Abstract
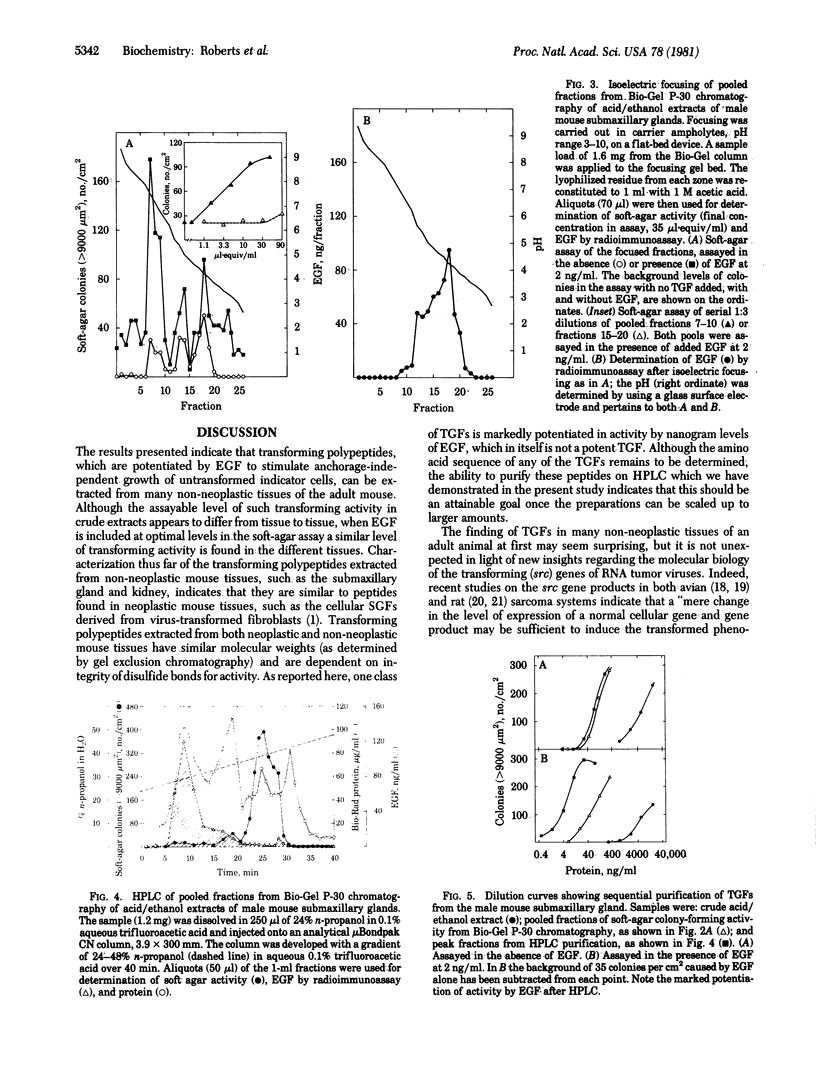
Proteins potentiated by epidermal growth factor (EGF) to induce a transformed phenotype in non-neoplastic rat kidney fibroblasts in cell culture have been isolated from many non-neoplastic tissues of the adult mouse, including submaxillary gland, kidney, liver, muscle, heart, and brain. They resemble previously described transforming growth factors (TGFs) isolated from neoplastic cells as follows: they are extractable by acid/ethanol and are acid-stable, low molecular weight (6000-10,000) polypeptides requiring disulfide bonds for activity; and they cause anchorage-independent growth of non-neoplastic indicator cells that will not grow in soft agar in their absence. Partial purification of these TGFs from submaxillary glands of male mice shows that they are distinct from EGF. Unlike previously described extracellular TGFs, but like certain cellular TGFs from neoplastic cells, they are potentiated by EGF in their ability to promote anchorage-independent growth. The isoelectric point of the submaxillary gland TGF protein is near neutrality. Chromatography on Bio-Gel P-30 followed by high-pressure liquid chromatography has resulted in a 22,000-fold overall purification. The most purified protein is active in inducing growth in soft agar at 1 ng/ml when assayed in the presence of EGF. The data add further evidence to the concept that neoplasia may result from a quantitative, rather than qualitative, alteration in non-neoplastic biochemical processes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barka T. Biologically active polypeptides in submandibular glands. J Histochem Cytochem. 1980 Aug;28(8):836–859. doi: 10.1177/28.8.7003006. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Byyny R. L., Orth D. N., Cohen S. Radioimmunoassay of epidermal growth factor. Endocrinology. 1972 May;90(5):1261–1266. doi: 10.1210/endo-90-5-1261. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., King L., Jr, Cohen S. Rapid enhancement of protein phosphorylation in A-431 cell membrane preparations by epidermal growth factor. J Biol Chem. 1979 Jun 10;254(11):4884–4891. [PubMed] [Google Scholar]
- Cifone M. A., Fidler I. J. Correlation of patterns of anchorage-independent growth with in vivo behavior of cells from a murine fibrosarcoma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1039–1043. doi: 10.1073/pnas.77.2.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Larco J. E., Reynolds R., Carlberg K., Engle C., Todaro G. J. Sarcoma growth factor from mouse sarcoma virus-transformed cells. Purification by binding and elution from epidermal growth factor receptor-rich cells. J Biol Chem. 1980 Apr 25;255(8):3685–3690. [PubMed] [Google Scholar]
- DeFeo D., Gonda M. A., Young H. A., Chang E. H., Lowy D. R., Scolnick E. M., Ellis R. W. Analysis of two divergent rat genomic clones homologous to the transforming gene of Harvey murine sarcoma virus. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3328–3332. doi: 10.1073/pnas.78.6.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson R. L., Purchio A. F., Erikson E., Collett M. S., Brugge J. S. Molecular events in cells transformed by Rous Sarcoma virus. J Cell Biol. 1980 Nov;87(2 Pt 1):319–325. doi: 10.1083/jcb.87.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. Protein phosphorylated by the RSV transforming function. Cell. 1980 Dec;22(3):647–648. doi: 10.1016/0092-8674(80)90539-5. [DOI] [PubMed] [Google Scholar]
- Kahn P., Shin S. I. Cellular tumorigenicity in nude mice. Test of associations among loss of cell-surface fibronectin, anchorage independence, and tumor-forming ability. J Cell Biol. 1979 Jul;82(1):1–16. doi: 10.1083/jcb.82.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure D. B., Ohasa S., Sato G. H. Factors in the rat submaxillary gland that stimulate growth of cultured glioma cells: identification and partial characterization. J Cell Physiol. 1981 May;107(2):195–207. doi: 10.1002/jcp.1041070205. [DOI] [PubMed] [Google Scholar]
- Montesano R., Drevon C., Kuroki T., Saint Vincent L., Handleman S., Sanford K. K., DeFeo D., Weinstein I. B. Test for malignant transformation of rat liver cells in culture: cytology, growth in soft agar, and production of plasminogen activator. J Natl Cancer Inst. 1977 Dec;59(6):1651–1658. doi: 10.1093/jnci/59.6.1651. [DOI] [PubMed] [Google Scholar]
- Ozanne B., Fulton R. J., Kaplan P. L. Kirsten murine sarcoma virus transformed cell lines and a spontaneously transformed rat cell-line produce transforming factors. J Cell Physiol. 1980 Oct;105(1):163–180. doi: 10.1002/jcp.1041050118. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Lamb L. C., Newton D. L., Sporn M. B., De Larco J. E., Todaro G. J. Transforming growth factors: isolation of polypeptides from virally and chemically transformed cells by acid/ethanol extraction. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3494–3498. doi: 10.1073/pnas.77.6.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Schaeffer W. I., Friend K. Efficient detection of soft agar grown colonies using a tetrazolium salt. Cancer Lett. 1976 May;1(5):259–262. doi: 10.1016/s0304-3835(75)97506-0. [DOI] [PubMed] [Google Scholar]
- Scheinberg D. A., Strand M. A brain membrane protein similar to the rat src gene product. Proc Natl Acad Sci U S A. 1981 Jan;78(1):55–59. doi: 10.1073/pnas.78.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Shih T. Y., Maryak J., Ellis R., Chang E., Lowy D. Guanine nucleotide binding activity of the src gene product of rat-derived murine sarcoma viruses. Ann N Y Acad Sci. 1980;354:398–409. doi: 10.1111/j.1749-6632.1980.tb27981.x. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Harris E. D., Jr Proliferative diseases. Am J Med. 1981 Jun;70(6):1231–1235. doi: 10.1016/0002-9343(81)90832-9. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Mitchell W. M., Cohen S. Epidermal growth factor. Physical and chemical properties. J Biol Chem. 1972 Sep 25;247(18):5928–5934. [PubMed] [Google Scholar]
- Todaro G. J., Fryling C., De Larco J. E. Transforming growth factors produced by certain human tumor cells: polypeptides that interact with epidermal growth factor receptors. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5258–5262. doi: 10.1073/pnas.77.9.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushiro H., Cohen S. Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A-431 cell membranes. J Biol Chem. 1980 Sep 25;255(18):8363–8365. [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]