Abstract
Dienyl diketones containing tethered acetates selectively undergo two different 1,6-conjugate addition-initiated cyclization cascades. One is a 1,6-conjugate addition/cyclization sequence with incorporation of the nucleophile, and the other is catalyzed by DABCO and is thought to proceed via a cyclic acetoxonium intermediate. The reaction behavior of substrates lacking the tethered acetate was also studied. The scope of both types of cyclization cascades, the role of the amine additive, and the factors controlling reactivity and selectivity in the two different reaction pathways is discussed.
Functionalized cyclopentenones containing quaternary stereogenic carbon centers are valuable building blocks for the preparation of interesting small molecules. Efficient, catalytic methods for the assembly of these targets via Nazarov cyclization have been studied extensively in recent years,1 and capitalize on the conservation of orbital symmetry2 as a means to control relative stereochemistry. Typically, either a Bronsted or a Lewis acid is used to promote the 4π cationic electrocyclization.
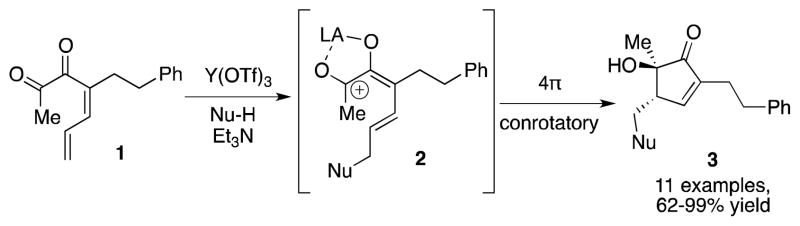
Recently, we reported that 1,6-conjugate addition of a nucleophile to dienyl diketone 1 could initiate Nazarov cyclization, via pentadienyl cation 2 (Scheme 1).3 Further study of nucleophile-initiated cyclization of this kind has revealed a new type of cascade cyclization, which occurs under neutral reaction conditions, yet produces a stereochemical result consistent with cationic electrocyclization. In this communication, we describe the two reaction pathways available to dienyl diketones upon treatment with nucleophiles, and present a mechanistic rationale for the unusual reactivity observed.
Scheme 1.
Conjugate Addition-Initiated Nazarov Cyclization
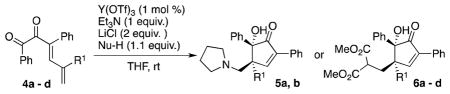
A series of diketone substrates of type 4 were prepared for examination in the conjugate addition-initiated reaction. Substrates in which R1 ≠ H were of particular interest, since conrotatory cyclization would install adjacent tertiary and all-carbon quaternary centers stereospecifically in these systems.
We found that conjugate addition-initiated Nazarov cyclization proceeded smoothly with pyrrolidine to give products of type 5 (Table 1, entries 1 and 3), and with dimethyl malonate to give products of type 6 (entries 2 and 4-7, and 9). Both triethylamine and DABCO (1,4-diazabicyclo[2.2.2]octane) could be used as the basic additive for the reaction of substrate 4b (entries 3 and 4). As would be expected in an electrocyclic reaction, only a single diastereoisomer was observed in each case. These reactions occur at ambient temperature, employ inexpensive reagents and catalysts, and are neither air-nor moisture-sensitive.
Table 1.
Lewis Acid-catalyzed Conjugate Addition/Electrocyclization Cascadea
| ||||
|---|---|---|---|---|
| entry | R1 = | nucleophile | base | yield (product; %) |
| 1 | 4a, H | pyrrolidine | Et3N | 5a; 87 |
| 2 | 4a, H | dimethylmalonate (DMM) | Et3N | 6a; 83 |
| 3 | 4b, Me | pyrrolidine | Et3N | 5b; 85 |
| 4 | 4b, Me | DMM | Et3N | 6b; 78 |
| 5 | 4b, Me | DMM | DABCOb | 6b; 72 |
| 6 | 4c, (CH2)2OAc | DMM | Et3N | 6c; 89 |
| 7 | 4c | DMM | DABCOb | see Table 2 |
| 8 | 4d, (CH2)3OAc | DMM | Et3N | 6d; 87 |
Reaction Conditions:
diketone 4 added to a stirring solution of Y(OTf)3 (1 mol %), LiCl (2 equiv.), base (1 equiv.), and the nucleophile in THF (1 M) at rt.
DABCO= 1,4-diazabicyclo[2.2.2]octane
In the case of dienyl diketone 4c, a dramatic change in reaction outcome was observed when DABCO was used instead of Et3N as the basic additive (Table 1, entry 8). None of the expected product 6c was obtained: instead, a new type of cyclization cascade took place resulting in cyclopentenone 7 (74 % yield, Table 2). Strangely, no incorporation of the malonate nucleophile had occurred. This result is especially surprising in light of the entry 5 result, in which use of DABCO as the basic additive uneventfully produced malonate adduct 7b (entry 4 vs. 5; Table 1).
Table 2.
Optimization of DABCO-Catalyzed Cyclization Cascade

| ||||
|---|---|---|---|---|
| entry | Lewis acid | base | catalyst loading (mol %) | yield (%) |
| 1 | Y(OTf)3 | DABCO | 100 | 92 |
| 2b | Y(OTf)3 | Et3N | 100 | 20 |
| 3c | Y(OTf)3 | DMAP | 100 | 83 |
| 4 | Y(OTf)3 | DBU | 100 | 64 |
| 5 | Y(OTf)3 | -- | -- | no reaction |
| 6 | -- | DABCO | 100 | 97 |
| 7 | -- | DABCO | 10 | 96 |
| 8 | -- | DABCO | 5 | 95 |
| 9c | -- | DABCO | 1 | 54 |
All reactions run at 1 M concentration in THF at rt for 8 h, except when otherwise noted.
Heating to 50 °C was required for the reaction to occur
Reaction took 24 h to reach completion.
When malonate was excluded from the reaction, the yield of product 7 improved to 92% (Table 2, entry 1). Further experimentation revealed that treatment of 4c with Y(OTf)3 and various amine bases also led to formation of 7 in good yield. While Et3N facilitated the reaction to some extent (20% yield; entry 2), long reaction times and elevated temperatures were required. Nucleophilic bases such as DMAP, DBU and DABCO (entries 3 and 4) were found to be efficient promoters. Still, DABCO was the optimal promoter, and we were interested to find that when the Lewis acid was excluded, an even higher yield of 7 was obtained (entry 6, 97% yield). Furthermore, DABCO facilitated the reaction at catalytic levels (entries 7-9), operating with catalyst loading as low as 1 mol % (54 % yield). However, 10 mol % was found to be the optimal loading, both in terms of yield (96%) and reaction time (1 hour). The reaction was equally efficient when run open to air.
Once optimized conditions were identified, substrate scope was evaluated (Table 3). Acetate 4d cyclized efficiently to produce cyclopentenone 8 in nearly quantitative yield (entry 2). Treatment of secondary acetates 4e and 4f gave 9 and 10, respectively, as single diastereoisomers (entries 3 and 4). Figure 1 shows the key nOe correlations used to assign the stereochemistry of compound 9. Acetate 4g underwent cyclization to give cyclopentenone 11 in 84% yield (entry 5). For this experiment, elevated temperature was required to facilitate the reaction.
Table 3.
| entry | dienyl diketone | product | yield (%) |
|---|---|---|---|
| 1 |
 4c |
 7 |
96% |
| 2c |
 4d |
 8 |
98% |
| 3d |
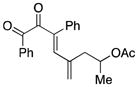 4e |
 9 |
92% |
| 4e |
 4f |
 10 |
91% |
| 5e |
 4g |
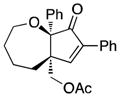 11 |
84% |
Reaction conditions: The diene was dissolved in THF (1 M) and DABCO (10 mol %) was added. The solution was stirred at rt until all starting material was consumed as judged by TLC.
Reaction time was 1 h unless otherwise stated.
Reaction time was 24 h.
Reaction time was 8 h.
Reaction was heated to 50 °C for 8 h.
Figure 1.
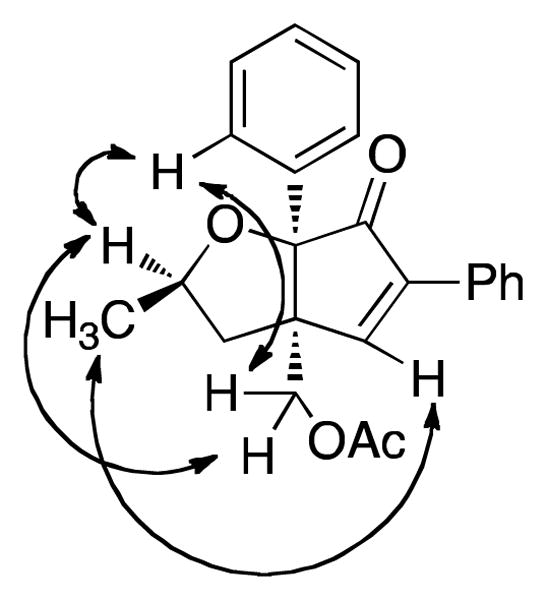
Key nOe correlations for compound 9
A mechanistic hypothesis consistent with the results shown in both Table 1 and Table 3 is presented in Scheme 2. In the absence of DABCO, 1,6-conjugate addition of either pyrrolidine or malonate leads to adducts 2 (Scheme 1), which undergo electrocyclization. In the presence of DABCO, 1,6-conjugate addition of DABCO4,5 to the dienyl diketone gives a zwitterionic DABCO adduct (see 12, Scheme 2). We hypothesize that this species does not undergo 4π electrocyclization, and if formed reversibly. Thus, when both malonate and DABCO are present, the malonate adduct 6b is formed (Table 1, entry 5), and DABCO acts as a simple base. However, if the substrate has an acetate tether (like 4c-4g, Table 3); another reaction pathway is available to DABCO adduct 12. In these cases, we propose that the allylic quaternary ammonium moiety of 12 is displaced by the pendant acetate to produce cyclic zwitterion 13, which contains a stereogenic center. The intramolecular SN2 reaction is related to the anchimeric assistance acetates sometimes demonstrate during solvolysis,6 and glycosylation, 7 but we could find only one example of a displacement reaction thought to proceed via a seven-membered acetoxonium intermediate.8 This stereocenter then controls the torquoselectivity of the subsequent electrocyclization, which produces spirocyclic zwitterions 14. The ring stereochemistry is consistent with conrotatory closure, and analogous to the results of cyclization of the type 2 intermediate to cyclopentenones 5 and 6. The cascade is concluded with intramolecular ether formation (Table 3). Remarkably, the proposal implies that seven, eight and nine-membered zwitterions 13 readily participate as intermediates in the cyclization cascades.
Scheme 2.
Proposed Mechanism for 1,6-Conjugate Addition/Cyclization Cascades
In conclusion, two different cyclization cascades can be carried out beginning with 1,6-conjugate addition to a dienyl diketone. One is Lewis acid-catalyzed, and involves incorporation of a nucleophile, producing highly substituted cyclopentenones containing a quaternary stereogenic center. The second is a novel, metal-free cascade catalyzed by 1,6-conjugate addition of DABCO, and involves three successive cyclizations: formation of a cyclic acetoxyonium, putative 4π electrocyclization, and opening of the acetoxonium to form a cyclic ether. The stereochemical outcome of both reactions is consistent with conrotatory electrocyclization, even though the DABCO-catalyzed cyclization cascade must proceed via a zwitterionic rather than a cationic intermediate.9 The study of other varieties of conjugate addition-initiated cascade cyclizations is currently underway in our laboratory.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (NIGMS R01 GM079364) and ARRA supplement (3R01GM079364-03S2) for funding this work. We are also grateful to Dr. Sandip Sur for assistance with the NOE experiments.
Footnotes
Supporting Information. Experimental procedure and characterization data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.For recent reviews on Nazarov cyclization, see: Pellissier H. Tetrahedron. 2005;61:6479–6517.Frontier AJ, Collison C. Tetrahedron. 2005;61:7577–7606.Tius MA. Eur J Org Chem. 2005;2005:2193–2206.Grant TN, Rieder CJ, West FG. Chem Commun. 2009:5676–5688. doi: 10.1039/b908515g.Nakanishi W, West FG. Curr Opin Drug Discov Dev. 2009;12:732–751.Shimada N, Stewart C, Tius MA. Tetrahedron. 2011;67:5851–5870. doi: 10.1016/j.tet.2011.05.062.Vaidya T, Eisenberg R, Frontier AJ. ChemCatChem. 2011;3:1531–1548.
- 2.Woodward RB, Hoffmann R. Angew Chem Int Ed. 1969;8:781–853. [Google Scholar]
- 3.Brooks JL, Caruana PA, Frontier AJ. J Am Chem Soc. 2011;133:12454–12457. doi: 10.1021/ja205440x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dienyl diketone substrates of type 4 are readily available via a convenient reaction sequence featuring reductive coupling of aryl glyoxals and enynes, using a protocol pioneered by Krische: Huddleston RR, Jang HY, Krische MJ. J Am Chem Soc. 2003;125:11488–11489. doi: 10.1021/ja030415v.Jang HY, Huddleston RR, Krische MJ. J Am Chem Soc. 2004;126:4664–4668. doi: 10.1021/ja0316566. See supporting information for details.
- 5.For use of DABCO in Morita-Baylis-Hilman reactions see: Basavaiah D, Rao AJ, Satyanarayana T. Chem Rev. 2003;103:811–892. doi: 10.1021/cr010043d.Declerck V, Martinez J, Lamaty F. Chem Rev. 2009;109:1–48. doi: 10.1021/cr068057c.Basavaiah D, Reddy BS, Badsara SS. Chem Rev. 2010;110:5447–5674. doi: 10.1021/cr900291g.
- 6.For other examples of reactions initiated by conjugate addition of DABCO see: Yeo JE, Yang X, Kim HJ, Koo S. Chem Commun. 2004;2:236–237. doi: 10.1039/b311951c.Franck X, Figadere B. Tetrahedron Lett. 2002;43:1449–1451.Lesch B, Brase S. Angew Chem Int Ed. 2004;43:115–118. doi: 10.1002/anie.200352154.
- 7.Capon B. Chem Soc Rev. 1964;18:45–111. [Google Scholar]
- 8.For a discussion of the role of remote acetates in glycosylation, see Crich D, Hu T, Cai F. J Org Chem. 2008;73:8942–8953. doi: 10.1021/jo801630m.and references therein.
- 9.Wilen SH, Delguzzo, Saferstein R. Tetrahedron. 1987;43:5089–5094. [Google Scholar]
- 10.For an example of a Nazarov cyclization that occurs under neutral conditions see: Douelle F, Tal L, Greaney MF. Chem Commun. 2005:660–662. doi: 10.1039/b415463k.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.