Abstract
Schnitzler syndrome is a rare plasma cell disorder the pathogenesis of which is still not fully understood. We evaluated the circulating levels of four major angiogenic cytokines (VEGF, angiogenin, angiopoietin-1 and angiopoietin-2) and six bone remodeling markers (sRANKL, osteoprotegerin, dickkopf-1, CTX, osteocalcin and bone-specific alkaline phosphatase-bALP) in 13 patients with Schnitzler syndrome. At diagnosis, patients had elevated angiogenic cytokines. The mean VEGF levels were almost 3.5-fold higher in Schnitzler syndrome compared to controls, while 10 of 13 patients had higher VEGF than the upper control value. Successful treatment led to a significant reduction in VEGF. Patients with Schnitzler syndrome had increased bone formation (high bALP, osteocalcin and osteoprotegerin) which was not balanced by an increase in bone resorption (normal CTX and sRANKL). These data support a role for VEGF as a new minor criterion in the diagnosis and follow up of Schnitzler syndrome, while the uncoupling of bone remodeling in favor of bone formation justifies the presence of bone densification.
Key words: Schnitzler syndrome, VEGF, angiogenesis, bone formation
Introduction
Schnitzler syndrome is a rare plasma cell disorder characterized by the presence of a monoclonal IgM immunoglobulin in association with a chronic urticarial skin rash and at least 2 of the following minor criteria: intermittent fever, arthralgia or arthritis, bone pain, enlarged lymph nodes, splenomegaly and/or hepatomegaly, increased neutrophil counts, increased erythrocyte sedimentation rate (ESR), and abnormal bone findings with imaging evidence of osteosclerosis.1,2
The pathogenesis of the syndrome is unclear, with only one described case of spontaneous remission.3 The presence of high levels of interleukins (ILs) in patients with Schnitzler syndrome4,5 suggests that this plasma-cell disorder may be an acquired auto-inflammatory disease due to an unregulated secretion of cytokines via interaction of a clonal product (e.g. the M-protein) with a key component of the IL-1 pathway. This is further supported by the successful treatment of the syndrome with anakinra,6 a synthetic analog of the endogenous IL-1 receptor antagonist, or with immuno-suppressant agents.7,8 However, the increased levels of the above inflammatory cytokines are not sufficient to explain several disease features, such as bone densification.9 Bone remodeling has never been evaluated in a series of patients with Schnitzler syndrome and there are very limited data in the literature on the coupling of bone formation and bone resorption in this disease. Furthermore, although angiogenesis is implicated in the pathogenesis of other plasma cell disorders, including multiple myeloma (MM)10 and POEMS syndrome,11 no information is available on the role of angiogenesis in Schnitzler syndrome. Therefore, the aim of this study was to evaluate angiogenic cytokines and bone remodeling in a large series of patients with Schnitzler syndrome in order to obtain a better understanding of the biology of the disease.
Design and Methods
Patients
We studied 13 patients (12 males and one female, median age 55 years, range 39-79 years) with a well characterized Schnitzler syndrome. Patients were diagnosed, treated and followed at the Hôpital Saint-Louis, Paris, France, and in Alexandra Hospital, Athens, Greece, between 1989 and 2009.
Serum had been collected from all patients at the time of diagnosis and at the time of best response to treatment; this was stored at −80°C until use. Time of best response was defined as the time of the best control of symptoms (mainly urticarial rash, bone pain and fever). All patients had given their written informed consent to the sampling and storage of their serum for research purposes.
At the time of diagnosis, patients had had a complete skeletal survey using conventional X-ray to evaluate bone involvement; 11 of 13 patients also had a technetium bone scintigraphy.
Measurement of angiogenic cytokines and bone remodeling markers
The following circulating angiogenic cytokines were evaluated at diagnosis and at the time of best response to therapy: i) vascular endothelial growth factor (VEGF); ii) angiogenin; and iii) angiopoietin-1 and angiopoietin-2. The following serum indices were measured at diagnosis to evaluate bone remodeling: i) the osteoclast regulators, soluble receptor activator of nuclear factor kappa-B ligand (sRANKL) and osteoprotegerin (OPG); ii) the osteoblast inhibitor dickkopf-1 (Dkk-1); iii) the bone resorption marker C-telopeptide of collagen type-1 (CTX); and iv) the bone formation markers, bone-specific alkaline phosphatase (bALP) and osteocalcin. Angiogenic cytokines and bone markers were measured using commercially available ELISA kits, according to the manufacturers' instructions, as previously described.12-14
The above molecules were also evaluated in 24 gender- and agematched healthy subjects (22 males and 2 females, median age 55 years, range 30-80 years) who served as controls. Each healthy control was examined to ensure that there was no evidence of bone disease: the presence of osteoporosis was excluded by bone densitometry (DXA) while the presence of osteoarthritis was excluded by X-ray. Furthermore, none of the healthy individuals had taken medication that could alter their normal bone turnover over the previous six months, or had infections or autoimmune disorder at the time of sampling; all had normal liver and renal function, there were no cases of heart disease and none were taking medication for hypertension.
The research protocol was approved by the institutional review board of Alexandra Hospital.
Statistical analysis
Wilcoxon's signed rank test was used to test for differences within groups. Pearson's (r) coefficient of correlation was used to test correlation between variables.
Results and Discussion
Patients' clinical characteristics
All patients presented with urticaria and monoclonal IgM-kappa protein (median M-peak 0.79 g/dL, range 0.1-2.36 g/dL). Other patients' clinical characteristics were typical for patients with Schnitzler syndrome2 (Figure 1). Four (30.7%) patients had documented sclerotic bone lesions in plain X-rays. All 11 patients who were evaluated with a technetium bone scintigraphy had high uptake in at least one site.
Figure 1.
Clinical features of patients with Schnitzler syndrome (except chronic urticarial rash and IgM monoclonal protein).
At diagnosis, 11 of 13 patients received various symptomatic therapies, including low-dose corticosteroids, that were ineffective. Two patients at diagnosis and 6 after initial symptomatic therapy were treated with the quinolone antibiotic pefloxacin, an established therapy for Schnitzler syndrome.15 Five of these 8 patients had complete response to pefloxacin. Seven patients were treated with anakinra, including 3 who did not respond to pefloxacin. All of them had a complete and sustained control of the disease.
Circulating angiogenic cytokines in Schnitzler syndrome
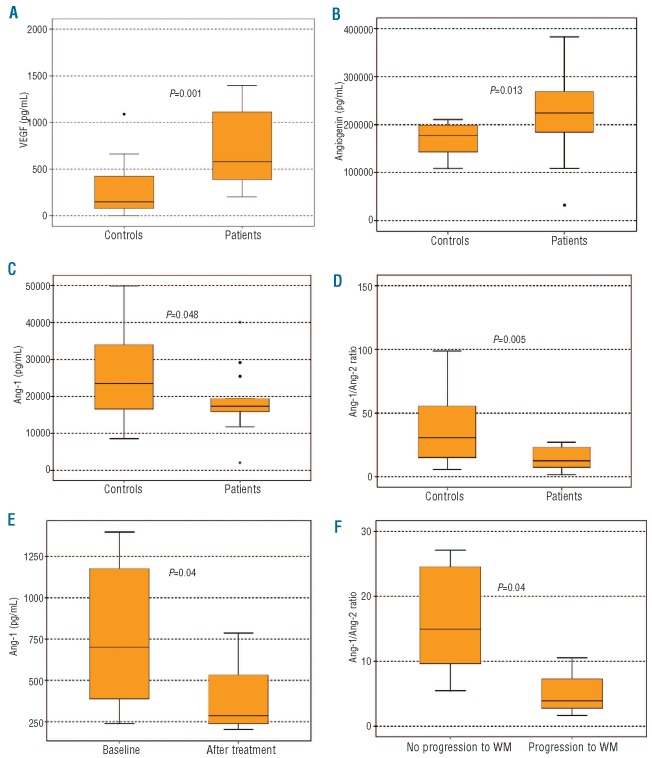
At diagnosis, patients with Schnitzler syndrome had elevated VEGF (809±598 pg/mL vs. 263±257 pg/mL; P=0.001) and angiogenin (221±96 ng/mL vs. 169±33; P=0.013) compared to healthy controls, while they had decreased angiopoietin-1 (18.7±9.1 ng/mL vs. 25.4±10.9 ng/mL; P=0.048) and angiopoietin-1 to angiopoietin-2 ratio (13.9±8.7 vs. 63.4±102.1; P=0.005) (Figure 2A-D). More specifically, 10 of 13 patients had higher VEGF values than the upper value of the controls, while 6 of 13 patients had higher values of angiogenin than the upper value of the controls. After successful treatment with pefloxacin or anakinra, VEGF levels decreased (387±207 pg/mL) compared to baseline values (P=0.04; Figure 2E). There were no significant modifications in the levels of the other angiogenic cytokines after successful therapy.
Figure 2.
At diagnosis patients with Schnitzler syndrome had increased circulating levels of VEGF (A) and angiogenin (B) compared to healthy controls and decreased angiopoietin-1 (C) and angiopoietin-1/angiopoietin-2 ratio (D) suggesting increased angiogenic activity. After successful treatment with pefloxacin or anakinra, VEGF levels decreased (387±207 pg/mL) compared to baseline values (P=0.04). (E) Three patients who progressed to overt WM had decreased levels of angiopoietin-1 to angiopoietin-2 ratio at diagnosis compared to all other patients (5.3±4.6 vs. 16.5±8.0; P=0.04, F).
With a median follow up of ten years, 3 (23%) patients with symptomatic disease requiring therapy developed Waldenström's macroglobulinemia (WM) at five, seven and 20 years post diagnosis. Interestingly, these patients had decreased levels of angiopoietin-1 (10.2±7.5 ng/mL) compared to all the other patients (21.2±8.2 ng/mL; P=0.04) at diagnosis and reduced angiopoietin-1 to angiopoietin-2 ratio (5.3±4.6 vs. 16.5±8.0; P=0.04; Figure 2F). Patients with other plasma cell dyscrasias, such as multiple myeloma (MM)11 and POEMS syndrome,12,16 also have increased circulating VEGF. Indeed, in POEMS syndrome, the increased level of circulating VEGF is considered to be a minor diagnostic criterion for the disease, while VEGF levels have been used for patient follow up.16 Furthermore, in other IgMmonoclonal gammopathies, such as in WM or in IgM monoclonal gammopathy of undetermined significance (IgMMGUS), the circulating VEGF levels have been found to be 2-2.7-fold higher than that of healthy controls.17 In our study, mean VEGF levels were almost 3.5-fold higher in patients with Schnitzler syndrome compared to healthy controls, while 10 of 13 patients had higher VEGF values than the upper VEGF value of the controls. In addition, VEGF was significantly reduced after successful treatment with anakinra or pefloxacin. Based on these data, we suggest that VEGF may be used as a valuable marker or even as a minor criterion for the diagnosis of Schnitzler syndrome. However, more patients with the syndrome should be studied before VEGF is established as a minor criterion of the disease.
The increased circulating VEGF in Schnitzler syndrome could be related to the IL-1 dysfunction which is characteristic of this disease.4 It has been reported that IL-1 activates inflammatory cells to produce endothelial cell activating factors, such as VEGF, and thus to promote angiogenesis.18 Furthermore, inhibition of IL-1 completely abrogated angiogenesis and reduced VEGF levels by 85% in an in vitro model.18 The reduction in VEGF levels in all our patients who were treated with anakinra (an IL-1 receptor antagonist) supports this hypothesis, although further confirmation is required.
In our study, patients with Schnitzler syndrome also had a reduced ratio of angiopoietin-1 to angiopoietin-2. Angiopoietin-1 and angiopoietin-2 are ligands for the Tie-2 receptor which is present on endothelial cells and endothelial progenitor cells. Angiopoietin-2 blocks the angiopoietin-1/Tie-2 signaling which serves to inhibit endothelial cell activation. Therefore, angiopoietin-2 facilitates endothelial activation in response to inducers of angiogenesis, such as VEGF. Reduced angiopoietin-1 to angiopoietin-2 ratio indicates high angiogenic activity and is also present in myeloma, where it correlates with advanced disease and poor prognosis.12 The reduced angiopoietin-1 to angiopoietin-2 ratio in our patients confirms that angiogenesis is elevated in Schnitzler syndrome and is implicated in its biology. Another interesting finding of our study is the correlation between low angiopoietin-1 to angiopoietin-2 ratio and progression of Schnitzler to WM. Although the number of our patients who progressed to WM was low (3 of 13; 23%), but similar to that reported in the literature (15%),2 and progression took place several years after diagnosis, this result may suggest that low angiopoietin-1 to angiopoietin-2 ratio at diagnosis may indicate a predisposition for evolution of the gammopathy towards an overt WM. However, this has to be confirmed prospectively in a larger number of patients.
Bone remodeling markers in Schnitzler syndrome
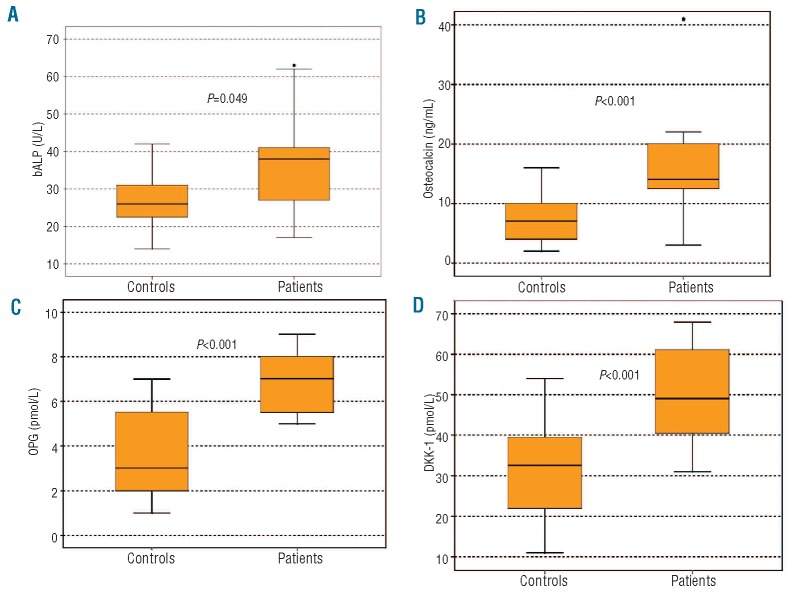
At diagnosis, patients with Schnitzler syndrome had increased serum levels of bALP (mean±SD: 36.5±15.0 IU/L vs. 26.8±7.1 IU/L; P=0.049), osteocalcin (20.5±18.6 ng/mL vs. 7.4±3.5 ng/mL; P <0.001), Dkk-1 (49.9±13.0 pmol/L vs. 30.9±11.1 pmol/L; P<0.001) and OPG (6.7±1.3 pmol/L vs. 3.5±1.8; P<0.001) compared to healthy controls (Figure 3). There were no differences between patients and controls in terms of sRANKL (0.28±0.22 pmol/L vs. 0.23±0.12 pmol/L; P=0.465) or CTX (0.32±0.25 ng/mL vs. 0.29±0.16 ng/mL; P=0.521).
Figure 3.
At diagnosis patients with Schnitzler syndrome had elevated serum bALP (A), osteocalcin (B), OPG (C) and Dkk-1 (D). The increase in bone formation markers (bALP, osteocalcin and OPG) in combination with normal bone resorption is responsible for the bone densification observed in Schnitzler syndrome.
The 4 patients with documented sclerotic lesions on conventional skeletal X-ray had significantly higher bALP (59.6±11.8 IU/L vs. 32.8±6.1 IU/L; P=0.04) and OPG (9.2±2.3 pmol/L vs. 5.7±0.8; P=0.03) compared to all others. In all patients, there was a strong positive correlation between OPG and VEGF (r=0.676 and P=0.016). No other significant correlations were observed between bone remodeling markers and angiogenic cytokines.
All patients had a high uptake on technetium bone scintigraphy while 30% of patients had sclerotic lesions with conventional skeletal X-ray, both suggestive of increased osteoblast function. This was further confirmed by the elevation of both markers of bone formation, bALP and osteocalcin that are produced directly by activated osteoblasts. On the other hand, there was no increase in bone resorption, as assessed by normal CTX and sRANKL levels, while there was a big increase in OPG (the decoy receptor of RANKL). These data suggest that there is an enhanced osteoblast function in Schnitzler syndrome which is not balanced by an increase in bone resorption, leading to bone densification and osteosclerotic lesions. Other IgM-gammopathies, i.e. WM and IgM-MGUS, have also altered bone remodeling19 but the increase in bone formation is not as evident as that observed in Schnitzler syndrome. The cause for this increased osteoblast function is obscure. IL-1 and IL-6, that participate in the pathogenesis of Schnitzler syndrome,2,4 are well known stimulators of osteoclast function20,21 while IL-1 directly inhibits osteoblast activity.22 During recent years, it has become evident that angiogenesis enhances osteogenesis and VEGF is able to increase bone formation through modulation of angiogenesis.23 In our cohort of 13 patients, we found a strong correlation between VEGF and osteoprotegerin, suggesting that elevated VEGF may be, at least partially, responsible for an increase in bone formation in Schnitzler syndrome.
In this study, we also found an increase in the osteoblast inhibitor Dkk-1. Dkk-1 is a Wnt signaling inhibitor which is up-regulated by osterix, an osteoblast-specific transcription factor required for bone formation.24 Therefore, the increase in Dkk-1 in Schnitzler syndrome may reflect a balancing effect on the increased activity of the osteoblasts.
Four patients who were treated with anakinra also had a bone scintigraphy at a median six months post-treatment. All patients showed a dramatic reduction in osteoblastic lesions but in none of them did the lesions completely disappear.
In conclusion, our analysis shows altered circulating angiogenic cytokines in Schnitzler syndrome reflecting increased angiogenic activity. Furthermore, we document enhanced bone formation with no alterations in bone resorption; this explains the presence of sclerotic bone lesions in this disease entity. Successful therapy with either anakinra or pefloxacin is associated with a reduction in the major angiogenic cytokine VEGF. These data support a potential role for VEGF in the diagnosis and follow up of patients with Schnitzler syndrome, and suggest that VEGF may be used as a minor criterion for the diagnosis of the disease.
Footnotes
The paper has been presented as a poster presentation at the ASH 2011 Annual Meeting [Blood 2011;118(21):abstract 1802].
Authorship and Disclosures: The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Lipsker D, Veran Y, Grunenberger F, Cribier B, Heid E, Grosshans E. The Schnitzler syndrome. Four new cases and review of the literature. Medicine (Baltimore). 2001;80(1):37-44 [DOI] [PubMed] [Google Scholar]
- 2.de Koning HD, Bodar EJ, van der Meer JW, Simon A, Schnitzler Syndrome Study Group Schnitzler syndrome: beyond the case reports: review and follow-up of 94 patients with an emphasis on prognosis and treatment. Semin Arthritis Rheum. 2007;37(3):137-48 [DOI] [PubMed] [Google Scholar]
- 3.Asli B, Brouet JC, Fermand JP. Spontaneous remission of Schnitzler syndrome. Ann Allergy Asthma Immunol. 2011;107(1):87-8 [DOI] [PubMed] [Google Scholar]
- 4.van Deuren M, Kroot JJ, Swinkels DW. Time-course analysis of serum hepcidin, iron and cytokines in a C282Y homozygous patient with Schnitzler's syndrome treated with IL-1 receptor antagonist. Haematologica. 2009;94(9):1297-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Migliorini P, Del Corso I, Tommasi C, Boraschi D. Free circulating interleukin-18 is increased in Schnitzler syndrome: a new autoinflammatory disease? Eur Cytokine Netw. 2009;20(3):108-11 [DOI] [PubMed] [Google Scholar]
- 6.Gran JT, Midtvedt Ø, Haug S, Aukrust P. Treatment of Schnitzler's syndrome with anakinra: report of three cases and review of the literature. Scand J Rheumatol. 2011;40(1):74-9 [DOI] [PubMed] [Google Scholar]
- 7.Ramadan KM, Eswedi HA, El-Agnaf MR. Schnitzler syndrome: a case report of successful treatment using the anti-CD20 monoclonal antibody rituximab. Br J Dermatol. 2007;156(5):1072-4 [DOI] [PubMed] [Google Scholar]
- 8.Bozeman S, Lewis S. Schnitzler syndrome successfully treated with methotrexate. Ann Allergy Asthma Immunol. 2008;101(2):219. [DOI] [PubMed] [Google Scholar]
- 9.Dinarello CA. Blocking IL-1 in systemic inflammation. J Exp Med. 2005;201(9):1355-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajkumar SV, Mesa RA, Fonseca R, Schroeder G, Plevak MF, Dispenzieri A, et al. Bone marrow angiogenesis in 400 patients with monoclonal gammopathy of undetermined significance, multiple myeloma, and primary amyloidosis. Clin Cancer Res. 2002;8(7):2210-6 [PubMed] [Google Scholar]
- 11.Kastritis E, Terpos E, Anagnostopoulos A, Xilouri I, Dimopoulos MA. Angiogenetic factors and biochemical markers of bone metabolism in POEMS syndrome treated with high-dose therapy and autologous stem cell support. Clin Lymphoma Myeloma. 2006;7(1):73-6 [DOI] [PubMed] [Google Scholar]
- 12.Terpos E, Anargyrou K, Katodritou E, Kastritis E, Papatheodorou A, Christoulas D, et al. Circulating angiopoietin-1 to angiopoietin-2 ratio is an independent prognostic factor for survival in newly diagnosed patients with multiple myeloma who received therapy with novel antimyeloma agents. Int J Cancer. 2012;130(3):735-42 [DOI] [PubMed] [Google Scholar]
- 13.Terpos E, Szydlo R, Apperley JF, Hatjiharissi E, Politou M, Meletis J, et al. Soluble receptor activator of nuclear factor kappaB ligandosteoprotegerin ratio predicts survival in multiple myeloma: proposal for a novel prognostic index. Blood. 2003;102(3):1064-9 [DOI] [PubMed] [Google Scholar]
- 14.Terpos E, Fragiadaki K, Konsta M, Bratengeier C, Papatheodorou A, Sfikakis PP. Early effects of IL-6 receptor inhibition on bone homeostasis: a pilot study in women with rheumatoid arthritis. Clin Exp Rheumatol. 2011;29(6):921-5 [PubMed] [Google Scholar]
- 15.Asli B, Bienvenu B, Cordoliani F, Brouet JC, Uzunhan Y, Arnulf B, et al. Chronic urticaria and monoclonal IgM gammopathy (Schnitzler syndrome): report of 11 cases treated with pefloxacin. Arch Dermatol. 2007;143(8):1046-50 [DOI] [PubMed] [Google Scholar]
- 16.D'Souza A, Hayman SR, Buadi F, Mauermann M, Lacy MQ, Gertz MA, et al. The utility of plasma vascular endothelial growth factor levels in the diagnosis and follow-up of patients with POEMS syndrome. Blood. 2011;118(17):4663-5 [DOI] [PubMed] [Google Scholar]
- 17.Anagnostopoulos A, Eleftherakis-Papaiakovou V, Kastritis E, Tsionos K, Bamias A, et al. Serum concentrations of angiogenic cytokines in Waldenstrom macroglobulinaemia: the ration of angiopoietin-1 to angiopoietin-2 and angiogenin correlate with disease severity. Br J Haematol. 2007;137(6):560-8 [DOI] [PubMed] [Google Scholar]
- 18.Carmi Y, Voronov E, Dotan S, Lahat N, Rahat MA, Fogel M, et al. The role of macrophage-derived IL-1 in induction and maintenance of angiogenesis. J Immunol. 2009;183(7):4705-14 [DOI] [PubMed] [Google Scholar]
- 19.Terpos E, Anagnostopoulos A, Kastritis E, Bamias A, Tsionos K, Dimopoulos MA. Abnormal bone remodelling and increased levels of macrophage inflammatory protein-1 alpha (MIP-1alpha) in Waldenström macroglobulinaemia. Br J Haematol. 2006;133(3):301-4 [DOI] [PubMed] [Google Scholar]
- 20.Lee YM, Fujikado N, Manaka H, Yasuda H, Iwakura Y. IL-1 plays an important role in the bone metabolism under physiological conditions. Int Immunol. 2010;22(10):805-16 [DOI] [PubMed] [Google Scholar]
- 21.Liu XH, Kirschenbaum A, Yao S, Levine AC. Cross-talk between the interleukin-6 and prostaglandin E(2) signaling systems results in enhancement of osteoclastogenesis through effects on the osteoprotegerin/receptor activator of nuclear factor-{kappa}B (RANK) ligand/RANK system. Endocrinology. 2005;146(4):1991-8 [DOI] [PubMed] [Google Scholar]
- 22.Stashenko P, Obernesser MS, Dewhirst FE. Effect of immune cytokines on bone. Immunol Invest. 1989;18(1-4):239-49 [DOI] [PubMed] [Google Scholar]
- 23.Samee M, Kasugai S, Kondo H, Ohya K, Shimokawa H, Kuroda S. Bone morphogenetic protein-2 (BMP-2) and vascular endothelial growth factor (VEGF) transfection to human periosteal cells enhances osteoblast differentiation and bone formation. J Pharmacol Sci. 2008;108(1):18-31 [DOI] [PubMed] [Google Scholar]
- 24.Zhang C, Dai H, de Crombrugghe B. Characterization of Dkk1 gene regulation by the osteoblast-specific transcription factor Osx. Biochem Biophys Res Commun. 2012;420(4):782-6 [DOI] [PMC free article] [PubMed] [Google Scholar]