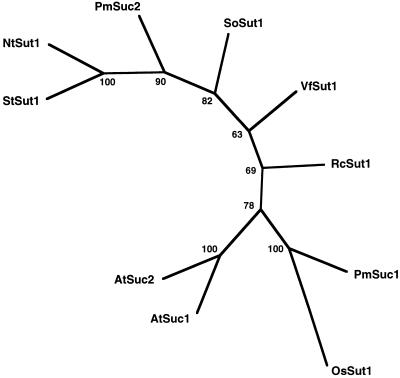
Consistent with the amphidiploidy of tobacco, two subclasses of 5′ clones with slight differences in the untranslated regions were identified. A hypothetical sequence composed of one of the 5′ sequences, the central fragment, and the overlapping 3′ sequence was constructed and deposited with the second 5′ end in the EMBL database (accession nos. X82276 and X82277). A computer-aided analysis of homologies between SUTs from different species shows that NtSUT1 is most similar to the potato StSUT1, indicating that it may serve a similar function (phloem loading) in tobacco as StSUT1 serves in potato (Fig. 1).
Figure 1.
Computer-aided homology analysis by PHYLIP (Felsenstein, 1993) of aligned SUTs from tobacco (NtSut1), potato (StSut1) (Riesmeier et al., 1993), spinach (SoSut1) (Riesmeier et al., 1992), Arabidopsis (AtSuc1 and AtSuc2) (Sauer and Stolz, 1994), Plantago major (PmSuc1 and PmSuc2) (Gahrtz et al., 1994, 1996), castor bean (RcSut1) (Weig and Komor, 1996), rice (OsSut1) (Hirose et al., 1997), and fava bean (VfSut1) (Weber et al., 1997). The comparison was restricted to the region in NtSUT1 from amino acid position 19 to 472. The numbers indicate the occurrence of a branch in 100 bootstrap replicates of a given data set. OsSut1 was used as the outgroup.
Expression of SUT1 in Tobacco
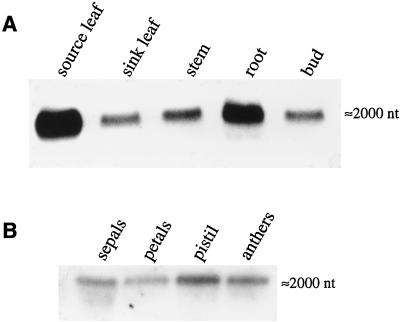
The central 1.2-kb fragment of NtSUT1 was used to analyze SUT1 mRNA levels in different tobacco organs. NtSUT1 was found to be highly expressed in mature leaves, whereas expression was low in developing sink leaves (Fig. 2A). Expression was also found in all other tissues analyzed, i.e. roots, stems, and all flower organs (Fig. 2, A and B). The ubiquitous expression may indicate that the transporter is important not only for phloem loading, but also for retrieval along the translocation path and/or for unloading processes. These results are consistent with immunolocalization and GUS expression data of SUT1 in tobacco (Kühn et al., 1997; B. Hirner and W.B. Frommer, unpublished results). Nevertheless, we cannot fully exclude the possibility of cross-hybridization with other, as-yet-unknown SUT mRNAs in tobacco.
Figure 2.
Northern-blot analysis of NtSUT1 expression in tobacco using stringent conditions (see Methods; probe, NtSut1, 1.2 kb). A, Expression in various parts of the plant (16.5 μg/lane). B, Expression in flower organs (5 μg/lane).
Antisense Repression of the SUT
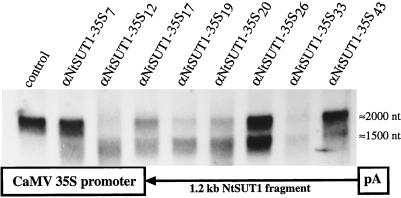
To determine the physiological role of SUT1, NtSUT1 was cloned in an antisense orientation behind the CaMV 35S promoter into a binary vector (Fig. 3). Leaf discs of tobacco were transformed with the chimeric construct and, after transfer of the regenerated plants to the greenhouse, 71 of 91 transformants showed no visible phenotypic peculiarities compared with the wild type. However, 10 plants developed a weak phenotype (e.g. αNtSUT1-35S46*), whereas 10 other plants clearly showed visible symptoms, as described below. αNtSUT1-35S12 and αNtSUT1-35S50 were the most strongly affected plants, whereas αNtSUT1-35S17, αNtSUT1-35S19, αNtSUT1-35S20, αNtSUT1-35S29, αNtSUT1-35S30, αNtSUT1-35S33, αNtSUT1-35S34, and αNtSUT1-35S55* were only intermediately affected. Several of these plants were tested at the RNA level using the central, 1.2-kb fragment of NtSUT1 as a probe. Only plants displaying symptoms (αNtSUT1-35S12, αNtSUT1-35S17, αNtSUT1-35S19, αNtSUT1-35S20, and αNtSUT1-35S33) showed a significant reduction in NtSUT1 mRNA (Fig. 3), whereas unaffected plants such as αNtSUT1-35S7, αNtSUT1-35S26, and αNtSUT1-35S43 had SUT1 mRNA levels similar to those of the wild type.
Figure 3.
Structure of the chimeric gene and analysis of transgenic tobacco plants with reduced expression of NtSUT1. Analysis of NtSUT1 mRNA expression in source leaves of transgenic and control plants. Total RNA (25 μg/lane) was hybridized with a radiolabeled NtSUT1 1.2-kb probe under stringent conditions (see Methods). Transcript sizes are given on the right. The hybridization signals at 1500 nt represent the antisense NtSUT1 mRNA.
Phenotypic Effects of SUT Inhibition
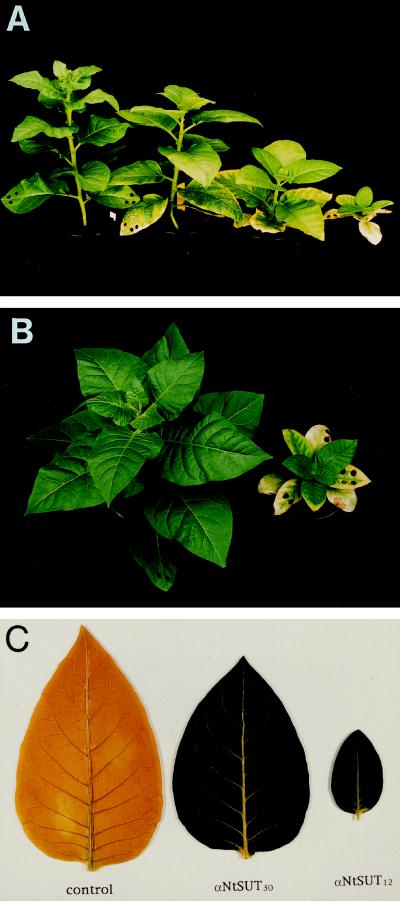
αNtSUT1-35S12 and αNtSUT1-35S50 were the most strongly affected plants. During maturation, leaves began to curl and rims and intercostal fields became chlorotic or even necrotic (Fig. 4, A and B). In contrast, sink leaves appeared unaffected. Both lines exhibited retarded leaf development and grew no taller than 10 cm. At this stage, development was arrested, without flower induction for longer than 12 months. αNtSUT1-35S55* was slightly less affected, also retarded in growth but eventually flowering and setting seeds (data not shown).
Figure 4.
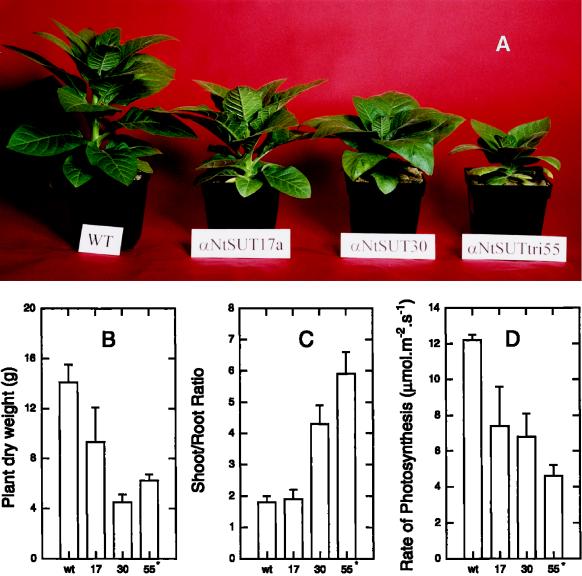
Development of symptoms in tobacco plants transformed with the SUT1 antisense construct. A, Transgenic tobacco plants after 7 weeks in the greenhouse (from left to right): wild type, αNtSUT1-35S33, αNtSUT1-35S30, and αNtSUT1-35S12. B, Top view of wild type (left) and αNtSUT1-35S50 (right). C, Starch accumulation as determined after 16 h of darkness for wild type (control), αNtSUT1-35S30, and αNtSUT1-35S12 by KI staining.
Three seed-producing transformants with weak to intermediate phenotypes, αNtSUT1-35S17, αNtSUT1-35S30, and αNtSUT1-35S55*, were grown in a controlled-growth environment and used for more detailed physiological analyses. In the controlled environment, these plants also showed the above-described phenotype (Fig. 5A). Dry-weight accumulation in lines αNtSUT1-35S30 and αNtSUT1-35S55* was greatly reduced, with line αNtSUT1-35S17 being intermediate in its growth response relative to lines αNtSUT1-35S30, αNtSUT1-35S55*, and the wild type (Fig. 5B). The same gradation was also seen in the shoot-to-root ratio; the amount of root produced per unit of shoot was lower in lines αNtSUT1-35S30 and αNtSUT1-35S55* compared with the wild type (Fig. 5C).
Figure 5.
Growth response of three selected antisense plants with weak to intermediate phenotypes. A, Phenotype; B, dry weight; C, shoot-to-root ratio; and D, photosynthesis of tobacco plants transformed with the SUT1 antisense construct. Rate of net photosynthesis of the youngest fully expanded leaf was measured at a growth irradiance of 500 μmol photons m−2 s−1. wt, Wild type; 17, αNtSUT1-35S17; 30, αNtSUT1-35S30; 55*, αNtSUT1-35S55*.
Biochemical and Physiological Analysis of SUT Antisense Plants
Retarded development and the phenotype of plants containing reduced amounts of SUT1 mRNA indicated that photosynthesis was affected and the export of carbohydrates from leaves was impaired. The rate of net photosynthesis at growth irradiance was reduced in all lines relative to controls, with the reduction in photosynthesis being greatest in lines showing the largest reduction in growth and the strongest phenotype (Fig. 5D; Table I). To determine directly whether the lower rates of sucrose transport from the leaves was responsible for reduced growth, rates of export of assimilate from leaf tissue, primarily sucrose, were analyzed (Fig. 6). 14CO2 was fed to leaves and the export of the fixed 14C was subsequently monitored. In wild-type plants the initial 14C export occurred at a fast rate, subsequently decreasing progressively. Export of 14C from wild-type leaves fitted well to a double-exponential equation (see Methods).
Table I.
Rate of sucrose export from mature leaves of wild type (WT), αNtSUT1-35S17 (17), αNtSUT1-35S30 (30), and αNtSUT1-35S55* (55)
| Plant | Rate of Net Photosynthesis | Sucrose | K01 | Export Rate |
|---|---|---|---|---|
| μmol m−2 s−1 | mmol m−2 | μs−1 | μmol m−2 s−1 | |
| WT | 14.1 | 3.8 | 975 | 3.7 |
| 17 | 11.8 | 4.0 | 844 | 3.3 |
| 30 | 6.7 | 12.5 | 50 | 0.6 |
| 55* | 4.3 | 21.5 | 6 | 0.1 |
Export was calculated from the measured rate of net photosynthesis, the measured sucrose concentration in the leaf, and the transfer coefficient (K01). For details, see Methods.
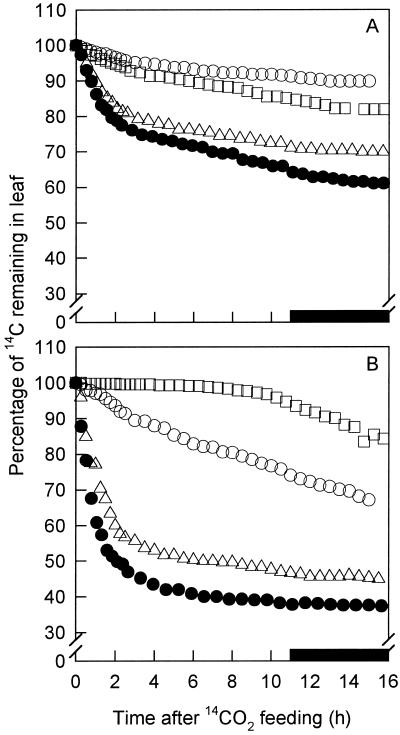
Figure 6.
The export of 14C from leaves of tobacco. Wild-type plants (•) and the transgenic lines αNtSUT1-35S17 (▵), αNtSUT1-35S30 (○), and αNtSUT1-35S55* (□) expressing antisense NtSUT1 mRNA. A, Data from young leaves; B, data from mature, fully expanded leaves. Leaves were fed with 14CO2 at 11:00 am and the export of 14C was followed with a Geiger-Müller tube positioned under the fed area of the leaf. Data are expressed as the maximum amount of isotope incorporated. The black bar on the x axis represents the dark period.
Both developing and mature leaves of αNtSUT1-35S17, αNtSUT1-35S30, and αNtSUT1-35S55* exported less radiolabeled carbon than the wild type (Fig. 6). Inhibition of 14C export became more pronounced as leaves matured (Fig. 6). Both the initial slope of 14C export and the second, slower phase of 14C export were lower in αNtSUT1-35S17, αNtSUT1-35S30, and αNtSUT1-35S55* than in the wild type. The rate of sucrose export was calculated from the rate of photosynthesis of the leaves used for efflux, from the sucrose concentrations measured in the leaves, and from the transfer coefficients calculated from the export data of mature leaves. Despite the fact that the concentration of sucrose increased in the leaves of antisense plants, the rate of sucrose export from those leaves was reduced compared with the wild type (Table I).
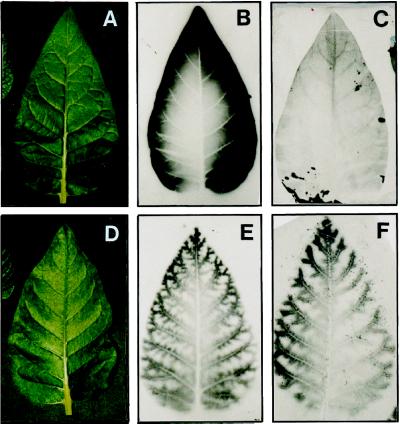
After the leaves of lines expressing antisense mRNA reached full expansion, a progressive development of chlorosis was observed in interveinal regions. The yellow sectoring started at the tips and moved to the base as the leaves aged, following a sink-to-source transition pattern (Fig. 7). All parts of wild-type leaves fixed 14CO2 relatively homogenously and then exported the majority of the isotope supplied after a 24-h chase period (Fig. 8, A–C). However, for transgenic lines (data shown for αNtSUT1-35S55*), the distribution of 14CO2 fixation was heterogenous after 5 min of photosynthesis (Fig. 8E) and resembled the pattern of chlorophyll distribution shown in older chlorotic leaves (Fig. 7). There was no evidence of chlorosis, however, in the younger leaves chosen for this experiment (Fig. 8D). There was also no evidence of export of label after a 24-h chase or of reallocation of label within the leaf, with 14C remaining localized around the major veins (Fig. 8F).
Figure 7.
Typical pattern of chlorosis observed in the interveinal regions of mature leaves from line αNtSUT1-35S30. This phenotype developed progressively after leaves attained full expansion and only in lines exhibiting a marked inhibition of sucrose export.
Figure 8.
Photographs (A and D) and autoradiographs (B, C, E, and F) of wild-type (A–C) and transgenic (D–F) plants. Leaves were allowed to fix 14CO2 for 5 min and were then detached from the plant (B and E) or allowed to remain attached for another 24 h to permit export of incorporated radioactivity (C and F).
Rates of 14CO2 incorporation into leaf discs were comparable to the rates of photosynthesis measured by IR gas analysis; both were reduced in antisense plants compared with wild-type controls (Table II). Partitioning of recently fixed photosynthate between the insoluble starch pool and the soluble sucrose pool, however, was unaffected in the transgenic lines. Thus, the observed reduction in photosynthesis was associated with a parallel reduction of both starch and sucrose synthesis (Table II).
Table II.
Rate of incorporation of 14CO2 and the partitioning of 14C into water-soluble and -insoluble fractions of leaf discs from wild-type (WT), αNtSUT1-35S17 (17), αNtSUT1-35S30 (30), and αNtSUT1-35S55* (55)
| Plant | Rate of 14C
Incorporation
|
Ratio | ||
|---|---|---|---|---|
| Total | Soluble fraction | Insoluble fraction | ||
| μmol m−2 s−1 | ||||
| WT | 15.3 ± 0.8 | 7.3 ± 0.2 | 7.9 ± 0.7 | 1.07 ± 0.08 |
| 17 | 12.8 ± 0.6 | 6.4 ± 0.3 | 6.4 ± 0.3 | 1.01 ± 0.03 |
| 30 | 12.0 ± 1.1 | 5.7 ± 0.4 | 5.6 ± 0.8 | 0.99 ± 0.03 |
| 55* | 11.3 ± 1.2 | 5.6 ± 0.6 | 4.6 ± 0.6 | 0.96 ± 0.11 |
14CO2 was fed at growth light intensity.
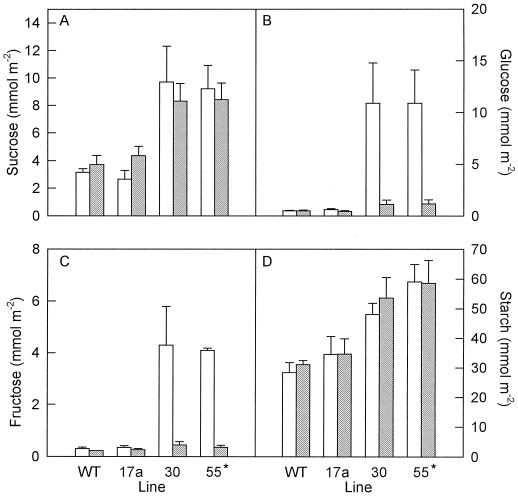
The concentration of soluble sugars increased in all three antisense lines and was greatest in line αNtSUT1-35S55* (Fig. 9, A–C). The concentration of sucrose and starch increased in the regions proximal and distal to major veins as carbon export was inhibited (Fig. 9, A and D). In lines αNtSUT1-35S30 and α NtSUT1-35S55*, Glc and Fru clearly accumulated in regions distal to major veins (Fig. 9, B and C).
Figure 9.
Concentration of soluble sugars and starch in leaves from tobacco plants transformed with antisense constructs of the SUT. Samples were taken 5 h into the photoperiod. WT, Wild type; 17a, αNtSUT1-35S17; 30, αNtSUT1-35S30; 55, αNtSUT1-35S55*. Data are means ± se. Open bars, Regions distal to major veins; shaded bars, equal regions proximal to major veins.
After they were kept in darkness for 16 h, wild-type plants had only low levels of starch, whereas antisense plants showed strong iodine staining, indicating that they were unable to mobilize excess carbohydrates accumulated during the previous day (Fig. 4C).
DISCUSSION
In general, two potential routes have been discussed for the loading of phloem with assimilates: the symplastic and apoplastic pathways. In terms of the classification of plants into symplastic or apoplastic loaders, Solanaceae represents a polytypic family encompassing species with relatively high plasmodesmata density between mesophyll and the sieve-element/companion-cell complex (intermediate type 1–2a; Datura, 1–10 plasmodesmata μm−2 interface) and species with low plasmodesmal connectivity (closed primitive type 2a; tobacco, 0.12 plasmodesmata μm−2 interface; potato, 0.08 plasmodesmata μm−2 interface; Gamalei, 1991). These data are supported by a detailed analysis of individual vein structure from potato leaves (McCauley and Evert, 1989).
A protein that could be responsible for phloem loading with sucrose was identified from potato via yeast complementation (Riesmeier et al., 1993). The tissue specificity (prevalence in the phloem), together with an observed increase in sucrose-transport activity during the sink-to-source transition of sugar beet leaves and the increase of SUT1 mRNA during leaf development from sink to source in potato, were taken as strong indications that the SUT is involved in phloem loading (Lemoine et al., 1992; Riesmeier et al., 1993).
Direct evidence for an apoplastic route of phloem loading in potato came from analysis of potato plants in which sucrose transport was inhibited by antisense repression of SUT1 (Riesmeier et al., 1994; Kühn et al., 1996; Lemoine et al., 1996). Inhibited plants were retarded in development and showed severe symptoms in mature leaves indicative of osmotic problems associated with carbohydrate accumulation. Furthermore, sucrose export from leaves was inhibited, leading to reduced root development and dramatically reduced tuber yield.
To study the role of SUT1 in tobacco, transgenic tobacco plants containing reduced amounts of endogenous NtSUT1 mRNA due to antisense inhibition were generated. Twenty of ninety-one transformants had reduced amounts of SUT1 mRNA and also displayed characteristic symptoms of leaf curling and chlorosis.
Feeding 14CO2 allowed us to monitor 14C export from leaves of αNtSUT1-35S17, αNtSUT1-35S30, αNtSUT1-35S55*, and wild-type plants. In wild-type plants the export of 14C from leaves occurred in two clear phases, fitting well to a double-exponential equation (see Methods). The export characteristics of lines αNtSUT1-35S30 and αNtSUT1-35S55* were markedly different from those of the wild type, whereas αNtSUT1-35S17 showed an intermediate response; all lines showed decreased export compared with the wild type. The degree to which 14C export was inhibited in the antisense lines increased as the leaves matured.
Export of radiolabeled carbon from leaves has previously been shown to occur in at least two phases and to fit well to exponential functions (Rocher and Prioul, 1987). Compartmental analysis of export of photosynthetically fixed 14C from mature leaves should allow pool sizes and rates of export of carbon from leaves to be calculated, but compartmental analysis relies on a set of assumptions (Rocher and Prioul, 1987; Zierler, 1981). Most of the assumptions do not hold in the antisense lines, and therefore pool sizes were not estimated from our export data. By measuring the concentration of sucrose in the leaves biochemically, the rate of sucrose export could be estimated (Table I). However, these data are likely to overestimate the rates of export, because sucrose is typically present in more than one pool in a leaf and only one pool will be directly available for export (Rocher and Prioul, 1987). Despite the fact that sucrose export from these leaves may have been overestimated because of its accumulation, the rates of export were still much lower than in the wild type, strongly supporting the proposed role of NtSUT1 in sucrose export from tobacco leaves.
The proportion of 14CO2 partitioned between sucrose and starch could also affect the rate and amount of 14C exported from a leaf. By measuring the short-term partitioning of 14C between soluble and insoluble fractions, it was shown that slower rates of carbon export from αNtSUT1-35S17, αNtSUT1-35S30, and αNtSUT1-35S55* were not due to a stimulation of the production of starch (Table II). Despite the fact that mature leaves of lines αNtSUT1-35S30 and αNtSUT1-35S55* exported much less than leaves of the wild type, the reduction in carbohydrate export was not lethal. However, plants with more pronounced phenotypes, such as αNtSUT1-35S12, exhibited stronger inhibition of phloem loading and did not produce seed.
Sugars accumulated in plants in which expression of antisense NtSUT1 mRNA led to reduced export of carbon from leaves. Accumulation of soluble sugars and starch in response to inhibition of carbon export from leaves is well documented (von Schaewen et al., 1990; Krapp et al., 1991; Kühn et al., 1996). However, in lines αNtSUT1-35S30 and αNtSUT1-35S55*, there was a clear difference between the concentration of hexoses distal and proximal to major veins.
Typically, when carbohydrates accumulate in leaves, down-regulation of photosynthesis occurs (Jang and Sheen, 1994). The rates of photosynthesis in αNtSUT1-35S17, αNtSUT1-35S30, and αNtSUT1-35S55* were lower than in the wild type. Furthermore, autoradiography showed that even before development of the mottled, chlorotic phenotype, photosynthesis was concentrated in the regions close to major veins and down-regulated in areas between the veins. Areas where photosynthesis had become down-regulated coincided with the areas where hexoses accumulated to high levels. Accumulation of hexoses has previously been implicated in the down-regulation of photosynthetic gene expression (Herbers et al., 1996).
These results further support the assumption that SUT1 is essential for sucrose export from leaves both during the day and at night, since even when sugar biosynthesis was reduced, soluble sugars accumulated and remained high after extended periods of darkness, suggesting that the antisense plants were unable to export stored sugar reserves.
Similar results, including a mottled leaf phenotype, were observed in detached spinach leaves fed carbohydrates (Krapp et al., 1991), indicating that accumulation of carbohydrate alone could induce the change in leaf phenotype. Molecular mechanisms that could bring about these changes have been demonstrated and include carbohydrate-induced repression of photosynthetic gene expression (Jang and Sheen, 1994). High leaf starch is also associated with this phenotype, but may not be a prerequisite for the appearance of the symptoms in wild-type plants. In a previous study, potato plants containing a similar antisense SUT1 construct also had a similar mottled leaf phenotype; however, areas of high or low photosynthesis did not correlate well with the pattern of starch deposition (Kühn et al., 1996), and plant growth was severely reduced, especially in the roots, resulting in a large increase in the shoot-to-root ratio. This agrees with the hypothesis that carbohydrate export to heterotrophic tissues was impaired, suggesting an important role of the SUT in tobacco for the export of sucrose from source leaves.
It will be important to determine the remaining sucrose transport activity in antisense plants to determine whether SUT1 activity may become limiting for photosynthesis under optimal conditions, e.g. at increased atmospheric CO2 levels, thus leading to the typical acclimation phenomena observed in various plants (Besford, 1990).
In summary, the results obtained with transgenic plants in which the SUT was inhibited clearly show that SUT1 is important for sucrose export from leaves, a strong antisense reduction being lethal. Because it is localized on sieve-element plasma membranes in tobacco and potato (Kühn et al., 1997), it is responsible for phloem loading both in tobacco, which has a large leaf area relative to the dry weight of reproductive organs, and in potato, which has a high tuber-harvest index, and thus appears to be a more general mechanism of phloem loading, at least for type 2a plants. However, low levels of NtSUT1 expression were also found in a number of different sink tissues, including stems and parts of the flower, indicating that SUT1 may have other functions, such as sucrose retrieval along the translocation path and possibly phloem unloading. It will be important in the future to determine the precise function of SUT1 expression in these tissues by cell-type-specific antisense experiments. Because sucrose, the major osmotic compound present in phloem sap, is thought to create the major driving force for mass flow in the phloem, antisense plants with reduced loading capacity should provide an excellent tool with which to study the mechanisms driving phloem translocation (Münch, 1930).
ACKNOWLEDGMENT
We are very grateful to Nicole Thiele for her excellent technical assistance.
Abbreviations:
- CaMV
cauliflower mosaic virus
- SUT
sucrose transporter
Footnotes
This work was supported by grants from the Biotechnology and Biological Science Research Council (no. 50/PO1777) and the Deutsche Forschungsgemeinschaft (SPP, Elevated CO2 and Transport, no. Fr989/5-2).
LITERATURE CITED
- Ap Rees T. Plant physiology: virtue on both sides. Curr Biol. 1994;4:557–559. doi: 10.1016/s0960-9822(00)00125-1. [DOI] [PubMed] [Google Scholar]
- Besford RT. The greenhouse effect: acclimation of tomato plants growing in high CO2, relative changes in Calvin cycle enzymes. J Plant Physiol. 1990;136:458–463. [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984;12:8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorer KJ, Loo DDF, Frommer WB, Wright EM. Transport mechanism of the cloned potato H+/sucrose transporter StSUT1. J Biol Chem. 1996;271:25139–25144. doi: 10.1074/jbc.271.41.25139. [DOI] [PubMed] [Google Scholar]
- Bush DR. Electrogenicity, pH-dependence, and stoichiometry of the proton-sucrose symport. Plant Physiol. 1990;93:1590–1596. doi: 10.1104/pp.93.4.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush DR. Proton-coupled sugar and amino acid transporters in plants. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:513–542. [Google Scholar]
- Felsenstein J (1993) PHYLIP (Phylogeny Interference Package), Version 3.5c. Department of Genetics, University of Washington, Seattle
- Frommer WB, Kühn C, Hirner B, Harms K, Martin T, Riesmeier JW, Schulz B (1996) Sugar transport in higher plants. In M Smallwood, JP Knox, DJ Bowles, eds, Membranes: Specialized Functions in Plants. BIOS Scientific Publishers, Oxford, UK, pp 319–335
- Gahrtz M, Schmelzer E, Stolz J, Sauer N. Expression of the PmSUC1 sucrose carrier gene from Plantago major L. is induced during seed development. Plant J. 1996;9:93–100. doi: 10.1046/j.1365-313x.1996.09010093.x. [DOI] [PubMed] [Google Scholar]
- Gahrtz M, Stolz J, Sauer N. A phloem-specific sucrose-H+ symporter from Plantago major L. supports the model of apoplastic phloem loading. Plant J. 1994;6:697–706. doi: 10.1046/j.1365-313x.1994.6050697.x. [DOI] [PubMed] [Google Scholar]
- Gamalei Y. Phloem loading and its development related to plant evolution from trees to herbs. Trees. 1991;5:50–64. [Google Scholar]
- Hamill JD, Rounsley S, Spencer A, Todd G, Rhodes MJC. The use of the polymerase chain reaction in plant transformation studies. Plant Cell Rep. 1991;10:221–224. doi: 10.1007/BF00232562. [DOI] [PubMed] [Google Scholar]
- Harms K, Wöhner RV, Schulz B, Frommer WB. Expression of plasma membrane H+-ATPase genes in potato. Plant Mol Biol. 1994;26:979–988. doi: 10.1007/BF00028864. [DOI] [PubMed] [Google Scholar]
- Herbers K, Meuwly P, Frommer WB, Métraux JP, Sonnewald U. Systemic acquired resistance mediated by the ectopic expression of invertase: possible hexose sensing in the secretory pathway. Plant Cell. 1996;8:793–803. doi: 10.1105/tpc.8.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt EJ (1966) Sand and Water Culture Methods Used in the Study of Plant Nutrition, Ed 2. Commonwealth Agricultural Bureau, Technical Communications No. 22, East Malling, Kent, UK
- Hibberd JM, Whitbread R, Farrar JF. Carbohydrate metabolism in source leaves of barley grown in 700 μmol mol−1 CO2 and infected with powdery mildew. New Phytol. 1996;133:659–671. [Google Scholar]
- Hirose T, Imaizumi N, Scofield GN, Furbank RT, Ohsugi R. cDNA cloning and tissue specific expression of a gene for sucrose transporter from rice (Oryza sativa L.) Plant Cell Physiol. 1997;38:1389–1396. doi: 10.1093/oxfordjournals.pcp.a029134. [DOI] [PubMed] [Google Scholar]
- Jang JC, Sheen J. Sugar sensing in higher plants. Plant Cell. 1994;6:1665–1679. doi: 10.1105/tpc.6.11.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köster-Töpfer M, Frommer WB, Rocha-Sosa M, Rosahl S, Schell J, Willmitzer L. A class II patatin promoter is under developmental control in both transgenic potato and tobacco plants. Mol Gen Genet. 1989;219:390–396. doi: 10.1007/BF00259611. [DOI] [PubMed] [Google Scholar]
- Krapp A, Quick WP, Stitt M. Ribulose-1,5-bisphosphate carboxylase-oxygenase, other Calvin-cycle enzymes, and chlorophyll decrease when glucose is supplied to mature spinach leaves via the transpiration stream. Planta. 1991;186:58–69. doi: 10.1007/BF00201498. [DOI] [PubMed] [Google Scholar]
- Kühn C, Franceschi VR, Schulz A, Lemoine R, Frommer WB. Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science. 1997;275:1298–1300. doi: 10.1126/science.275.5304.1298. [DOI] [PubMed] [Google Scholar]
- Kühn C, Quick WP, Schulz A, Sonnewald U, Frommer WB. Companion cell-specific inhibition of the potato sucrose transporter SUT1. Plant Cell Environ. 1996;19:1115–1123. [Google Scholar]
- Lemoine R, Gallet O, Galliard C, Frommer WB, Delrot S. Plasma membrane vesicles from source and sink leaves. Plant Physiol. 1992;100:1150–1156. doi: 10.1104/pp.100.3.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine R, Kühn C, Thiele N, Delrot S, Frommer WB. Antisense inhibition of the sucrose transporter: effects on amount of carrier and sucrose transport activity. Plant Cell Environ. 1996;19:1124–1131. [Google Scholar]
- McCauley MM, Evert RF. Minor veins of the potato (Solanum tuberosum L.) leaf: ultrastructure and plasmodesmatal frequency. Bot Gaz. 1989;150:351–368. [Google Scholar]
- Münch E. Die Stoffbewegungen in der Pflanze. Jena, Germany: Gustav Fischer; 1930. [Google Scholar]
- Quick WP, Neuhaus HE, Stitt M. Increased pyrophosphate is responsible for restriction of sucrose synthesis after supplying fluoride to spinach leaf discs. Biochim Biophys Acta. 1989;973:263–271. [Google Scholar]
- Riesmeier JW, Hirner B, Frommer WB. Potato sucrose transporter expression in minor veins indicates a role in phloem loading. Plant Cell. 1993;5:1591–1598. doi: 10.1105/tpc.5.11.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier JW, Willmitzer L, Frommer WB. Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J. 1992;11:4705–4713. doi: 10.1002/j.1460-2075.1992.tb05575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier JW, Willmitzer L, Frommer WB. Antisense repression of the sucrose transporter affects assimilate partitioning in transgenic potato plants. EMBO J. 1994;13:1–7. doi: 10.1002/j.1460-2075.1994.tb06229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocher JP, Prioul JL. Compartmental analysis of assimilate export in a mature maize leaf. Plant Physiol Biochem. 1987;25:531–540. [Google Scholar]
- Sauer N, Stolz J. SUC1 and SUC2: two sucrose transporters from Arabidopsis thaliana: expression and characterization in baker's yeast and identification of the histidine-tagged protein. Plant J. 1994;6:67–77. doi: 10.1046/j.1365-313x.1994.6010067.x. [DOI] [PubMed] [Google Scholar]
- Stitt M, McLilley R, Gerhardt R, Heldt HW. Metabolic levels in specific cells and subcellular compartments of plant leaves. Methods Enzymol. 1989;174:518–552. [Google Scholar]
- von Schaewen A, Stitt M, Schmidt R, Sonnewald U, Willmitzer L. Expression of a yeast-derived invertase in the cell wall of tobacco and Arabidopsis plants leads to accumulation of carbohydrate and inhibition of photosynthesis and strongly influences growth and phenotype of transgenic tobacco plants. EMBO J. 1990;9:3033–3044. doi: 10.1002/j.1460-2075.1990.tb07499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J, Kühn C, Tegeder M, Frommer WB. Sucrose transport in plants. Int Rev Cytol. 1997;178:41–71. doi: 10.1016/s0074-7696(08)62135-x. [DOI] [PubMed] [Google Scholar]
- Weber H, Borisjuk L, Heim U, Sauer N, Wobus U. A role for sugar transporters during seed development: molecular characterization of a hexose and a sucrose carrier in fava bean seeds. Plant Cell. 1997;9:895–908. doi: 10.1105/tpc.9.6.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weig A, Komor E. An active sucrose carrier (Scr 1) that is predominantly expressed in the seedling of Ricinus communis L. J Plant Physiol. 1996;147:685–690. [Google Scholar]
- Zhou JJ, Theodoulou F, Sauer N, Sanders D, Miller AJ. A kinetic model with ordered cytoplasmic dissociation for SUC1, an Arabidopsis H+/sucrose cotransporter expressed in Xenopus oocytes. J Membr Biol. 1997;159:113–125. doi: 10.1007/s002329900275. [DOI] [PubMed] [Google Scholar]
- Zierler K. A critic of compartmental analysis. Annu Rev Biophys Bioeng. 1981;10:531–562. doi: 10.1146/annurev.bb.10.060181.002531. [DOI] [PubMed] [Google Scholar]