Abstract
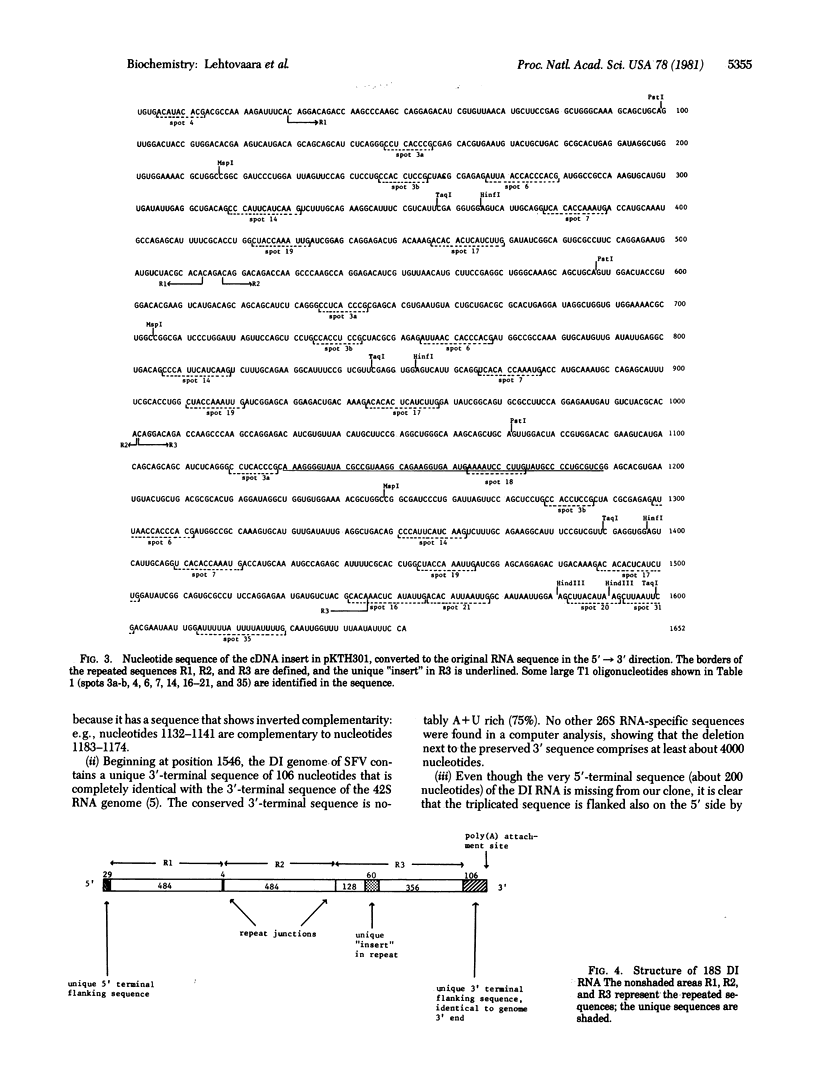
The nucleotide sequence of a nearly full-length cloned cDNA copy of an 18S defective interfering (DI) RNA of Semliki Forest virus has been determined. This corresponded to a major virus-specific cytoplasmic RNA species at the 11th undiluted passage of the virus in BHK cells. The 1652-nucleotide-long sequence consists of a unique 5'-terminal sequence followed by three tandem 484-nucleotide repeat units derived from the 5' two-thirds of the viral genome and a unique sequence of 106 nucleotides preceding the poly(A) of the 3' terminus. One of the tandem 484-nucleotide repeat units contains an extra segment of 60 nucleotides. Hybridization experiments showed that the cloned cDNA was colinear with an 18S DI RNA and that it contained an approximately 320-nucleotide-long segment colinear with the viral genomic RNA. Analysis of 18S DI RNA oligonucleotide fingerprints revealed that the molecule studied and the heterogeneous DI RNA population contain similar repeated sequences. The mechanism by which the DI RNAs are generated is not known, but it seems likely that multiple internal deletions and duplications are involved.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruton C. J., Porter A., Kennedy S. I. Defective-interfering particles of Semliki Forest virus: intracellular events during interference. J Gen Virol. 1976 Jun;31(3):397–416. doi: 10.1099/0022-1317-31-3-397. [DOI] [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohner D., Monroe S., Weiss B., Schlesinger S. Oligonucleotide mapping studies of standard and defective Sindbis virus RNA. J Virol. 1979 Feb;29(2):794–798. doi: 10.1128/jvi.29.2.794-798.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan J. B., Pastan I., de Crombrugghe B. Sequence rearrangement and duplication of double stranded fibronectin cDNA probably occurring during cDNA synthesis by AMV reverse transcriptase and Escherichia coli DNA polymerase I. Nucleic Acids Res. 1980 Jul 11;8(13):3055–3064. doi: 10.1093/nar/8.13.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed G. C., Davoli D. Molecular biology of papovaviruses. Annu Rev Biochem. 1977;46:471–522. doi: 10.1146/annurev.bi.46.070177.002351. [DOI] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. Nucleotide sequence of cdna coding for Semliki Forest virus membrane glycoproteins. Nature. 1980 Nov 20;288(5788):236–241. doi: 10.1038/288236a0. [DOI] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. The capsid protein of Semliki Forest virus has clusters of basic amino acids and prolines in its amino-terminal region. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6376–6380. doi: 10.1073/pnas.77.11.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Wallis J. Colony hybridization. Methods Enzymol. 1979;68:379–389. doi: 10.1016/0076-6879(79)68027-8. [DOI] [PubMed] [Google Scholar]
- Guild G. M., Stollar V. Defective interfering particles of Sindbis virus. V. Sequence relationships between SVSTD 42 S RNA and intracellular defective viral RNAs. Virology. 1977 Mar;77(1):175–188. doi: 10.1016/0042-6822(77)90416-0. [DOI] [PubMed] [Google Scholar]
- Jeppesen P. G. Separation and isolation of DNA fragments using linear polyacrylamide gradient gel electrophoresis. Methods Enzymol. 1980;65(1):305–319. doi: 10.1016/s0076-6879(80)65041-1. [DOI] [PubMed] [Google Scholar]
- Kaback D. B., Angerer L. M., Davidson N. Improved methods for the formation and stabilization of R-loops. Nucleic Acids Res. 1979 Jun 11;6(7):2499–2317. doi: 10.1093/nar/6.7.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. I., Bruton C. J., Weiss B., Schlesinger S. Defective interfering passages of Sindbis virus: nature of the defective virion RNA. J Virol. 1976 Sep;19(3):1034–1043. doi: 10.1128/jvi.19.3.1034-1043.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. I. Sequence relationships between the genome and the intracellular RNA species of standard and defective-interfering Semliki Forest virus. J Mol Biol. 1976 Dec;108(2):491–511. doi: 10.1016/s0022-2836(76)80132-5. [DOI] [PubMed] [Google Scholar]
- Lee T. N., Nathans D. Evolutionary variants of simian virus 40: replication and encapsidation of variant DNA. Virology. 1979 Jan 30;92(2):291–298. doi: 10.1016/0042-6822(79)90134-x. [DOI] [PubMed] [Google Scholar]
- Lehtovaara P., Ulmanen I., Käriäinen L., Keränen S., Philipson L. Synthesis and processing of Semliki Forest virus-specific nonstructural proteins in vivo and in vitro. Eur J Biochem. 1980 Dec;112(3):461–468. doi: 10.1111/j.1432-1033.1980.tb06108.x. [DOI] [PubMed] [Google Scholar]
- Lundquist R. E., Sullivan M., Maizel J. V., Jr Characterization of a new isolate of poliovirus defective interfering particles. Cell. 1979 Nov;18(3):759–769. doi: 10.1016/0092-8674(79)90129-6. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nomoto A., Jacobson A., Lee Y. F., Dunn J., Wimmer E. Defective interfering particles of poliovirus: mapping of the deletion and evidence that the deletions in the genomes of DI(1), (2) and (3) are located in the same region. J Mol Biol. 1979 Feb 25;128(2):179–196. doi: 10.1016/0022-2836(79)90125-6. [DOI] [PubMed] [Google Scholar]
- Ou J. H., Strauss E. G., Strauss J. H. Comparative studies of the 3'-terminal sequences of several alpha virus RNAs. Virology. 1981 Mar;109(2):281–289. doi: 10.1016/0042-6822(81)90499-2. [DOI] [PubMed] [Google Scholar]
- Pettersson R. F. 5'-Terminal nucleotide sequence of Semliki forest virus 18S defective interfering RNA is heterogeneous and different from the genomic 42S RNA. Proc Natl Acad Sci U S A. 1981 Jan;78(1):115–119. doi: 10.1073/pnas.78.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson R. F., Hewlett M. J., Baltimore D., Coffin J. M. The genome of Uukuniemi virus consists of three unique RNA segments. Cell. 1977 May;11(1):51–63. doi: 10.1016/0092-8674(77)90316-6. [DOI] [PubMed] [Google Scholar]
- Pettersson R. F., Söderlund H., Käriäinen L. The nucleotide sequences of the 5'-terminal T1 oligonucleotides of Semliki-Forest-virus 42-S and 26-S RNAs are different. Eur J Biochem. 1980 Apr;105(3):435–443. doi: 10.1111/j.1432-1033.1980.tb04518.x. [DOI] [PubMed] [Google Scholar]
- Ravetch J. V., Horiuchi K., Zinder N. D. DNA sequence analysis of the defective interfering particles of bacteriophage f1. J Mol Biol. 1979 Mar 5;128(3):305–318. doi: 10.1016/0022-2836(79)90090-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Stark C., Kennedy S. I. The generation and propagation of defective-interfering particles of Semliki Forest virus in different cell types. Virology. 1978 Aug;89(1):285–299. doi: 10.1016/0042-6822(78)90060-0. [DOI] [PubMed] [Google Scholar]
- Stollar V. Defective interfering particles of togaviruses. Curr Top Microbiol Immunol. 1979;86:35–66. doi: 10.1007/978-3-642-67341-2_2. [DOI] [PubMed] [Google Scholar]
- Wengler G., Wengler G., Gross H. S. Replicative form of Semliki Forest virus RNA contains an unpaired guanosine. Nature. 1979 Dec 13;282(5740):754–756. doi: 10.1038/282754a0. [DOI] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]






