Abstract
Age-associated central arterial wall stiffness is linked to extracellular matrix (ECM) remodeling, including fibrosis and vascular calcification. Angiotensin II induces both matrix metalloproteinase type 2 (MMP2) and calpain-1 expression and activity in the arterial wall. But the role of calpain-1 in MMP2 activation and ECM remodeling remains unknown. Dual histo-immunolabeling demonstrates co-localization of calpain-1 and MMP2 within old rat vascular smooth muscle cells. Over-expression of calpain-1 induces MMP2 transcripts, protein levels and activity, in part, by increasing the ratio of membrane-type 1 MMPs to tissue inhibitor of metalloproteinases 2. These effects of calpain-1 over-expression-induced MMP2 activation are linked to increased collagen I and III production and vascular calcification. In addition, over-expression of calpain-1 also induces transforming growth factor-β1/Smad signaling, elastin degradation, alkaline phosphatase activation and total calcium content, but reduces the expression of calcification inhibitors, osteopontin and osteonectin, in cultured vascular smooth muscle cells in vitro and in carotid artery rings ex vivo. Furthermore, both calpain-1 and collagen II increase with aging within human aortic intima. Interestingly, in aged human aortic wall, both calpain-1 and collagen II are highly expressed in arteriosclerotic plaque areas compared to grossly normal areas. Cross-talk of two proteases, calpain-1 and MMP2, leads to secretion of active MMP2, which modulates ECM remodeling via enhancing collagen production and facilitating vascular calcification. These results establish calpain-1 as a novel molecular candidate to retard age-associated ECM remodeling and its attendant risk for hypertension and atherosclerosis.
Keywords: calpain-1, matrix metalloproteinase type 2, vascular calcification, fibrosis, aging
INTRODUCTION
It is well known that aging induces several changes in arterial structure and function and is the major independent risk factor for cardiovascular diseases i.e. hypertension, atherosclerosis, and stroke.1-5 An age-associated increase in central arterial wall stiffness, that is manifested as increases in pulse wave velocity, is attributable to extracellular matrix (ECM) remodeling, including increased collagen content and crosslinking, elastin fragmentation,1,6,7 and vascular calcification (VC).8-15 ECM remodeling that accompanies advancing age is initially induced by a phenotypic shift of vascular smooth muscle cells (VSMC) within the arterial wall from the contractile to synthetic state16 in vivo, and is associated with arterial wall inflammation.
VC is a very common salient feature of age-associated ECM remodeling. Accumulating evidence indicates that VC is not a passive phenomenon, but rather is controlled by a tightly regulated balance of factors that promote and inhibit calcification.11,12 Synthetic VSMC express many of the calcification-regulatory proteins commonly found in bone.17,18 Among these are transforming growth factor 1 (TGF-β1),19,20 bone morphogenetic protein-2 (BMP-2),21 collagen II, elastin,13,14 osteopontin (OPN) and osteonectin (ON).22
Angiotensin II (Ang II) and its downstream molecules, i.e. matrix metalloprotease 2 (MMP2),23,24 TGFβ125 and calpain-116 are involved in VSMC phenotype transition. ECM alterations appear to be closely linked to exaggerated arterial wall MMP2 activation.26 We have shown previously that activated MMP2 is increased within the aged rat,24 nonhuman primate,23 and human26 aorta. Increased MMP2 facilitates TGF-β1 activation, and these two molecules form part of a signaling loop to induce collagen production in the central arterial wall.25 MMP2 mediated elastin degradation also triggers VC in the media of the central arterial wall.14
Calpain-1 is a Ca2+-activated, intracellular cysteine protease that carries out limited proteolytic cleavage of a diverse range of cellular substrates.27 Our previous studies have shown that rat aortic wall calpain-1 transcripts, protein and activity increase with aging. 16 That calpain-1 plays an important role in Ang II-induced VSMC MMP2 activation and invasion,16 suggests a possible link between the VSMC intracellular protease, calpain-1, and MMP2, a major secreted protease. But the role of calpain-1 in age-associated ECM remodeling has not been investigated. In the present study, we hypothesize that calpain-1 activity contributes to ECM remodeling that accompanies advancing age by inducing MMP2 activation, which facilitates induction of collagen I, II, III, TGFβ1, elastin fragmentation and reduction of OPN and ON.
Materials and Methods
See the online-only Data Supplement.
Results
Calpain-1 induces MMP2 activity by modifying the MT1MMP/TIMP2 ratio
We first investigated whether calpain-1 regulates MMP2 activation in early passage old rat VSMC. Dual histo-immunolabeling shows that calpain-1 colocalizes with MMP2 (Figure 1A). Over-expression of calpain-1 by adenovirus infection increases VSMC MMP2 transcripts (Figure S1A), protein (Figure 1B), and activity (Figure 1C). The respective increases in young (8 mo) VSMC were 34%, 107% and 20% (p<0.05), above the GFP control, and are similar to levels in old (30 mo) untreated VSMC. Over-expression of calpastatin, the endogenous calpain inhibitor, reduces MMP2 activities in both young and old VSMC (Figure 1D).
Figure 1. Calpain-1 induces MMP2 activation in VSMC.

A. Photomicrographs of dual labeling for calpain-1 (green) and matrix metalloproteinase-2 (MMP2, red) within old rat VSMC; merged image (right panel). Nuclei are counterstained with DAPI (blue). Magnification: X400. B. Representative Western blot and average MMP2 protein levels in young (8 mo) and old (30 mo) rat VSMC infected with GFP or CANP1 for 48 hrs. C. Representative gelatin zymograph and average MMP2 activity from young (8 mo) and old (30 mo) rat VSMC infected with GFP or CANP1 for 48 hrs. D. Representative gelatin zymograph and average MMP2 activity from young (8 mo) and old (30 mo) rat VSMC infected with control or calpastatin (CAST) adenoviruses. *: p<0.05 compared to GFP infected young VSMC, n=3. †: p<0.05 compared to young blank VSMC, n=3. # p<0.05 compared to GFP infected old VSMC, n=3.
To further clarify the role of calpain-1 in the regulation of MMP2 activation, we studied the calpain-1 effects on the MMP2 activation inhibitor, TIMP2, and activator, MT1MMP. 28 Real-time PCR shows that over-expression of calpain-1 in young VSMC decreases TIMP-2 transcripts by 38%, and increases MT1MMP transcripts by 32% (Figure S1A). Furthermore, over-expression of calpain-1 decreases of TIMP-2 protein levels by 31%, and increases MT1MMP protein by 48% (Figure 2A, 2B). Thus, over-expression of calpain-1 increases the MT1MMP/TIMP2 ratio (Figure 2C), which induces MMP2 activation.
Figure 2. Calpain-1 induces MT1MMP/TIMP2 ratio in VSMC.

Representative western blot (A) and average protein levels of TIMP2 and MT1MMP from VSMC infected by GFP or CANP1 adenoviruses for 48 hrs. *: p<0.05 compared to GFP infected 8-mo VSMC, n=3. # p<0.05 compared to GFP infected old VSMC, n=3.
Interestingly, that calpain-1 is augmented by MMP2 inhibition in vitro and in vivo (Online data supplement), suggests that MMP2 is downstream of calpain-1signaling.
Over-expression of calpain-1 induces collagen I, II and III expressions and reduces elastin
Fibrosis, due mainly to collagen over-deposition, is a major characteristic of the ECM central arterial wall remodeling with aging. Transcript levels of collagen type I (Col I, Col1a1) and type III (Col III, Col3a1) in old rat aortic wall (30-mo) increase by 29% and 60%, respectively, compared to young (8-mo) (Figure S2A). Compared to control GFP virus, over-expression of calpain-1 by CANP1 infection in young rat VSMC increases Col I and Col III transcripts levels by 54% and 59%, respectively, (Figure S2B) and protein levels of Col I and Col III to 2.18- and 1.99-fold, respectively, (Figure 3A), and these protein levels are comparable to those of old control cells.
Figure 3. Over-expression of calpain-1 induces collagen type I, II and III, and reduces elastin expression levels in VSMC.

A. Representative western blots of collagen I and collagen III in VSMC after infected by GFP or CANP1 adenoviruses for 48 hrs. B. Photomicrographs (X400) of collagen II protein staining (brown color) from young (8-mo) and old (30-mo) rat aortic wall. Nuclei are counterstained with hematoxylin (blue color). L = lumen; and M = media. C and D. Representative Western blots and average protein levels collagen II (C) and elastin (D) in young VSMC infected with GFP or CANP1 for 48 hours. *: p<0.05 compared to GFP infected 8-mo VSMC, n=3.
Collagen II is the major collagen within cartilage.29 Interestingly, histo-immunostaining shows that collagen II levels are increased in old compared to the young aortic wall (Figure 3B). Over-expression of calpain-1 in young VSMC enhances collagen II levels (Figure 3C), and reduces elastin (Figure 3D).
Calpain-1 induces VSMC calcification
Compared to the GFP control, over-expression of calpain-1 (Figure 4A, middle left panel) induces young VSMC calcification in high phosphate containing medium (Figure 4A, upper left panel), and the degree of calcification achieved is similar to the old GFP infected control (Figure 4A, lower left panel). That over-expression of TIMP2 abolishes the calpain-1 effects on VSMC calcification (Figure 4A, middle right panel) suggest that the calpain-1 effect to increase calcification is mediated via its effect to increase MMP2 activity.
Figure 4. Over-expression of calpain-1 induces VSMC calcification.

A. Representative Alizarin Red S Staining showing that compared to GFP control (top left panel) over-expression of calpain-1 induces local calcification in young (8-mo) VSMC (middle left panel) in high phosphate (2.0 mmol/L) calcification medium. Resultant levels in these treated young VSMC are comparable to the levels of old GFP control (30-mo, lower left panel). Calpain-1 induced calcification is inhibited by TIMP2 (lower right panel). Red deposits indicate deposits of phosphate-containing mineral. GFP infected VSMC without phosphate (lower right panel) is a negative control. Representative Western blots and average protein levels of TGF-β1 (B), and Smad 2/3 (C) in young (8-mo) and old (30-mo) VSMC infected with GFP or CANP1 for 48 hours. *: p<0.05 compared to GFP infected 8-mo VSMC, n=3.
We next investigate calpain-1 effects on factors that regulate calcification. Prior studies indicate that calcification markers alkaline phosphatase (ALP) and total calcium content are both increased in the aged aortic wall.29-32 Over-expression of calpain-1 in young VSMC increases secreted ALP activity by 35% (0.248± 0.039 vs 0.183± 0.021 IU/L, p<0.05, n=3) and total calcium content by 72% (7.92 ± 0.45 vs 4.60 ± 0.26 ug Ca2+/mg total protein, p<0.05, n=3), i.e. up to the levels observed in old VSMC (6.774± 1.44 ug Ca2+/mg total protein, n=3). Over-expression of calpain-1 in young rat VSMC also induces TGF-β1 (Figure 4B) and smad 2/3 activation (Figure 4C), up to the levels in old control cells. Over-expression of calpain-1 in young VSMC, however, has no effects on BMP-2 expression (Figure S3).
OPN and ON are two major inhibitors of calcification. In early-passage old (30-mo) rat VSMC, both OPN and ON protein levels are reduced by 39% and 36% compared to young (8-mo) (Figure 5A, B). Over-expression of calpain-1 in young VSMC decreases OPN by 75% and ON by 39%, resulting in similar levels as those in old rat VSMC. Conversely, infection of young VSMC with CAST, an endogenous inhibitor of calpain-1, increases OPN and ON by 52% and 55% (Figure 5A, B). These results indicate that increased calpain-1 overrides the effects of calcification inhibitors in the vessel wall. Interestingly, over-expression of TIMP2 can partially block calpain-1 effects on ON, but not on OPN (Figure 5C, D).
Figure 5. Over-expression of Calpain-1 down-regulates osteoponin (OPN) and osteonectin (ON) levels in VSMC, while over-expression of calpastatin, a specific inhibitor of calpain-1 has the opposite effects.
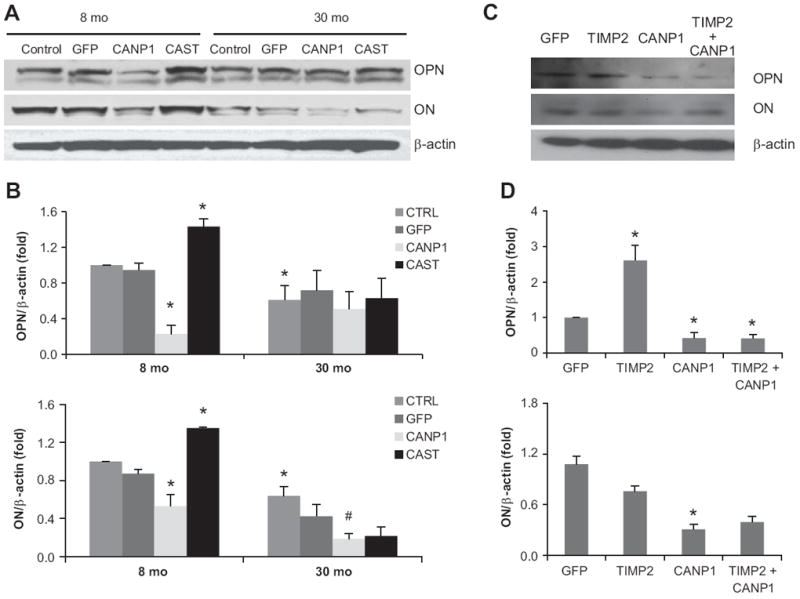
Representative western blot (A, C) and average protein levels (B, D) of OPN and ON in VSMC infected by GFP or CANP1 or CAST or TIMP2 adenoviruses for 48 hrs. *: p<0.05 compared to GFP infected 8-mo VSMC, n=3. # p<0.05 compared to GFP infected old VSMC, n=3.
Calpain-1 induces ECM remodeling in carotid aortic rings ex vivo
Over-expression of calpain-1 and TIMP2 are validated by western blot in cultured rat carotid artery rings (Figure 6A). Over-expression of calpain-1 induces collagen I, II, III, TGF-β1 and Smad 2/3, but reduces elastin, OPN and ON (Figure 6). Interestingly, Over-expression of TIMP2 blocks, in part, calpain-1 effects on these proteins (Figure 6).
Figure 6. Over-expression of calpain-1 regulates ECM remodeling, particularly fibrosis and vascular calcification in cultured carotid artery rings.

Representative western blot of TIMP2, calpain-1, elastin, TGFβ1, (Figure 6A), Smad 2/3, Collagen I, II, III, (Figure 6B) and OPN and ON (Figure 6C) in cultured carotid artery rings isolated from 8-mo old rat after infected by GFP or CANP1 or TIMP2 adenoviruses for 48 hrs.
Both calpain-1 and collagen II levels increase in human aortic intima with aging, particularly the atherosclerotic areas
Both calpain-1 and collagen II levels are significantly increased in aged versus young human aortic intima (Figure 7A). Furthermore, in atherosclerotic areas of aged human aortae, in which calcification is distinguished by Alizarin red staining, both calpain-1 and collagen II are also highly expressed (Figure 7B). Importantly, calpain-1 activity also significantly increases within atherosclerotic areas compared to grossly normal areas (Figure 7C).
Figure 7. Both calpain-1 and collagen II are increased in human aortic intima and arthrosclerosis area.

A. Representative western blot and average protein levels of calpain-1 and collagen II in human aortic intima. B. Photomicrographs (X400) of calpain-1, collagen II protein staining (brown color) within arthrosclerosis area from old human aortic wall. Nuclei were counterstained with hematoxylin (blue color). Representative Alizarin Red S staining showing local calcification within the same atherosclerotic area. C. calpain-1 activity is significantly increased in the atherosclerotic area compared to normal area in human aortic wall. *: p<0.05 compared to young ones (A) or normal area (C) in human aortic wall, n=3.
Discussion
The major findings of our study indicate a role of calpain-1 to increase vascular calcification and fibrosis, via an effect to increase MMP2 activation (Figure S4).
The first novel finding is that calpain-1 induces MMP2 activation, in part, by modifying the MT1MMP/TIMP2 ratio. MMP2 activity is dramatically elevated in the central arterial wall with advancing age1-6, 23,24 and plays a key role to facilitate age-associated ECM remodeling. MT1MMP is the most potent activator and TIMP2 the most important inhibitor of MMP2 activity.28 Our previous studies showed that calpain-1 facilitates an Ang II signaling induced increase in VSMC migration, and that this effect can be blocked by MMP2 inhibition.16 The present study demonstrates that calpain-1 induces MMP2 transcripts, protein and activities in rat VSMC, and that calpastatin, the endogenous inhibitor of calpain-1, reduces both cell associated and secreted MMP2 activities in VSMC. The present results, for the first time, also show that over-expression of calpain-1 increases MT1MMP and reduces TIMP2 levels in VSMC, resulting in an increased ratio of MT1MMP/TIMP2, which favors induction of MMP2 activation.
The second novel finding is that over-expression of calpain-1 in young VSMC increases Col I and III transcripts and proteins levels. This result is consistent with a previous report, showing that over-expression of calpastatin prevents Ang II–dependent perivascular fibrosis by collagen fibrils.33 The ECM structural framework is essential for the functional properties of blood vessels.6 Our prior work demonstrated that the intima of the older aorta is markedly and diffusely thickened compared to that in younger rat aorta, and harbors increased levels of MMP2, and collagen I and III proteins. 28 Ang II signaling triggers collagen I and III expression and MMP2 activation,18 and calpain-1 is one of the important signal transducers of Ang II signaling, that regulates MMP2 activation.16 The present studies extend these findings in the aortic wall in vivo to VSMC in vitro and carotid aortic ring ex vivo. Unlike Collagen Types I and III, collagen Type II is a main component of cartilage matrix proteins, and a marker for cartilaginous metaplasia in arteries.15 The present study also shows that collagen II is increased with aging in both rat aortic wall and human aortic intima, particularly within atherosclerotic areas.
The third novel finding of our study is that calpain-1 accelerates vascular calcification, and that this effect is counteracted, at least in part, by TIMP2 over-expression. Our results also show that calpain-1 induces expression of molecules and factors that promote calcification, including TGF-β1/smad elevation, elastin degradation, collagen II production, and reduces expression of molecules that inhibit calcification, including OPN and ON.
A growing body of evidence suggests VC is complex, and involves multiple steps and mediators.9 With advancing age VC is triggered by inflammatory cytokines.8 New pathways regulating calcification including TGFβ1, OPN, ON are the focus of many current studies.11,12 Over-expression of calpain-1 enhances secretion of activated ALP, total calcium content and collagen II, hallmarks of VC.29-31 The present results are consistent with the prior observation that calpain-1 activation is involved in atherosclerotic lesions of the human carotid artery,34 a highly potent calcification locus, and also rodent osteoblastic cell differentiation.35
Medial calcification is also associated with elastic fiber fragmentation, appearing initially as linear deposits along elastic lamellae.13,14 Our finding that calpain-1 induces elastin degradation, via induction of MMP2 activation, supports the idea that MMP-mediated elastic fiber degradation contributes to calcification. Further, the calpain-1 induced increases in Col I and III provide fertile soil for calcium deposition.
It is known that TGF-β1 plays a crucial role in bone matrix production and many fibrocalcific lesions, including calcific aortic valve stenosis.19, 20 Calpain releases mature TGF-β from the latent form of TGF-β (LAP) in test tube experiments.36 In the aged arterial wall TGF-β1 interaction with MMP2 creates a proinflammatory niche. 25 We have previously demonstrated that MMP2 increases active TGF-β by cleaving TGF-β-LAP also. The present results, which demonstrate that calpain-1 induces TGF-β1/smad signaling, suggest that a new interactive signaling module, involving calpain-1/MMP2/TGF-β1, participates in calcification within aged VSMC.
OPN and ON inhibit mineralization via direct binding to crystal surfaces to prevent any further propagation.22 OPN is an inhibitor of crystal growth during VSMC calcification. Our results demonstrate that calpain-1 prevents inhibitory effects of OPN and ON’s on VC, and that this inhibition is partially inhibited by MMP2 inhibition. Interestingly, over-expression of the calpain-1 inhibitor, calpastatin, induces both OPN and ON in young VSMC.
Age-associated arterial remodeling results, in part, from proinflammatory Ang II signaling cascades, that involve calpain-1, MMP-2/9, monocyte chemoattractant protein-1 (MCP-1), TGF-β1 activation and milk fat globule epidermal growth factor-8 (MFG-E8). 4 The major focus of the present study is to perturb VSMC isolated from young rats to determine the extent to which this shifts their phenotype to that observed in untreated cells with advancing age. But aging is associated with a complex remodeling of the arterial wall and is comprised of changes in multiple steps of multiple signaling pathways over a prolonged period. Thus, over-expression of one molecule in young vascular cells may not, per se, mimic completely quantitatively or qualitatively the chronic changes that occur and vary over time in numerous pathways. For example, when changes occur in one pathway compensations may occur in other pathways to counteract the effects.
Age-associated arterial stiffness is, to a large extent, attributable to ECM remodeling, including fibrosis and VC. It has been shown that several risk-factors i.e. high blood pressure, diabetes mellitus, smoking, are related to arterial stiffness and its link to cardiovascular diseases. Ang II, the major biologically active component of the renin-angiotensin system, contributes to the regulation of vascular tone, and blood pressure. Calpain-1 is induced by Ang II in vitro, ex vivo and in vivo. 16 The present study extends AngII/calpain-1 effects on MMP2 activation and remodeling, which are important in hypertension. Arterial wall stiffening in type 2 diabetes patients has a significant impact on cardiac function and peripheral vascular manifestations. Furthermore, myocardial hypertrophy and fibrosis in diabetic mice are attenuated by a reduction of calpain function. 37 Chronic exposure to cigarette smoke via calpain inhibition attenuates pulmonary artery endothelial cells angiogenesis, tube formation, cell migration, and proliferation and inhibits wound repair.38 Our data in the arterial wall are consistent with these results and suggest that over-expression of calpain-1 induces ECM remodeling including fibrosis and VC not only during aging but also in chronic diseases. Thus targeted inhibition of calpain represents a potential novel therapeutic strategy for retarding age-associated central arterial remodeling and its attendant high risk for chronic arterial disease.
Perspectives
An age-associated increase in central arterial wall stiffness is, in large measure, attributable to ECM remodeling, including increased collagen content and crosslinking, 1,6,7 and VC.8-15 The present study investigates the role of calpain-1 in age-associated ECM remodeling. Our results indicate that calpain-1, via its effect on MMP2 activity, induces collagen production and participates in multiple steps of VC with advancing age. Calpain-1 induces collagen II, TGF-β1/Smad signaling, elastin degradation, and reduction of VC inhibitors, OPN and ON. Collectively, these observations strongly suggest that calpain-1 plays a pivotal role in central arterial ECM remodeling during aging, at least in part, by regulating MMP2 activation. Thus calpain-1 inhibition is an attractive novel strategy to retard central arterial ECM remodeling with advancing age and its attendant increased risk for hypertension and atherosclerosis.
Supplementary Material
Supplementary Material
Novelty and Significance.
1. What is New?
The expression of calpain-1 is increased in human aortic intima with advancing age, particularly within atherosclerotic plaque areas. Calpain-1 induces fibrosis and calcification partially through MMP2 activation in young VSMC in vitro and in young carotid artery rings ex vivo.
2. What is Relevant?
An age-associated increase in central arterial wall stiffness is attributable, in large measure, to ECM remodeling. This study strongly indicates that calpain-1 plays a pivotal role in MMP2 activation and ECM remodeling, including fibrosis and vascular calcification.
3. Summary/Conclusion
Calpain-1 inhibition is an attractive novel strategy to retard vascular ECM remodeling that underlies age-associated remodeling and its attendant diseases i.e. hypertension and atherosclerosis.
Acknowledgments
Sources of Funding
This research was supported by the Intramural Research Program of the National Institute on Aging.
Footnotes
Disclosures
None
References
- 1.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 2.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 3.Wang M, Monticone RE, Lakatta EG. Arterial aging: a journey into subclinical arterial disease. Curr Opin Nephrol Hypertens. 2010;19:201–207. doi: 10.1097/MNH.0b013e3283361c0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang M, Khazan B, Lakatta EG. Central Arterial Aging and Angiotensin II Signaling. Curr Hypertens Rev. 2010;6:266–281. doi: 10.2174/157340210793611668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakatta EG, Wang M, Najjar SS. Arterial aging and subclinical arterial disease are fundamentally intertwined at macroscopic and molecular levels. Med Clin North Am. 2009;93:583–604. doi: 10.1016/j.mcna.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacob MP. Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed Pharmacother. 2003;57:195–202. doi: 10.1016/s0753-3322(03)00065-9. [DOI] [PubMed] [Google Scholar]
- 7.Brooke BS, Karnik SK, Li DY. Extracellular matrix in vascular morphogenesis and disease: structure versus signal. Trends Cell Biol. 2003;13:51–56. doi: 10.1016/s0962-8924(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 8.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–1059. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 9.Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–2948. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–1170. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 11.Shao JS, Cai J, Towler DA. Molecular mechanisms of vascular calcification: lessons learned from the aorta. Arterioscler Thromb Vasc Biol. 2006;26:1423–1430. doi: 10.1161/01.ATV.0000220441.42041.20. [DOI] [PubMed] [Google Scholar]
- 12.Dellegrottaglie S, Sanz J, Rajagopalan S. Molecular determinants of vascular calcification: a bench to bedside view. Curr Mol Med. 2006;6:515–524. doi: 10.2174/156652406778018653. [DOI] [PubMed] [Google Scholar]
- 13.Lee JS, Basalyga DM, Simionescu A, Isenburg JC, Simionescu DT, Vyavahare NR. Elastin calcification in the rat subdermal model is accompanied by up-regulation of degradative and osteogenic cellular responses. Am J Pathol. 2006;168:490–498. doi: 10.2353/ajpath.2006.050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey M, Pillarisetti S, Jones P, Xiao H, Simionescu D, Vyavahare N. Involvement of matrix metalloproteinases and tenascin-C in elastin calcification. Cardiovasc Pathol. 2004;13:146–155. doi: 10.1016/S1054-8807(04)00009-2. [DOI] [PubMed] [Google Scholar]
- 15.Kuivaniemi H, Tromp G, Prockop DJ. Mutations in fibrillar collagens (types I, II, III, and XI), fibril-associated collagen (type IX), and network-forming collagen (type X) cause a spectrum of diseases of bone, cartilage, and blood vessels. Hum Mutat. 1997;9:300–315. doi: 10.1002/(SICI)1098-1004(1997)9:4<300::AID-HUMU2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Jiang L, Wang M, Zhang J, Monticone RE, Telljohann R, Spinetti G, Pintus G, Lakatta EG. Increased aortic calpain-1 activity mediates age-associated angiotensin II signaling of vascular smooth muscle cells. PLoS One. 2008;3:e2231. doi: 10.1371/journal.pone.0002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofbauer LC, Brueck CC, Shanahan CM, Schoppet M, Dobnig H. Vascular calcification and osteoporosis--from clinical observation towards molecular understanding. Osteoporos Int. 2007;18:251–259. doi: 10.1007/s00198-006-0282-z. [DOI] [PubMed] [Google Scholar]
- 18.Olesen P, Nguyen K, Wogensen L, Ledet T, Rasmussen LM. Calcification of human vascular smooth muscle cells: associations with osteoprotegerin expression and acceleration by high-dose insulin. Am J Physiol Heart Circ Physiol. 2007;292:H1058–1064. doi: 10.1152/ajpheart.00047.2006. [DOI] [PubMed] [Google Scholar]
- 19.Clark-Greuel JN, Connolly JM, Sorichillo E, Narula NR, Rapoport HS, Mohler ER, 3rd, Gorman JH, 3rd, Gorman RC, Levy RJ. Transforming growth factor-beta1 mechanisms in aortic valve calcification: increased alkaline phosphatase and related events. Ann Thorac Surg. 2007;83:946–953. doi: 10.1016/j.athoracsur.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Nesti LJ, Caterson EJ, Li WJ, Chang R, McCann TD, Hoek JB, Tuan RS. TGF-beta1 calcium signaling in osteoblasts. J Cell Biochem. 2007;101:348–359. doi: 10.1002/jcb.21180. [DOI] [PubMed] [Google Scholar]
- 21.Rennenberg RJ, Schurgers LJ, Kroon AA, Stehouwer CD. Arterial calcifications. J Cell Mol Med. 2010;14:2203–2210. doi: 10.1111/j.1582-4934.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gadeau AP, Chaulet H, Daret D, Kockx M, Daniel-Lamazière JM, Desgranges C. Time course of osteopontin, osteocalcin, and osteonectin accumulation and calcification after acute vessel wall injury. J Histochem Cytochem. 2001;49:79–86. doi: 10.1177/002215540104900108. [DOI] [PubMed] [Google Scholar]
- 23.Wang M, Takagi G, Asai K, Resuello RG, Natividad FF, Vatner DE, Vatner SF, Lakatta EG. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension. 2003;41:1308–1316. doi: 10.1161/01.HYP.0000073843.56046.45. [DOI] [PubMed] [Google Scholar]
- 24.Wang M, Zhang J, Spinetti G, Jiang LQ, Monticone R, Zhao D, Cheng L, Krawczyk M, Talan M, Pintus G, Lakatta EG. Angiotensin II activates matrix metalloproteinase type II and mimics age-associated carotid arterial remodeling in young rats. Am J Pathol. 2005;167:1429–1442. doi: 10.1016/S0002-9440(10)61229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M, Zhao D, Spinetti G, Zhang J, Jiang LQ, Pintus G, Monticone R, Lakatta EG. Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (TGF-beta1) and TGF-beta1-type II receptor signaling within the aged arterial wall. Arterioscler Thromb Vasc Biol. 2006l;26:1503–1509. doi: 10.1161/01.ATV.0000225777.58488.f2. [DOI] [PubMed] [Google Scholar]
- 26.Wang M, Zhang J, Jiang LQ, Spinetti G, Pintus G, Monticone R, Kolodgie FD, Virmani R, Lakatta EG. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension. 2007;50:219–227. doi: 10.1161/HYPERTENSIONAHA.107.089409. [DOI] [PubMed] [Google Scholar]
- 27.Nixon RA. The calpains in aging and aging-related diseases. Ageing Res Rev. 2003;2:407–418. doi: 10.1016/s1568-1637(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 28.Wang M, Lakatta EG. Altered regulation of matrix metalloproteinase-2 in aortic remodeling during aging. Hypertension. 2002;39:865–873. doi: 10.1161/01.hyp.0000014506.13322.66. [DOI] [PubMed] [Google Scholar]
- 29.Wallin R, Wajih N, Greenwood GT, Sane DC. Arterial calcification: a review of mechanisms, animal models, and the prospects for therapy. Med Res Rev. 2001;21:274–301. doi: 10.1002/med.1010. [DOI] [PubMed] [Google Scholar]
- 30.Lomashvili KA, Garg P, Narisawa S, Millan JL, O’Neill WC. Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: potential mechanism for uremic vascular calcification. Kidney Int. 2008;73:1024–1030. doi: 10.1038/ki.2008.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schoppet M, Shanahan CM. Role for alkaline phosphatase as an inducer of vascular calcification in renal failure? Kidney Int. 2008;73:989–991. doi: 10.1038/ki.2008.104. [DOI] [PubMed] [Google Scholar]
- 32.Trion A, Schutte-Bart C, Bax WH, Jukema JW, van der Laarse A. Modulation of calcification of vascular smooth muscle cells in culture by calcium antagonists, statins, and their combination. Mol Cell Biochem. 2008;308:25–33. doi: 10.1007/s11010-007-9608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Letavernier E, Perez J, Bellocq A, Mesnard L, de Castro Keller A, Haymann JP, Baud L. Targeting the calpain/calpastatin system as a new strategy to prevent cardiovascular remodeling in angiotensin II-induced hypertension. Circ Res. 2008;102:720–728. doi: 10.1161/CIRCRESAHA.107.160077. [DOI] [PubMed] [Google Scholar]
- 34.Gonçalves I, Nitulescu M, Saido TC, Dias N, Pedro LM, E Fernandes JF, Ares MP, Pörn-Ares I. Activation of calpain-1 in human carotid artery atherosclerotic lesions. BMC Cardiovasc Disord. 2009;9:26. doi: 10.1186/1471-2261-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray SS, Grisanti MS, Bentley GV, Kahn AJ, Urist MR, Murray EJ. The calpain-calpastatin system and cellular proliferation and differentiation in rodent osteoblastic cells. Exp Cell Res. 1997;233:297–309. doi: 10.1006/excr.1997.3550. [DOI] [PubMed] [Google Scholar]
- 36.Abe M, Oda N, Sato Y. Cell-associated activation of latent transforming growth factor-beta by calpain. J Cell Physiol. 1998;174:186–193. doi: 10.1002/(SICI)1097-4652(199802)174:2<186::AID-JCP6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Ma J, Zhu H, Singh M, Hill D, Greer PA, Arnold JM, Abel ED, Peng T. Targeted inhibition of calpain reduces myocardial hypertrophy and fibrosis in mouse models of type 1 diabetes. Diabetes. 2011;60:2985–2894. doi: 10.2337/db10-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su Y, Cao W, Han Z, Block ER. Cigarette smoke extract inhibits angiogenesis of pulmonary artery endothelial cells: the role of calpain. Am J Physiol Lung Cell Mol Physiol. 2004;287:L794–L800. doi: 10.1152/ajplung.00079.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


