Abstract
Hepcidin levels are high and iron absorption is limited in acute malaria. The mechanism(s) that regulate hepcidin secretion remain undefined. We have measured hepcidin concentration and cytokines in 100 Kenyan children with acute falciparum malaria and different degrees of anemia. Hepcidin was increased on admission and fell significantly one week and one month after treatment. The association of hepcidin with hemoglobin was not linear and hepcidin was very low in severe malarial anemia. Parasite density, IL-10 and IL-6 were significantly associated with hepcidin concentration. Hepcidin response to acute malaria supports the notion of iron sequestration during acute malaria infection and suggests that iron administration during acute malaria is futile. These data suggest iron supplementation policies should take into account the high hepcidin levels and probable poor utilization of iron for up to one week after treatment for the majority of patients with acute malaria.
Key words: hepcidin, Plasmodium falciparum malaria, anemia, iron supplementation
Introduction
In sub-Saharan Africa, malaria, hookworm, hemoglobinopathies and nutritional deficiencies may contribute to anemia.1,2 Frequently the etiology of anemia is uncertain and many cases are treated as iron deficiency anemia.
Mass administration of iron supplementation in a malariaendemic area is problematic. In a landmark study in Zanzibar, routine supplementation of iron and folic acid in pre-school children was associated with severe illness and death.3 It is, therefore, crucial to understand the appropriate timing of iron supplementation after acute illness.
Both iron absorption and release of iron from macrophages are tightly regulated by hepcidin.4 Hepcidin inhibits iron absorption and its release from macrophages by down-regulating the concentration of ferroportin.5 Hepcidin is up-regulated by inflammation6,7 and down-regulated by iron deficiency or hypoxia.8
Hepcidin levels are increased during malaria, at least in part due to stimulation of peripheral blood mononuclear cells by malaria-infected erythrocytes.9 Hepcidin inhibits P. falciparum liver-stage development and may decrease iron absorption during malaria infection.10-12 However, the mechanism, magnitude and duration of this elevated hepcidin response have not been defined in children with different degrees of malarial anemia.
To understand the role, duration and regulation of elevated hepcidin in blood stage infection, we studied hepcidin and potential host and parasite factors that may mediate hepcidin secretion in Kenyan children with acute malarial anemia.
Design and Methods
Patients and recruitment
The clinical study, approved by the National Ethical Committee of Kenya, was undertaken at Kilifi District Hospital between October 2001 and September 2002.13 Briefly, consecutive children aged six months to ten years attending outpatient clinics were screened for fever (>38.5°C), anemia, and P. falciparum parasitemia. Children with fever and any parasitemia were allocated to four different groups according to Hb concentration: severe (Hb < 5 g/dL), moderate (Hb 5- 6.9 g/dL), mild anemia (Hb 7-9.9 g/dL) and Hb higher than 10 g/dL. Children treated with antimalarials during the week before admission were excluded from the study.
Laboratory measurements
Peripheral blood films were stained with New Methylene Blue to count reticulocytes. Plasma concentrations of TNF-α, IL-10, IFN-γ, IL-12p70, erythropoietin (Epo) and soluble transferrin receptor (sTFR) were measured by ELISA Quantikine® kits (R&D Systems, UK). Hepcidin-25 levels were measured using Hepcidin- 25 (human) – Enzyme Immunoassay kit (Bachem, UK). Plasma samples were collected, aliquoted and stored according to good clinical and laboratory practice (GLCP) guidelines. The plasma aliquots used had been thawed twice. All plasma measurements were performed in stored samples. Cytokine concentrations were measured in 2004 and hepcidin was measured in 2010.
Statistical analyses
Independent t-tests or Mann-Whitney tests were used to compare means between independent variables/groups with a significance level α of 5%. Multiple groups were compared by means or medians analysis of variance (ANOVA) or the non-parametric equivalent (Kruskal-Wallis test). Pearson's or Spearman's (nonparametric) correlations were calculated to measure the association between variables. The relative contribution of the independent variables in the regression analysis was measured by the standardized regression coefficient (beta). Hepcidin concentration was log-transformed for the univariate and multiple linear regression analysis. Data were analyzed using Stata software version 11.0 (Stata Corporation, Texas, USA).
Results and Discussion
Hepcidin profiles during acute infection and convalescence
We measured plasma hepcidin concentration in 100 children with acute malaria on admission, one week and one month after treatment. Hepcidin levels were increased above baseline even in non-anemic children and highest in children with Hb concentration of 7-9.9 g/dL (Figure 1). Unexpectedly, children who were severely anemic (n=29) had the lowest median concentration of hepcidin (1.12 ng/mL, IQR 0.39-11.4) and very low median reticulocyte counts (47.9×109 ml−1, IQR 24.8-58.7) for the degree of anemia. Hepcidin concentration in children with severe anemia was significantly lower than in children with Hb over 5 g/dL (P<0.05).
Figure 1.
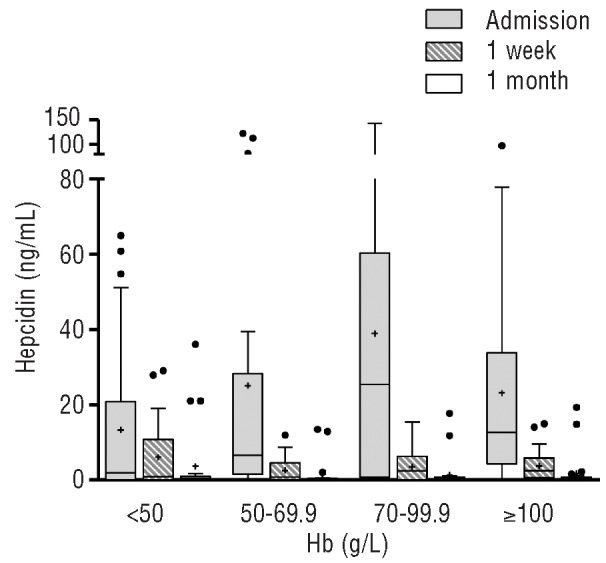
Concentration of plasma hepcidin in Kenyan children with acute P. falciparum malaria on admission and follow-up visits after treatment. Hb: hemoglobin concentration. Box plot indicates median and 25th and 75th percentiles. + indicates mean, · indicate distribution outliers.
Hepcidin levels fell significantly one week and one month after treatment compared with levels on admission (P<0.001). The median (IQR) hepcidin concentration in children with acute malaria was 8.57 (1.00-39.05) ng/mL, and declined swiftly to 1.69 (0-6.31) ng/mL one week after treatment and 0.43 (0.37-0.75) ng/mL a month later (Figure 1). None of the children died and the mean (SD) Hb concentration at one month was 10.06 (1.51) g/dL. A significant minority of children had raised hepcidin levels even one month after infection (Figure 1).
It appears that malaria infection stimulates hepcidin11,14-16 and this is associated with a lower reticulocyte response due, at least in part, to iron restriction to developing erythroid precursors. However, here we show for the first time that hepcidin secretion appears to be blunted as severe anemia develops. Therefore, in severe anemia, availability of iron should no longer restrict erythropoiesis, although the erythropoietic response in children with severe malarial anemia is greatly impaired,17,18 and hypoxemia might have contributed to a further decrease in hepcidin concentration in this group.8 The ineffective erythropoietic response, cell-cycle arrest and apoptosis observed in malarial anemia have been associated with raised proinflammatory cytokines, hemozoin and lipoperoxides. 13,19,20 Thus, the severely dyserythropoietic and hyperplastic erythroid marrow in malaria results not only from increased erythropoietic drive and the inhibitory effect of toxic and pro-inflammatory factors, but also from an inadequate iron supply.
Hepcidin and parasitological and immunological variables
We investigated whether the pro-inflammatory cytokines and the anti-inflammatory cytokine (IL-10) were associated with hepcidin concentration on admission (Table 1). TNF, IL-10, IL-6 and parasite density were significantly associated with hepcidin on admission after adjusting for baseline Hb concentration (Table 2). Parasite density was strongly correlated with TNF (r=0.34, P<0.001), IL-6 (r=0.54, P<0.001) and IL-10 (r= 0.56, P<0.001). The regression analysis adjusted for parasite density showed that IL-10 (β: 0.43, P<0.001) and IL-6 (β: 0.34, P=0.004) were significantly associated with hepcidin concentration on admission. Plasma hepcidin was not associated with γ-IFN and IL-12.
Table 1.
Variables associated with plasma hepcidin in children with acute malaria. Correlation analysis of variables associated with hepcidin concentration on admission. r indicates Pearson's correlation coefficient; Beta indicates standardized regression coefficient.

Table 2.
Variables associated with plasma hepcidin in children with acute malaria. Univariate analysis unadjusted and adjusted for hemoglobin concentration and parasite density using plasma hepcidin as dependent variable, and cytokines and parasite density as independent variables. r indicates Pearson's correlation coefficient; Beta indicates standardized regression coefficient.
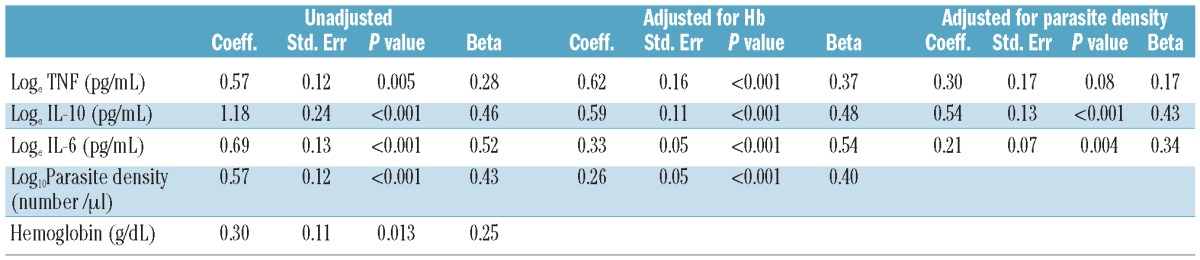
There is substantial evidence to show that IL-6 stimulates hepcidin secretion from hepatocytes.7 Here, IL-10 levels were very closely associated with parasitemia and hepcidin concentration. The effect of IL-10 on iron metabolism is poorly understood. Indeed, a large randomized placebo-controlled study of IL-10 as an adjuvant treatment in inflammatory bowel disease showed a dose-dependent decrease of hemoglobin in patients receiving IL-10 with doses from 1 to 20 μg/kg body weight.21 Our data now suggest a plausible explanation for this observation although the mechanism(s) of increased hepcidin levels by IL-10 remain to be explored.
Iron supplementation and malaria
Our findings support the hypothesis that in malarial anemia iron is not depleted but sequestered. Iron stores cannot be assessed from analysis of peripheral blood in children presenting with malaria and anemia. Previous studies in Kenyan children indicated that bone marrow iron depletion was rare with severe anemia.22 After infection, a low hepcidin level may help with mobilizing iron and our data show that increased reticulocyte counts after admission following treatment of the acute malaria episode correlates with low hepcidin concentration.
There has been considerable controversy regarding the role of iron supplementation in the management of children with malaria. Numerous guidelines suggest all children with malaria should be given oral iron.23 Perhaps these prescriptions are prompted by the high prevalence of iron deficiency in many communities, the diagnostic uncertainty surrounding the exact etiology of anemia in many tropical settings, and the difficultly of clinical review after discharge from hospital. However, indiscriminate iron supplementation in malaria endemic areas is not always efficacious but potentially harmful.3,24 Furthermore, iron is not absorbed or incorporated into hemoglobin during acute infection.12 Finally, our results show raised hepcidin levels directly related to malaria infection for up to a week after treatment has commenced and longer in some cases. In the light of this evidence, iron supplementation should be given to only those children with evident iron deficiency after parasitemia has subsided. Furthermore, the response to iron should be monitored to avoid giving iron when it is not being absorbed and/or utilized.
Acknowledgments
we thank the study participants and the clinical and technical staff for their help. We thank Dr. Alexander H. Drakesmith, Dr. Kathryn Maitland and Dr. Thomas N. Williams for comments, encouragement and advice.
Funding: CC-P is supported by the Medical Research Council (Clinician Scientist Fellowship: G0701885). DJR is supported by the National Health Service Blood and Transplant (NHSBT), National Institutes of Health Research (NIHR) Biomedical Research Centres funding scheme and NIHR Programme Grant NIHR-RP-PG-0310-1004-AN. The research was carried out at the Wellcome Trust Centre for Human Genetics (Oxford, UK), the NSHBT, the Oxford Centre and the Kenyan Institute of Medical Research (Kilifi).
Footnotes
Authorship and Disclosures: The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Lamikanra AA, Brown D, Potocnik A, Casals-Pascual C, Langhorne J, Roberts DJ. Malarial anaemia: of mice and men. Blood. 2007;110(1):18-28 [DOI] [PubMed] [Google Scholar]
- 2.Nweneka CV, Doherty CP, Cox S, Prentice A. Iron delocalisation in the pathogenesis of malarial anaemia. Trans R Soc Trop Med Hyg. 2009;104(3):175-84 [DOI] [PubMed] [Google Scholar]
- 3.Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community- based, randomised, placebo-controlled trial. Lancet. 2006;367(9505):133-43 [DOI] [PubMed] [Google Scholar]
- 4.Finch C. Regulators of iron balance in humans. Blood. 1994;84(6):1697-702 [PubMed] [Google Scholar]
- 5.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090-3 [DOI] [PubMed] [Google Scholar]
- 6.Armitage AE, Eddowes LA, Gileadi U, Cole S, Spottiswoode N, Selvakumar TA, et al. Hepcidin regulation by innate immune and infectious stimuli. Blood. 2011;118(15):4129-39 [DOI] [PubMed] [Google Scholar]
- 7.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113(9):1271-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piperno A, Galimberti S, Mariani R, Pelucchi S, Ravasi G, Lombardi C, et al. Modulation of hepcidin production during hypoxia-induced erythropoiesis in humans in vivo: data from the HIGHCARE project. Blood. 2010;117(10):2953-9 [DOI] [PubMed] [Google Scholar]
- 9.Portugal S, Carret C, Recker M, Armitage AE, Goncalves LA, Epiphanio S, et al. Hostmediated regulation of superinfection in malaria. Nat Med. 2011;17(6):732-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armitage AE, Pinches R, Eddowes LA, Newbold CI, Drakesmith H. Plasmodium falciparum infected erythrocytes induce hepcidin (HAMP) mRNA synthesis by peripheral blood mononuclear cells. Br J Haematol. 2009;147(5):769-71 [DOI] [PubMed] [Google Scholar]
- 11.Cercamondi CI, Egli IM, Ahouandjinou E, Dossa R, Zeder C, Salami L, et al. Afebrile Plasmodium falciparum parasitaemia decreases absorption of fortification iron but does not affect systemic iron utilization: a double stable-isotope study in young Beninese women. Am J Clin Nutr. 2010;92(6):1385-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prentice AM, Doherty CP, Abrams SA, Cox SE, Atkinson SH, Verhoef H, et al. Hepcidin is the major predictor of erythrocyte iron incorporation in anemic African children. Blood. 2012;119(8):1922-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casals-Pascual C, Kai O, Cheung JO, Williams S, Lowe B, Nyanoti M, et al. Suppression of erythropoiesis in malarial anaemia is associated with hemozoin in vitro and in vivo. Blood. 2006;108(8):2569-77 [DOI] [PubMed] [Google Scholar]
- 14.de Mast Q, Nadjm B, Reyburn H, Kemna EH, Amos B, Laarakkers CM, et al. Assessment of urinary concentrations of hepcidin provides novel insight into disturbances in iron homeostasis during malarial infection. J Infect Dis. 2009;199(2):253-62 [DOI] [PubMed] [Google Scholar]
- 15.de Mast Q, van Dongen-Lases EC, Swinkels DW, Nieman AE, Roestenberg M, Druilhe P, et al. Mild increases in serum hepcidin and interleukin-6 concentrations impair iron incorporation in haemoglobin during an experimental human malaria infection Br J Haematol. 2009;145(5):657-64 [DOI] [PubMed] [Google Scholar]
- 16.Howard CT, McKakpo US, Quakyi IA, Bosompem KM, Addison EA, Sun K, et al. Relationship of hepcidin with parasitaemia and anaemia among patients with uncomplicated Plasmodium falciparum malaria in Ghana. Am J Trop Med Hyg. 2007;77(4):623-6 [PubMed] [Google Scholar]
- 17.Abdalla SH. Hematopoiesis in human malaria. Blood Cells. 1990;16(2-3):401-16; discussion 17-9. [PubMed] [Google Scholar]
- 18.Abdalla S, Weatherall DJ, Wickramasinghe SN, Hughes M. The anaemia of P. falciparum malaria. Br J Haematol. 1980;46(2):171-83 [DOI] [PubMed] [Google Scholar]
- 19.Skorokhod OA, Caione L, Marrocco T, Migliardi G, Barrera V, Arese P, et al. Inhibition of erythropoiesis in malaria anaemia: role of hemozoin and hemozoingenerated 4-hydroxynonenal. Blood. 2010;116(20):4328-37 [DOI] [PubMed] [Google Scholar]
- 20.Lamikanra AA, Theron M, Kooij TW, Roberts DJ. Hemozoin (malarial pigment) directly promotes apoptosis of erythroid precursors. PLoS One. 2009;4(12):e8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tilg H, Ulmer H, Kaser A, Weiss G. Role of IL-10 for induction of anaemia during inflammation. J Immunol. 2002;169(4):2204-9 [DOI] [PubMed] [Google Scholar]
- 22.Newton CR, Warn PA, Winstanley PA, Peshu N, Snow RW, Pasvol G, et al. Severe anaemia in children living in a malaria endemic area of Kenya. Trop Med Int Health. 1997;2(2):165-78 [DOI] [PubMed] [Google Scholar]
- 23.WHO Guidelines for the Management of Common Illnesses with Limited Resources, 2005
- 24.Calis JC, Phiri KS, Faragher EB, Brabin BJ, Bates I, Cuevas LE, et al. Severe anaemia in Malawian children. N Engl J Med. 2008;358(9):888-99 [DOI] [PubMed] [Google Scholar]


