Abstract
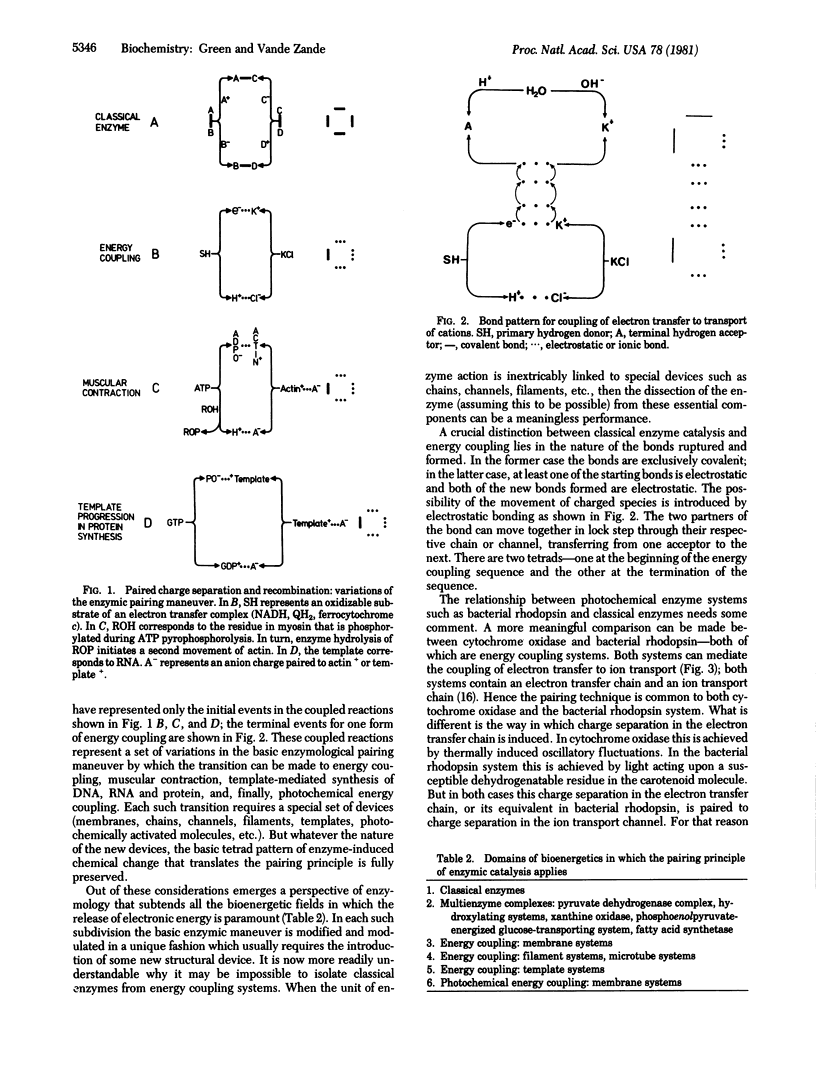
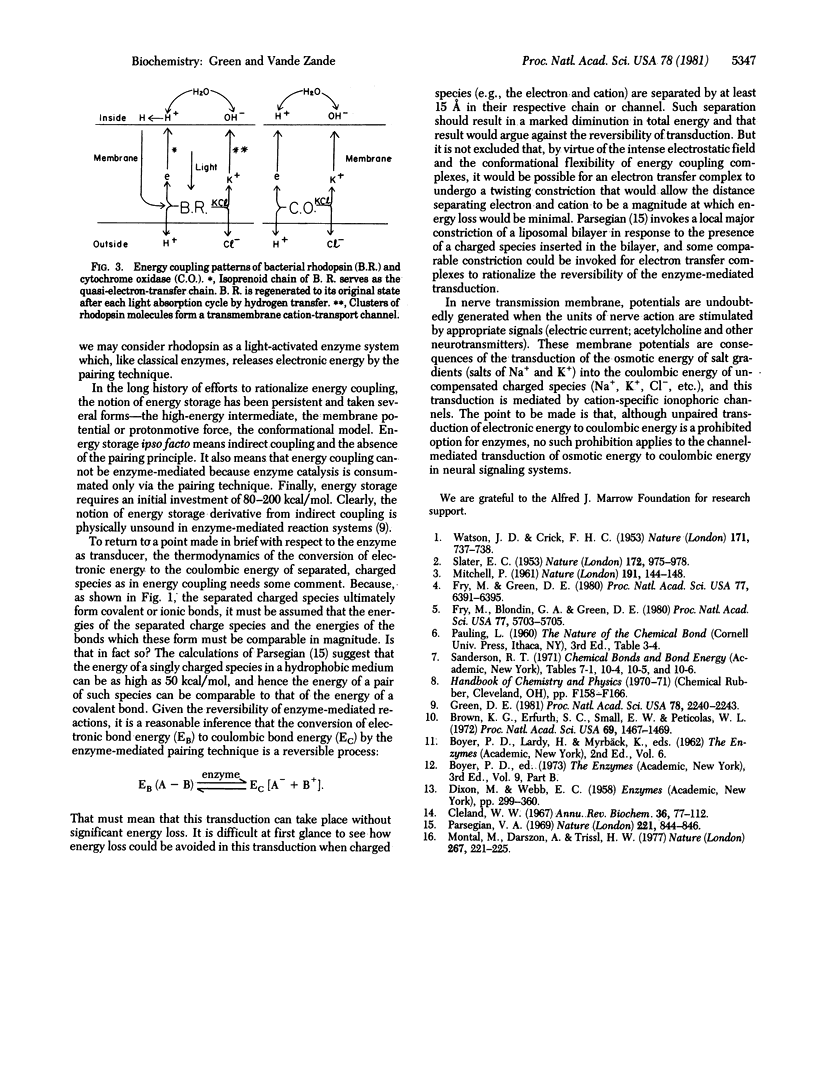
Electronic energy (chemical bond energy) is the exclusive source of utilizable energy in biological systems. The release of this energy is mediated enzymically. The energy required to rupture a single covalent or ionic bond is prohibitively high under physiological conditions [in the range of 80-200 kcal/mol (1 kcal = 4.18 kJ)]. By the technique of the pairing of bond rupture (two juxtaposed bonds ruptured simultaneously) and the pairing of bond formation, enzymes can bypass the huge thermodynamic barrier to chemical change inherent in rupture of a single bond and operate within thermal limits. Enzymes accordingly can be conceived of as the energy machines that translate this principle. The principle of this transduction is that the energy required for forming a new covalent bond can fall within thermal limits when the original charged atom partner to the bond is displaced by a substitute charged atom under conditions in which the charge field of the bond remains constant during the substitution. In the transition from classical enzymology to energy coupling, muscular contraction, template-dependent replication, etc., new dimensions and possibilities are added to the basic enzymatic machinery. Specialized molecular devices (membranes, filaments, channels, templates, etc.) have to be introduced to make possible these extensions and permutations of enzymology. But it is demonstrable that the basis pairing principle is fully preserved during any of these modifications or extensions. Long range movement--of an ion, a filament, or a template--is the most important property introduced into classical enzymology in the transition to energy coupling systems.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown K. G., Erfurth S. C., Small E. W., Peticolas W. L. Conformationally dependent low-frequency motions of proteins by laser Raman spectroscopy. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1467–1469. doi: 10.1073/pnas.69.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry M., Blondin G. A., Green D. E. Relation between enzymic catalysis and energy coupling. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5703–5705. doi: 10.1073/pnas.77.10.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry M., Green D. E. Ion-transport chain of cytochrome oxidase: the two chain-direct coupling principle of energy coupling. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6391–6395. doi: 10.1073/pnas.77.11.6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. E. A critique of the chemosmotic model of energy coupling. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2240–2243. doi: 10.1073/pnas.78.4.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Montal M., Darszon A., Trissl H. W. Transmembrane channel formation in rhodopsin-containing bilayer membranes. Nature. 1977 May 19;267(5608):221–225. doi: 10.1038/267221a0. [DOI] [PubMed] [Google Scholar]
- Parsegian A. Energy of an ion crossing a low dielectric membrane: solutions to four relevant electrostatic problems. Nature. 1969 Mar 1;221(5183):844–846. doi: 10.1038/221844a0. [DOI] [PubMed] [Google Scholar]
- SLATER E. C. Mechanism of phosphorylation in the respiratory chain. Nature. 1953 Nov 28;172(4387):975–978. doi: 10.1038/172975a0. [DOI] [PubMed] [Google Scholar]
- WATSON J. D., CRICK F. H. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature. 1953 Apr 25;171(4356):737–738. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]



