Abstract
The orbitofrontal cortex (OFC) is crucial for the inhibition of extraneous stimuli, evaluation of aversive information, and emotional regulation – all behaviors impaired in cocaine addiction. Previous studies suggest that cocaine-addicted subjects have decreased basal activity in the orbitofrontal cortex (OFC). In this study, we examined regional cerebral blood flow (rCBF) during a saline infusion in two independent populations of abstinent cocaine- (and mostly nicotine-) addicted (n=33 and 26) and healthy control (n=35 and 20) men and women. Isolated rCBF decreases (p<0.001) were observed in the left caudolateral OFC, as well as left superior temporal cortex, in cocaine-addicted subjects relative to controls in both cohorts and bilaterally in the combined cohort. An anatomically defined region of the caudolateral OFC showed similar findings and were evident in both male and female addicted subjects. The reliability of these findings across two cohorts reveals a functional disruption in the lateral OFC, a brain region implicated in the evaluation of behavior-terminating stimuli. This may contribute to an addicted individual’s persistent drug use despite negative consequences.
INTRODUCTION
More than 75% of addicted individuals return to substance use within one year following treatment (Crits-Christoph et al., 1999; McLellan et al., 2000). Relapse occurs despite numerous physically and/or emotionally devastating experiences. The persistence of substance use in the face of an overwhelming intimate knowledge of its dangers is indicative of serious deficits in decision-making. These deficits include a remarkable inability to appropriately assess and/or adapt one’s behavior in response to future risk. Deficits in these neurocognitive processes have been identified in a range of substance use disorders, both prior to (Schepis et al., 2008) and following substance use (Adinoff et al., 2007; Verdejo-Garcia et al., 2008). Inhibitory and decision-making deficits predict subsequent relapse risk, supporting relevance for these impairments in continued substance use(Moeller et al., 2001; Passetti et al., 2011; Streeter et al., 2008; Turner et al., 2009).
The orbitofrontal cortex (OFC) is critical in these decision-making processes, most notably for the weighing of stimulus values and negative outcomes. Intact assessment skills provide for the mental dexterity required when an individual is confronted with changing contingencies (Dolan, 2007; Fellows, 2007; Rolls, 2004), offering an organism the opportunity to alter previously chosen actions in the face of more or less rewarding outcomes (Chudasama et al., 2003; Clarke et al., 2008; Dias et al., 1997; Elliott et al., 2000a). Persons with OFC lesions exhibit deficits in these tasks, for example, perseverating in the selection of previously rewarding stimuli despite the subsequent experience of large losses (Bechara et al., 2000; Hornak et al., 2004). Even healthy individuals with smaller bilateral OFC gray matter volumes have higher scores on the Barratt Impulsivity Scale (BIS-11), a self-report measure of impulsivity (Matsuo et al., 2009). The behavior consequences of these deficits correspond to the impairments observed in addicted individuals, namely the inability to cease previously rewarding substance use in spite of the negative outcomes now experienced.
Both preclinical and clinical investigations have supported a role for the OFC in addictive processes, particularly with respect to cocaine addiction (London et al., 2000; Porrino et al., 2007; Volkow and Fowler, 2000). Bilateral lesions of the OFC altered both conditioned cue-induced and cocaine-primed reinstatement in rats (Fuchs et al., 2004) and, when coupled with lesions of the basolateral amygdala, unilateral lesions of the OFC attenuated drug context-induced cocaine seeking (Lasseter et al., 2010). Conversely, chronic cocaine administration induced deficits in rats similar to those observed with OFC lesioning (Schoenbaum et al., 2004). In humans, decreases in baseline OFC glucose metabolism (Volkow et al., 1993) regional cerebral blood flow (rCBF) (Adinoff et al., 2001), and gray matter volume (Alia-Klein et al., 2011; Franklin et al., 2002; Matochik et al., 2003) are evident in recently abstinent cocaine-addicted individuals. Further, OFC activity, assessed by positron emission tomography (PET), is decreased in cocaine-addicted subjects and increased in control subjects during performance on the Stroop Task (a measure of disinhibition) (Goldstein et al., 2001) whereas right OFC activity is increased in cocaine-addicted subjects, relative to controls, during performance of the Iowa Gambling Task (Bolla et al., 2003). The OFC is also implicated in multiple studies assessing cue-induced craving in cocaine-addicted individuals (Bonson et al., 2002; Kilts et al., 2004; Risinger et al., 2005; Volkow et al., 2010).
Given these insights, important questions remain. The OFC encompasses a sizable region of the brain and distinct roles have been proposed for these various subregions, particularly the medial and lateral OFC. The medial OFC has been associated with reward sensitivity and evaluation and the lateral OFC with punishment reactivity and behavior modification (Kringelbach and Rolls, 2003; O’Doherty et al., 2001). For instance, self-described “chocolate lovers” activated the medial OFC during chocolate ingestion in a non-satiated state, during which chocolate would presumably be experienced as rewarding (Small et al., 2001). In contrast, chocolate ingestion administered in a satiated state, when ingestion might be perceived as unpleasant, induced activation of the lateral OFC (Small et al., 2001). Bacher et al. (Gearhardt et al., 2011) recently reported that medial OFC activation to a milkshake cue is highly associated with scores on the Yale Food Addiction Scale. The left lateral OFC, on the other hand, showed less activation during food intake in those with high Yale Food Addiction scores compared to those with low scores. The attribution of the negative valence associated with “No” is also associated with increased responsivity in the lateral OFC (Alia-Klein et al., 2007). In studies of addiction, Fuchs et al. (2004) found that lateral OFC lesions increased, whereas medial OFC lesions decreased, cocaine-primed reinstatement in rats. Thus, functional imaging studies in healthy controls and behavioral studies in OFC lesioned patients suggest subregional specificity of OFC function.
Another area of ambiguity involves sex differences in OFC function, a relevant area of interest given the influence of gender in craving, drug use, abstinence and relapse (Fattore et al., 2008; Greenfield et al., 2003; Kilts et al., 2004) and the differential OFC response exhibited by men and women during a risk-delay task (Bolla et al., 2004). In this latter study, males more strongly activated the right ventromedial PFC; females more strongly activated the left dorsolateral PFC. In addition, we had previously reported that abstinent male cocaine-addicted subjects exhibit decreased rCBF in the lateral OFC, relative to healthy male controls, whereas female cocaine-addicted subjects show decreased rCBF in the medial OFC relative to healthy sex-matched controls. Finally, some investigators have reported an increase (Ernst et al., 2000; Gottschalk and Kosten, 2002) or no difference (Childress et al., 1999), rather than a decrease, in resting OFC activity in cocaine-addicted patients, offering a conflicting picture of OFC deficits in cocaine addiction.
To assist in clarifying these issues, two temporally distinct cohorts of cocaine-addicted and healthy control participants, enrolled in two separate studies, were assessed for rCBF during the administration of saline. RCBF was assessed using single photon emission computerized tomography (SPECT). Data from the first cohort has previously been reported (Adinoff et al., 2006); however, the present analyses utilizes a more stringent level of statistical significance (p<0.001 vs. p<0.01), the SPECT images are co-registered with the subject’s MRI prior to transformation in to MNI space, and the OFC regions are anatomically defined. The second cohort offered the opportunity to determine the reliability of our previous finding using a population recruited after the first cohort but utilizing the same recruitment sites, inclusion/exclusion criteria, and SPECT camera. We hypothesized that cocaine-addicted participants in both cohorts would 1) show a decrease in rCBF in the lateral and medial OFC relative to healthy controls, and 2) the decrease in lateral OFC would be specific to the male cocaine-addicted participants whereas the decrease in medial OFC would be specific to the female cocaine-addicted participants.
MATERIALS AND METHODS
Participants
Participants were 24 to 48 years old. All participants underwent a medical history and physical examination, DSM-IV Structured Clinical Interview, clinical laboratory tests, and urine drug screen. Lifetime cocaine and previous ninety day cocaine use and other substance use history was obtained from cocaine-dependent participants using the Time Line Follow Back (TLFB) (Sobell and Sobell, 1978). After a complete description of the study to the participants, consent was obtained. All subjects (except the first 10 cocaine-addicted and 10 healthy control participants in Cohort 1) underwent high-resolution T1-weighted magnetic resonance imaging (MRI) to rule-out overt cerebral pathology and to enhance spatial normalization of the SPECT images. Participants were financially compensated for their participation. Approval for the study was obtained from the Institutional Review Board of the University of Texas Southwestern Medical Center at Dallas and the VA North Texas Health Care System.
Cocaine-addicted Participants
Patients were recruited from patients requesting treatment for cocaine dependence at the Veteran’s Administration Medical Center in Dallas, Homeward Bound, Inc., and Nexus Recovery Center. All subjects endorsed cocaine as their primary drug of choice. Addicted subjects were hospitalized as soon as possible after their last reported use of cocaine and remained in a structured, residential unit until the study was completed. All subjects were evaluated between two weeks and six weeks abstinence. Exclusion criteria included a substance use disorder (except cocaine or nicotine) within the previous twelve (Cohort 1) or six (Cohort 2) months, present use of any central nervous system active medications (including all psychotropics), major medical or neurological disorders, lifetime presence of a non-substance use related Affective, Anxiety, or Psychotic Disorders, or Organic Brain Syndrome. In addition, subjects in Cohort 1 denied a lifetime history of withdrawal from alcohol, sedative-hypnotics, or opioids. Women were all pre-menopausal. A negative pregnancy test was obtained on all female subjects prior to SPECT scanning.
Healthy Control Participants
Controls were recruited through local ads in newspapers, the Internet, and notices on bulletin boards. Exclusion criteria for healthy controls included the medical criteria as noted for the cocaine-addicted subjects, as well as, a lifetime history of substance use or other Axis I disorder (except nicotine or caffeine abuse or dependence). Healthy controls with a first-degree relative or two or more second-degree relatives with a substance use disorder were also excluded.
Study Sessions
Study sessions took place at the Nuclear Medicine Center or the Clinical Trials Office at the University of Texas Southwestern Medical Center at Dallas. Participants from two different studies were investigated.
Cohort 1
35 control (17 women) and 33 cocaine-addicted (10 women) subjects participated in two study sessions to assess limbic sensitivity to the local anesthetic procaine (Adinoff et al., 2001; Adinoff et al., 2006). This study was conducted in a single blind, fixed-order (saline first, procaine second) design.
Cohort 2
20 control (10 women) and 26 cocaine-addicted (10 women) subjects participated in four study sessions to assess cholinergic and 5HT3 receptor system function. In each session, either the cholinergic agonist physostigmine, the muscarinic antagonist scopolamine, the 5HT3 antagonist ondansetron, or saline was administered (Adinoff et al., 2010). Subjects were blind to the order of pharmacologic challenge.
In both cohorts 20 mCi of 99mTc HMPAO (Nycomed/Amersham, Princeton New Jersey) was administered over 30 seconds followed by 10 ml saline flush over 30 seconds to assess rCBF. Infusion took place with the intravenous line draped behind a curtain so that subjects were not aware of the exact time of the infusion. SPECT scans were obtained in the Nuclear Medicine Center 90 minutes following 99mTc HMPAO administration. This allowed time for tracer activity to clear from blood and non-brain tissues. Because 99mTc HMPAO is extracted during the first arterial pass and remains distributed in the brain in proportion to rCBF for many hours, this image represents rCBF at the time of radiotracer administration and not at the time of scan acquisition.
Due to concern that the rCBF response following saline administration may reflect anticipation of receiving a medication, a third true resting condition was obtained in a subset of subjects from Cohort 1 between seven and eleven days following their saline scan (Adinoff et al., 2003). The paradigm was the same as that described for the saline infusion except that subjects were informed that no medication would be administered. Eleven (1 female) cocaine-addicted and eleven (5 females) control participants participated in the resting scan.
SPECT Imaging
SPECT images were acquired on a PRISM 3000S 3-headed SPECT camera (Picker International, Cleveland, OH) using ultra high-resolution fan-beam collimators (reconstructed resolution of 6.5mm) in a 128 × 128 matrix in three-degree increments. 20mCi of 99mTc HMPAO was administered for each scan, and total scan duration was 20 minutes. Image reconstruction was performed in the transverse domain using back-projection with a ramp filter. For our system voxels in reconstructed images were 1.9mm3. Reconstructed images were smoothed with a 4th-order Butterworth three-dimensional filter, attenuation corrected using a Chang first-order method with ellipse size adjusted for each slice.
Statistical Analysis
Demographic and Clinical Measures
Data were analyzed with SPSS for Windows version 18.0 (SPSS, Inc. Chicago, IL). Parametric statistics were used to examine the demographic characteristics of the sample and the substance use metrics of the patient groups. Nominal data were analyzed via chi square crosstabs analysis. Demographics variables that were significantly different between groups (race, gender, and age) were considered as covariates in the combined analyses. Nicotine use was not considered as a covariate due to its existence occurring primarily within one group (cocaine-addicted). Pearson product moment correlations were conducted to assess the relationship between clinical variables of interest and OFC rCBF.
Image Analysis
To more accurately register SPECT images, a rigid-body coregistration of the SPECT scan to a skull-stripped T1-weighted high-resolution (.8 × .8 × 1.5 mm) structural MRI scan of the same subject moved the SPECT image into the same space as the MRI. Spatial transformation parameters were then calculated using the SPM5 segmentation algorithm to warp the MRI into standard MNI space. The same transformation was then applied to the coregistered SPECT image and output images were resliced 2mm3 voxels. The image were smoothed to a final resolution of 10mm and normalized to whole brain counts (to correct for individual variability in global cerebral blood flow). All images were smoothed to a final resolution of 10mm and normalized to whole brain counts (to correct for individual variability in global cerebral blood flow). For the 20 participants who did not receive an MRI, SPECT images were resliced to 2mm3 voxels and co-registered to a PET template in MNI space (Talairach and Tournoux, 1988). Voxel-wise analyses of saline-induced effects on rCBF were analyzed using Statistical Parametric Mapping (SPM2) (Wellcome Department of Cognitive Neurology, University College, London, England) with a statistical threshold of p<0.001 for voxel z scores and a cluster size no smaller than 50 voxels. Analyses of change in rCBF were measured relative to global cerebral blood flow.
RESULTS
Demographic and Clinical Measures (Table 1)
Table 1.
Demographic, Clinical, and Caudolateral Orbitofrontal Counts/Voxel in Control and Cocaine-addicted Cohortsa
| Cohort 1 | Cohort 2 | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Demographic Characteristics | Control (n = 35) | Cocaine-Addicted (n = 33) | t, x2 or F value | P-value | Control (n = 20) | Cocaine-Addicted (n = 26) | t, x2 or F value | P-value |
| Age | 33.0 ± 6.7 | 38.3 ± 5.2 | 3.6b | 0.001 | 34.3 ± 7.1 | 38.2 ± 6.6 | 1.9b | 0.06 |
| Gender | 16 male | 23 male | 2b | 0.05 | 10 male | 16 male | 0.8b | 0.44 |
| Race, n (%) | 24.4c | 0.001 | 6.1c | 0.05 | ||||
| Black | 6 | 25 | 9 | 20 | ||||
| White | 20 | 7 | 9 | 6 | ||||
| Hispanic | 6 | 1 | 2 | 0 | ||||
| Asian | 3 | 0 | 0 | 0 | ||||
| Cigarette packs/years | 0.14 ± 0.35 | 12.6 ± 17.6 | 3.8b | 0.001 | 0.24 ± 0.72 | 14.6 ± 13.7 | 4.4b | 0.001 |
|
| ||||||||
| Cocaine use in days, 90 days prior to treatment | 53 ± 30 | 70 ± 25 | ||||||
| Cocaine use in $, 90 days prior to treatment | 1750 ± 1597 | 1754 ± 1198 | ||||||
| Cocaine use in days, lifetime | 227361 ± 403888 | 259997 ± 298266 | ||||||
| Cocaine use in $, lifetime | 1750 ± 1597 | 1859 ± 1269 | ||||||
| Left OFC counts/voxele | 75.0 ± 4.1 | 71.4 ± 5.4 | 6.42d | 0.02 | 72.9 ± 3.6 | 69.0 ± 4.8 | 7.16d | 0.02 |
| Right OFC counts/voxele | 73.4 ± 4.0 | 70.8 ± 4.8 | 4.58d | 0.04 | 72.1 ± 4.8 | 69.3 ± 4.4 | 1.63d | 0.21 |
values reported as mean ± SD
values obtained by t-test
values obtained by x2-test
values obtained by univariate analysis of variance
relative rCBF determined as counts/voxel normalized to whole brain counts in anatomically defined caudolateral OFC, adjusted for age, race, and gender
Gender distribution did not significantly differ in either cohort. Both cocaine-addicted cohorts were, on average, four to five years older than their respective controls. Cocaine-addicted cohorts also consisted of significantly more African-American subjects than their respective controls and almost all cocaine-addicted subjects in both cohorts smoked cigarettes whereas the control groups did not. Cocaine-addicted cohorts did not significantly differ from one another in age (t = 0.70, P= 0.94), gender (t = 0.65, P = 0.52), race (x2 = 0.81, P = 0.67), nicotine use (t = 0.46, P = 0.64), dollars spent on cocaine in the 90 days prior to treatment (t = 0.04, P = 0.97), lifetime dollars spent on cocaine (t = 0.34, P = 0.74) or lifetime days used of cocaine (t = 0.28, P = 0.78), although Cohort II used cocaine on significantly more days prior to treatment than Cohort 1 (t = 2.3, P = 0.03). The two control cohorts did not differ from one another in age (t = 0.69, P = 0.49), gender (t = 0.30, P = 0.76), or race (x2 = 6.14, P = 0.10).
Decreased rCBF in cocaine-addicted vs. healthy control subjects
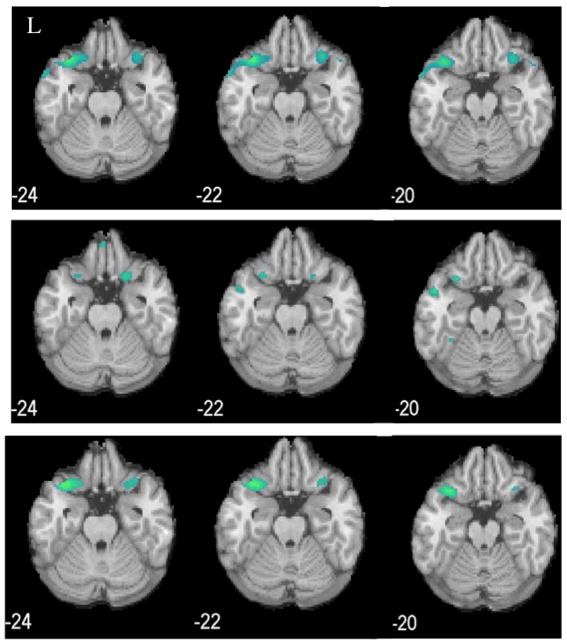
Cohort 1 cocaine-addicted participants, relative to healthy control participants, showed significant clusters of decreased rCBF in the left posterior lateral (caudolateral) OFC adjacent to a cluster in the left superior temporal gyrus (MNI coordinates; cluster size: −38, 18, −28; 196), the left middle temporal gyrus (−56, 6, −26; 146) and the right caudolateral OFC (28, 20-, 20; 55) (Fig. 1, top panel). Cohort 2 cocaine-addicted participants showed decreased rCBF in the left caudolateral OFC (−28, 16, −20; 143; Fig. 1, middle panel) in a region nearly identical to that observed in Cohort 1. A cluster was again observed in the left superior temporal cortex as well as in the symmetric right superior temporal cortex (28, 12, −34; 144). Since both cohorts demonstrated a similar, relatively isolated decrease in the left OFC and were demographically similar, the two cohorts were assessed as a combined sample. The combined cohort demonstrated a decrease in both the left (−36, 18, −28; 768) and right (30, 16, −34; 325) caudolateral OFC with adjacent clusters in bilateral superior temporal gyri and the left middle temporal gyrus (−64, −14, −12; 58) (Fig. 1, bottom panel).
Fig. 1.
Decreased rCBF (blue clusters, p<0.001) in cocaine-addicted, relative to control, participants in Cohort 1 (top panel), Cohort 2 (middle panel), and combined cohorts (bottom panel). L=left. MNI coordinates of z axis are noted.
Counts/voxel in anatomically derived caudolateral OFC
Although our previous work(Adinoff et al., 2001; Adinoff et al., 2006) and the above SPM comparisons all reveal group differences in similar OFC regions, the exact size and location of these regions varies slightly depending on the number of individuals included in an analysis and the specific cohort. To avoid using an idiosyncratic region specific to a given population, an anatomically consistent region of the OFC was defined that overlapped with the data-driven regions describing group differences in the right and left OFC rCBF (Fig. 2). The caudolateral OFC ROIs were defined as the right and left lateral one-third of the orbital frontal gyrus and the adjacent inferolateral aspect of the inferior frontal gyrus (Fig. 2, red and gray clusters). These ROIs contained Brodmann area (BA) 47 and the lateral components of BA10 and 11.
Fig. 2.
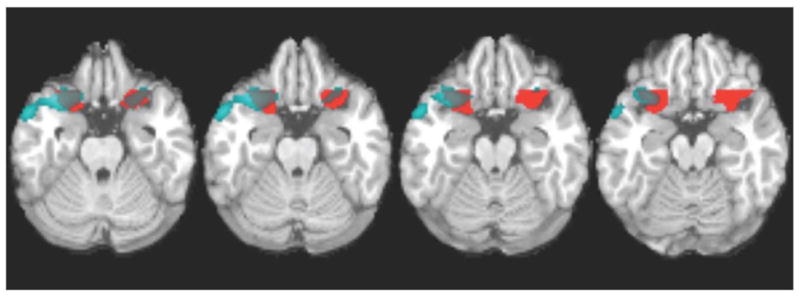
Anatomically derived caudolateral OFC. Blue and gray clusters denote areas of decreased rCBF (p<0.001) in cocaine-addicted (n=55), relative to healthy control (n=59), participants (as seen in the bottom panel of Fig. 1). Red and gray clusters denote anatomically defined caudolateral OFC. Gray clusters denote areas of overlap between decreased rCBF in cocaine-addicted relative to controls and anatomically defined caudolateral OFC. MNI coordinates of z axis are noted.
The left and right anatomically defined OFC regions (counts/voxel) were then compared between groups (adjusting for age, gender and race as covariates). Significant differences were observed in the left caudolateral OFC in both cohorts and the right OFC in Cohort 1 only (Table 1 and Fig. 3). The combined cohort also showed a significant group difference in both the left (F = 13.2, df = 1, P < 0.001) and right (F = 5.23, df = 1, P < 0.03) caudolateral OFC. As gender differences in OFC rCBF were previously reported in Cohort 1, we conducted post-hoc comparisons in the combined sample within each gender (adjusted for age and race). Both men and women showed a significant difference in the left (men: F = 4.39, P = 0.04; women: F = 5.40, P < 0.03) but not right (men: F = 3.06, P < 0.09; women: F = 0.85, P = 0.36) caudolateral OFC (although a statistical trend was noted in the men). Whole brain post-hoc comparisons in the combined cohort showed decreased left and right OFC rCBF (p<0.001) in male cocaine-addicted participants relative to male controls (Fig. 4, bottom panel). Female cocaine-addicted subjects, relative to female healthy controls, demonstrated decreased rCBF in the left OFC (p<0.01) (Fig. 5, bottom panel). At less stringent levels of significance, cohort specific gender comparisons also showed decreased caudolateral OFC rCBF for both men (Cohort 1 at p<0.005: Fig. 4, top panel; Cohort 2 at p<0.01: Fig. 4, middle panel) and women (Cohort 2 at p<0.005: Fig. 5, middle panel). The only exception was women in Cohort 1, who showed a decrease in medial OFC as previously reported (Adinoff et al., 2006).
Fig. 3.
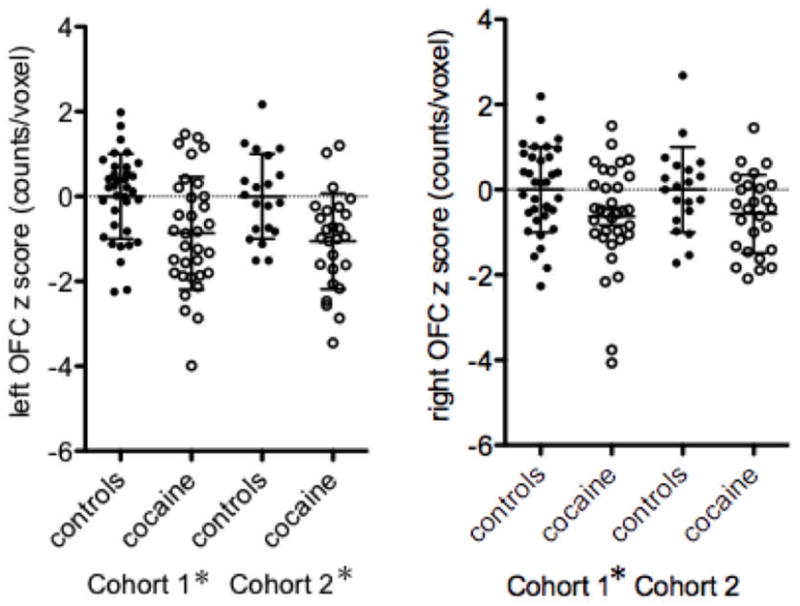
Caudolateral left (left panel) and right (right panel) orbitofrontal cortex (OFC) counts/voxel in control (closed circles) and cocaine-addicted (open circles) participants in Cohort 1 and 2. As mean counts/voxel in both groups were somewhat lower in Cohort 2 relative to Cohort 1, individual counts/voxel were converted to z-scores relative to the respective control group for a specific cohort. Caudolateral OFC was defined as in Fig. 2. *p<0.05 comparison within cohort, adjusted for age, sex, and gender.
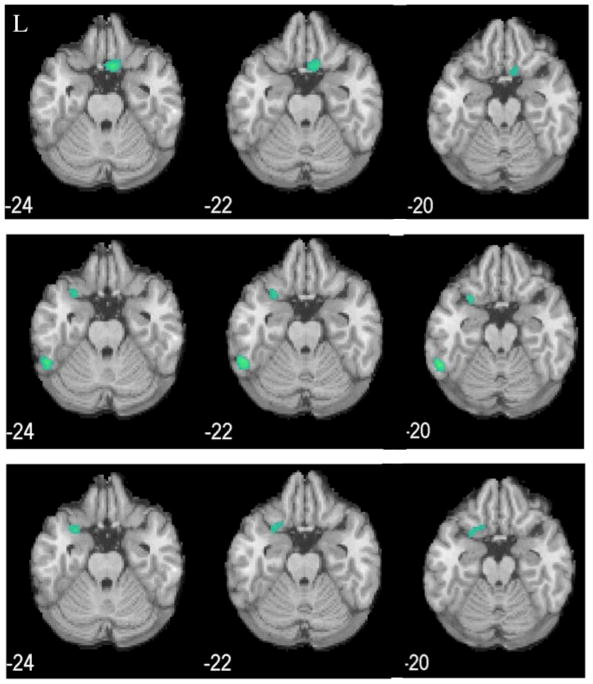
Fig. 4.
Decreased rCBF (blue clusters) in cocaine-addicted, relative to control, male participants in Cohort 1 (top panel, p<0.005), Cohort 2 (middle panel, p<0.01), and combined cohorts (bottom panel, p<0.001). L=left. MNI coordinates of z axis are noted.
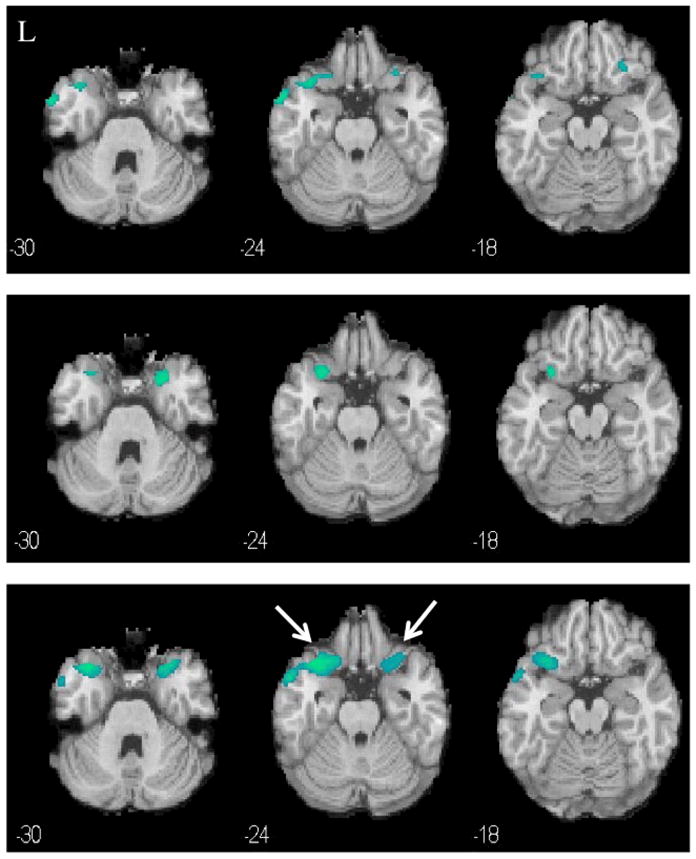
Fig. 5.
Decreased rCBF (blue clusters) in cocaine-addicted, relative to control, female participants in Cohort 1 (top panel, p<0.005), Cohort 2 (middle panel, p<0.005), and combined cohorts (bottom panel, p<0.01). L=left. MNI coordinates of z axis are noted.
Correlations with Clinical Variables
Measures of cocaine use (lifetime number of cocaine days used, lifetime dollars spent on cocaine, lifetime dollars spent on cocaine/lifetime number of days used, dollars spent and number of days used in the 90 days prior to treatment, and dollars spent on cocaine/days used cocaine in the 90 days prior to treatment, age of first use, total years used) and nicotine use (total pack years) were correlated with counts/voxel in the anatomically defined left and right caudolateral OFC (Fig. 6). There were no significant associations (P < 0.15) between OFC rCBF and any of these variables.
Fig. 6.
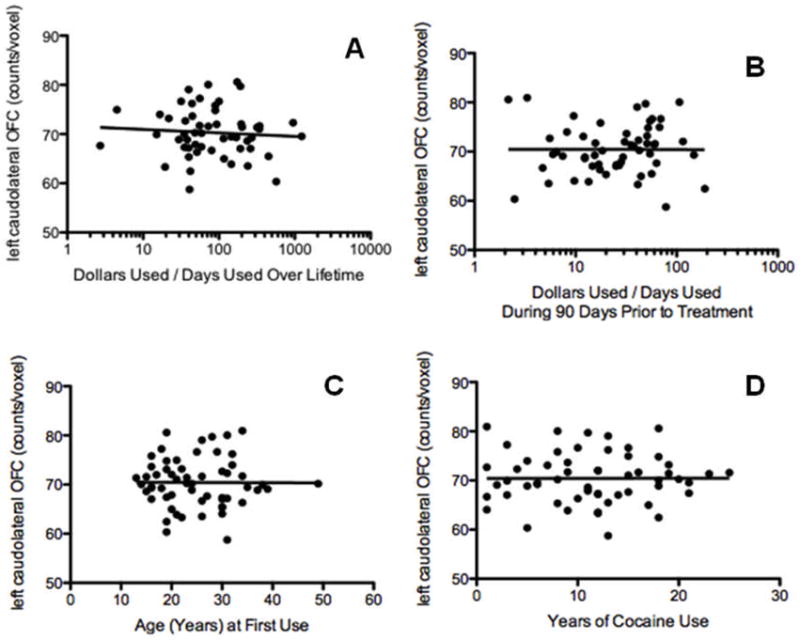
Correlation between left caudolateral OFC rCBF (counts/voxel) and cocaine use variables: A) dollars used/days used during lifetime (r=0.09, p=0.49). B) dollars used/days used over 90 days prior to treatment (r=0.003, p=0.98), C) age first use of cocaine (r=0.004, p=0.97), and D) years of cocaine use (r=0.0007, p=0.99).
Relationship of Saline and Resting rCBF
rCBF was obtained in both cohorts during a placebo saline infusion which subjects were informed may contain a pharmacologically active medication. To explore whether a true resting measure of rCBF differed from the rCBF values obtained during the saline infusion, a subgroup of Cohort 1 received a resting SPECT scan in which subjects knew no active pharmacologic challenge would occur. There was a significant correlation between rCBF obtained during saline infusion and at rest in both the left (r =0.89, df = 21, P < 0.001) and right (r = 0.76, df = 21; P < 0.001) anatomically defined caudolateral OFC. OFC rCBF was also lower during rest in the subgroup of cocaine-dependent, relative to the control, participants in both the left (t = 3.06, P = 0.006) and right (t = 2.81, P < 0.02) caudolateral OFC.
DISCUSSION
We have now replicated an earlier finding of decreased posterior lateral OFC rCBF in male cocaine-addicted subjects in a second cohort of cocaine-addicted and healthy control participants. In addition, we have isolated this decrease to a relatively specific, anatomically defined, and clinically relevant region of the OFC. These group differences also now extend to both male and female cocaine-addicted subjects. These findings reveal a disruption in OFC function in cocaine-dependent men and women and support a role for the OFC, particularly the caudolateral OFC, in the pathology of cocaine addiction.
The lateral OFC appears to assess stimuli that advise the cessation of an ongoing behavior (Elliott et al., 2000a; Kringelbach and Rolls, 2004). For example, the lateral OFC, in clusters overlapping with the anatomically defined caudolateral OFC portrayed in Fig. 2, is activated during alcohol tasting (left and right OFC) (Filbey et al., 2008a), risk-taking (left and right OFC) (Elliott et al., 2000b), exposure to a negatively valenced “No” (right OFC) (Alia-Klein et al., 2007), and during losing relative to winning (left and right OFC) (Elliott et al., 2010). While the significance of a decrease in resting caudolateral OFC rCBF in cocaine-addicted subjects is uncertain, it may portend functional disruptions in the assessment of punishments that would otherwise guide the subject to cease behaviors resulting in these negative outcomes. Of potential relevance is a rodent study demonstrating increased cocaine-primed reinstatement following lateral OFC lesions (Fuchs et al., 2004). Thus, there may be widespread neuronal as well as behavioral consequences of altered lateral OFC functional. MRI studies of the functional connectivity of the caudolateral OFC during rest in addicted subjects might determine whether alterations in this region are related to more widely distributed disruptions in network associations. Decreases in rCBF may also reflect, at least in part, reductions in gray matter volume in the OFC recently reported by Alia-Klein et al. (2011). However, there was no atrophy observed in our cocaine-addicted cohorts and it is unlikely that atrophy, if present, would exhibit such an isolated effect across a wide population of cocaine-addicted subjects. Furthermore, while corrections of SPECT rCBF and PET measures of glucose metabolism (rCGM) for atrophy reduce the magnitude of rCBF/rCGM abnormalities (typically no more than 25%) (Matsuda et al., 1999; Quarantelli et al., 2004), it does not eliminate observed findings.
The caudolateral OFC ROIs were defined as the right and left lateral one-third of the orbital frontal gyrus and the adjacent inferolateral aspect of the inferior frontal gyrus. Although commonly used imaging atlases do not describe the caudolateral OFC per se, the voxels inside the defined caudalateral OFC ROI are all associated with the OFC described by the Talairach Atlas (Talairach and Tournoux, 1988) (both by visual inspection of the atlas and by query using the Talairach Daemon (www.talairach.org/applet). The defined ROI also overlaps with the orbital part of the inferior frontal gyrus in the Anatomical Automatic Labeling SPM Atlas (Tzourio-Mazoyer et al., 2002).
In the first cohort, we had previously reported a decrease in the lateral OFC in cocaine-addicted men and in the medial OFC in cocaine-addicted women (Adinoff et al., 2006). In the present study, gender comparisons were conducted post-hoc on differences observed in the group comparison (i.e. lateral OFC). This approach, combined with more stringent statistical criteria, did not reveal group differences in the medial OFC or gender differences in the lateral OFC for the combined sample. The significant decrease in left caudolateral OFC rCBF observed in female cocaine-addicted subjects of the combined cohorts suggests the null finding in our previous report was due to insufficient power. In toto, these findings suggest that caudolateral OFC is similarly decreased in both male and female cocaine-addicted subjects relative to healthy controls. It is worth noting that while the findings are clearly stronger in the left rather than right lateral OFC, the extant literature does not suggest a clinical relevance to this finding. For example, fMRI studies report that inhibitory efforts induce bilateral (Wager et al., 2004), right only (Levesque et al., 2003) or left only (Bishop et al., 2004; Ochsner et al., 2004) activation in healthy controls. Thus, the relevance of our left dominant findings is uncertain.
The neurobiological mechanisms underlying the decrease in caudolateral OFC are unknown. Cocaine may alter rCBF through its direct or indirect effects upon the aminergic or cholinergic subcortical fibers innervating the OFC (Morecraft et al., 1992). Mesocortical dopaminergic neurons project to the prefrontal cortex (Berger et al., 1991) and dopamine levels are markedly higher in the OFC relative to non-prefrontal regions in the rat frontal lobe(Slopsema et al., 1982). Volkow et al. (2001; 1993) has shown that reduced OFC glucose metabolism (which is highly correlated with rCBF) at rest is positively associated with decreased striatal D2 receptor availability in both cocaine- and amphetamine-addicted subjects, although why mesocortical dopamine would preferentially affect the caudolateral OFC is uncertain. Filbey et al. (2008b) reported that heavy drinkers with DRD4 variable number of tandem repeats greater than seven had a significantly greater BOLD response to alcohol cues in the left lateral orbitofrontal cortex relative to those with less than 7 repeats, suggesting a specificity of dopaminergic input into this region.
Recent or lifetime use of cocaine did not correlate with the decrease in caudolateral OFC rCBF. The absence of a relationship may suggest that reduced OFC rCBF reflects a premorbid risk factor for the development of cocaine dependence. Alternately, the retrospective measures utilized may not accurately assess prior cocaine use or the cocaine use measures may not capture what may be the most relevant variables [e.g. highest use of cocaine over a short period of time, amount of use during periods of heightened vulnerability, cocaine use concomitant with alcohol (i.e cocaethylene) and/or nicotine use].
Strengths of our study include a relatively large number of cocaine-addicted and healthy control subjects in both cohorts, replication using similar recruitment methods and subject populations, and the inclusion of populations without other substance use, psychiatric, or medical co-morbidity studied during a relatively circumscribed period of abstinence. The duration of abstinence (2–6 weeks) was sufficient to avoid the acute toxic effects of cocaine and the more severe effects of early cocaine withdrawal. The use of an anatomically defined region allows a straightforward method of replication by other investigators. The exclusion of any other recent substance use diagnosis (except nicotine) within the previous 12 (Cohort 1) or 6 (Cohort 1) months, as well as the exclusion of a lifetime history of alcohol, benzodiazepine, or opioid withdrawal in Cohort 1, minimize the likelihood that other substances may have been major confounds. However, as prior lifetime substance use (except cocaine and nicotine) with and without cocaine use was not quantified, the possible contribution of other substance use cannot be excluded.
Methodological shortcomings include the use of nicotine by almost all of the cocaine-addicted and almost none of the control participants. Thus, group differences may have been due to either nicotine alone or the combined effects of nicotine and cocaine. Although cocaine-addicted subjects were slightly, albeit significantly, older and were more likely to be African-American relative to the control subjects, significant group differences in both the left and right caudolateral OFC rCBF persisted when these demographic variables were considered as covariates. Administration of saline during both scans may have produced an expectation effect in both samples. However, participants were unable to observe the infusion and, therefore, were not aware of the exact time of radiotracer administration. In addition, caudolateral OFC rCBF during saline administration in Cohort 1 was highly correlated with the same regions’ rCBF during a subsequent resting scan. Further, even when considered separately, resting caudolateral OFC rCBF was lower in the subgroup of cocaine-addicted studied at rest. Limits of spatial resolution and the ability to only determine relative measures of OFC rCBF are inherent in our SPECT methodology and camera. It should also be noted that our SPECT technique assessed relative (to whole brain), not absolute, rCBF differences between groups.
The present findings are relatively unique in that they document very similar differences between two distinct groups of cocaine-addicted and healthy control subjects. Thus, it is unlikely that our finding is peculiar to a particular group of either cocaine-addicted or comparison subjects. The isolation of the caudolateral OFC as an area of disruption will allow future studies to focus upon this region while considering individual differences in OFC sulcal variations and gray matter volume as well as its association with both neurocognitive measures of impulsivity and decision-making and clinical outcome assessments. Although neuroimaging studies utilizing radioligands are not allowed in healthy children, functional MRI studies will be useful in determining if alterations in the caudolateral OFC represent a pre-existing vulnerability to cocaine use or dependence and/or how long it persists following abstinence. Finally, somewhat similar findings in alcohol (Volkow et al., 1994) and methamphetamine (Volkow et al., 2001) addicted subjects suggest that caudolateral OFC alterations should be considered as a potential phenotypic signature of addiction.
Acknowledgments
The authors thank the staff of the Substance Abuse Team at the VA North Texas Health Care System, Homeward Bound, Inc., and the Nexus Recovery Center for their support in the screening and recruitment of study subjects. This study was funded by DA10218, DA11434, and DA023203 from the National Institute on Drug Abuse and supported by the VA North Texas Health Care System. Ceretec (HMPAO) was generously supplied by GE Healthcare.
Footnotes
Authors Contribution
Dr. Adinoff was responsible for study concept, design and oversight and manuscript preparation. Ms. Braud assisted with statistical analysis and manuscript preparation. Dr. Devous contributed to study design and manuscript preparation. Mr. Harris was responsible for image analyses. All authors critically reviewed the content and approved the final version for publication.
Conflicts of Interest
None of the authors report any relevant conflicts of interest.
Ms. Braud and Mr. Harris report no outside financial interests.
Dr. Devous is on the Scientific Advisory Board of AVID Radiopharmaceuticals and receives research support AVID Radiopharmaceuticals.
Dr. Adinoff receives grant/research support from NIAAA, NIDA, and Department of Veterans Affairs.
References
- Adinoff B, Devous MD, Best SM, George MS, Alexander D, Payne K. Limbic responsiveness to procaine in cocaine-addicted subjects. Am J Psychiatry. 2001;158:390–398. doi: 10.1176/appi.ajp.158.3.390. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Devous MD, Sr, Cooper DB, Best SE, Chandler P, Harris T, Cervin CA, Cullum CM. Resting regional cerebral blood flow and gambling task performance in cocaine-dependent subjects and healthy comparison subjects. Am J Psychiatry. 2003;160:1892–1894. doi: 10.1176/appi.ajp.160.10.1892. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Devous MD, Sr, Williams MJ, Best SE, Harris TS, Minhajuddin A, Zielinski T, Cullum M. Altered neural cholinergic receptor systems in cocaine-addicted subjects. Neuropsychopharmacology. 2010;35:1485–1499. doi: 10.1038/npp.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Rilling LM, Williams MJ, Schreffler E, Schepis TS, Rosvall T, Rao U. Impulsivity, neural deficits, and the addictions: the “oops” factor in relapse. J Addict Dis. 2007;26(Suppl 1):25–39. doi: 10.1300/J069v26S01_04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Williams MJ, Best SE, Harris TS, Chandler P, Devous MD., Sr Sex differences in medial and lateral orbitofrontal cortex hypoperfusion in cocaine-dependent men and women. Gend Med. 2006;3:206–222. doi: 10.1016/s1550-8579(06)80209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alia-Klein N, Goldstein RZ, Tomasi D, Zhang L, Fagin-Jones S, Telang F, Wang GJ, Fowler JS, Volkow ND. What is in a word? No versus Yes differentially engage the lateral orbitofrontal cortex. Emotion. 2007;7:649–659. doi: 10.1037/1528-3542.7.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alia-Klein N, Parvaz MA, Woicik PA, Konova AB, Maloney T, Shumay E, Wang R, Telang F, Biegon A, Wang GJ, Fowler JS, Tomasi D, Volkow ND, Goldstein RZ. Gene × Disease Interaction on Orbitofrontal Gray Matter in Cocaine Addiction. Arch Gen Psychiatry. 2011;68:283–294. doi: 10.1001/archgenpsychiatry.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Berger B, Gaspar P, Verney C. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends Neurosci. 1991;14:21–27. doi: 10.1016/0166-2236(91)90179-x. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Sex-related differences in a gambling task and its neurological correlates. Cereb Cortex. 2004;14:1226–1232. doi: 10.1093/cercor/bhh083. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Robbins TW, Roberts AC. Lesions of the medial striatum in monkeys produce perseverative impairments during reversal learning similar to those produced by lesions of the orbitofrontal cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:10972–10982. doi: 10.1523/JNEUROSCI.1521-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crits-Christoph P, Siqueland L, Blaine J, Frank A, Luborsky L, Onken LS, Muenz LR, Thase ME, Weiss RD, Gastfriend DR, Woody GE, Barber JP, Butler SF, Daley D, Salloum I, Bishop S, Najavits LM, Lis J, Mercer D, Griffin ML, Moras K, Beck AT. Psychosocial treatments for cocaine dependence: National Institute on Drug Abuse Collaborative Cocaine Treatment Study. Arch Gen Psychiatry. 1999:493–502. doi: 10.1001/archpsyc.56.6.493. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: restriction to novel situations and independence from “on-line” processing. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ. Keynote address: revaluing the orbital prefrontal cortex. Ann N Y Acad Sci. 2007;1121:1–9. doi: 10.1196/annals.1401.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Agnew Z, Deakin JF. Hedonic and informational functions of the human orbitofrontal cortex. Cereb Cortex. 2010;20:198–204. doi: 10.1093/cercor/bhp092. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex. 2000a;10:308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. J Neurosci. 2000b;20:6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L, Oropilla G, Gustavson A, Speck O. Cerebral perfusion abnormalities in abstinent cocaine abusers: a perfusion MRI and SPECT study. Psychiatry Res. 2000;99:63–74. doi: 10.1016/s0925-4927(00)00056-1. [DOI] [PubMed] [Google Scholar]
- Fattore L, Altea S, Fratta W. Sex differences in drug addiction: a review of animal and human studies. Womens Health (Lond Engl) 2008;4:51–65. doi: 10.2217/17455057.4.1.51. [DOI] [PubMed] [Google Scholar]
- Fellows LK. The role of orbitofrontal cortex in decision making: a component process account. Ann N Y Acad Sci. 2007;1121:421–430. doi: 10.1196/annals.1401.023. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, Du YP, Hutchison KE. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008a;33:1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Ray L, Smolen A, Claus ED, Audette A, Hutchison KE. Differential neural response to alcohol priming and alcohol taste cues is associated with DRD4 VNTR and OPRM1 genotypes. Alcohol Clin Exp Res. 2008b;32:1113–1123. doi: 10.1111/j.1530-0277.2008.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, Brownwell KD. Neural correlates of food addiction. Arch Gen Psychiatry. 2011;68:808–816. doi: 10.1001/archgenpsychiatry.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, Wang GJ, Fowler JS, Rajaram S. Addiction changes orbitofrontal gyrus function: involvement in response inhibition. Neuroreport. 2001;12:2595–2599. doi: 10.1097/00001756-200108080-00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk PC, Kosten TR. Cerebral perfusion defects in combined cocaine and alcohol dependence. Drug Alcohol Depend. 2002;68:95–104. doi: 10.1016/s0376-8716(02)00109-6. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Manwani SG, Nargiso JE. Epidemiology of substance use disorders in women. Obstet Gynecol Clin North Am. 2003;30:413–446. doi: 10.1016/s0889-8545(03)00072-x. [DOI] [PubMed] [Google Scholar]
- Hornak J, O’Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, Polkey CE. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. J Cogn Neurosci. 2004;16:463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. Neural correlates of rapid reversal learning in a simple model of human social interaction. Neuroimage. 2003;20:1371–1383. doi: 10.1016/S1053-8119(03)00393-8. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Lasseter HC, Wells AM, Xie X, Fuchs RA. Interaction of the Basolateral Amygdala and Orbitofrontal Cortex is Critical for Drug Context-Induced Reinstatement of Cocaine-Seeking Behavior in Rats. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry. 2003;53:502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- London ED, Ernst M, Grant S, Bonson K, Weinstein A. Orbitofrontal cortex and human drug abuse: Functional imaging. Cereb Cortex. 2000;10:334–342. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Liu Y, Mahankali S, Pu Y, Mahankali A, Wang J, DeFronzo RA, Fox PT, Gao JH. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes. 1999;48:1801–1806. doi: 10.2337/diabetes.48.9.1801. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat. 2001;21:193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Geula C, Mesulam MM. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. The Journal of comparative neurology. 1992;323:341–358. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Passetti F, Clark L, Davis P, Mehta MA, White S, Checinski K, King M, Abou-Saleh M. Risky decision-making predicts short-term outcome of community but not residential treatment for opiate addiction. Implications for case management. Drug Alcohol Depend. 2011 doi: 10.1016/j.drugalcdep.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Smith HR, Nader MA, Beveridge TJ. The effects of cocaine: a shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1593–1600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarantelli M, Berkouk K, Prinster A, Landeau B, Svarer C, Balkay L, Alfano B, Brunetti A, Baron JC, Salvatore M. Integrated software for the analysis of brain PET/SPECT studies with partial-volume-effect correction. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2004;45:192–201. [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, Ross TJ, Amen SL, Sanfilipo M, Hoffmann RG, Bloom AS, Garavan H, Stein EA. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage. 2005;26:1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Schepis TS, Adinoff B, Rao U. Neurobiological processes in adolescent addictive disorders. Am J Addict. 2008;17:6–23. doi: 10.1080/10550490701756146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci. 2004;19:1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- Slopsema JS, van der Gugten J, de Bruin JP. Regional concentrations of noradrenaline and dopamine in the frontal cortex of the rat: dopaminergic innervation of the prefrontal subareas and lateralization of prefrontal dopamine. Brain Res. 1982;250:197–200. doi: 10.1016/0006-8993(82)90970-2. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC. Behavioral treatment of alcohol problems. Plenum Press; NewYork: 1978. [Google Scholar]
- Streeter CC, Terhune DB, Whitfield TH, Gruber S, Sarid-Segal O, Silveri MM, Tzilos G, Afshar M, Rouse ED, Tian H, Renshaw PF, Ciraulo DA, Yurgelun-Todd DA. Performance on the Stroop predicts treatment compliance in cocaine-dependent individuals. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2008;33:827–836. doi: 10.1038/sj.npp.1301465. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotactic atlas of the human brain. Thieme Medical Publishers, Inc; New York: 1988. [Google Scholar]
- Turner TH, LaRowe S, Horner MD, Herron J, Malcolm R. Measures of cognitive functioning as predictors of treatment outcome for cocaine dependence. J Subst Abuse Treat. 2009;37:328–334. doi: 10.1016/j.jsat.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Hitzemann R, Fowler JS, Overall JE, Burr G, Wolf AP. Recovery of brain glucose metabolism in detoxified alcoholics. Am J Psychiatry. 1994;151:178–183. doi: 10.1176/ajp.151.2.178. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Telang F, Fowler JS, Pradhan K, Jayne M, Logan J, Goldstein RZ, Alia-Klein N, Wong C. Methylphenidate attenuates limbic brain inhibition after cocaine-cues exposure in cocaine abusers. PLoS ONE. 2010;5:e11509. doi: 10.1371/journal.pone.0011509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]