Abstract
Background
Flavopiridol is a protein-bound, cytotoxic, cyclin dependent kinase inhibitor. A phase II trial of flavopiridol followed by ara-C and mitoxantrone with flavopiridol given by 1-h bolus for adults with newly-diagnosed, poor-risk acute myelogenous leukemia yielded 67% complete remission with median disease-free survival of 13.6 months.
Design and Methods
We compared bolus flavopiridol (50 mg/m2/day, Arm A) versus 'hybrid' flavopiridol (30 mg/m2 over 30 min followed by 40 mg/m2 over 4 h, Arm B) followed by ara-C and mitoxantrone in 78 patients (39 per arm) with newly diagnosed, poor-risk acute myelogenous leukemia. To mitigate imbalance, patients were stratified by presence or absence of secondary leukemia and therapy for antecedent disorder.
Results
Death at or before Day 60 occurred in 8% of patients per arm. Complete remission plus complete remission with incomplete recovery was 68% (Arm A, 62%; Arm B, 74%) overall, and 65% or over in both arms for patients with secondary leukemia and leukemia with adverse genetics. In Arm A 91% and in Arm B 86% of patients received chemotherapy and/or allogeneic transplantation in complete remission. Median overall survival for all remission patients has not been reached for either arm, with median disease free survival of 13.6 months for Arm A and of 12.0 months for Arm B.
Conclusions
Both flavopiridol schedules produce comparably encouraging results in adults with poor-risk acute myelogenous leukemia. Given the greater ease of bolus administration, we are conducting a randomized phase II study of bolus flavopiridol followed by ara-c and mitoxantrone versus conventional induction therapy for patients aged 70 years and under with intermediate or poor-risk acute myelogenous leukemia. This study is registered at www.clinicaltrials.gov as #NCT 00407966.
Key words: acute myelogenous leukemia, poor risk, flavopiridol, ara-C, mitoxantrone
Introduction
Adults with newly diagnosed acute myelogenous leukemia (AML) with poor-risk features have a poor prognosis in terms of achievement and duration of complete remission (CR). The poor-risk features include secondary AML [i.e. treatment-related or arising from myelodysplastic syndromes (MDS) or myeloproliferative disorders (MPD) and AML with adverse cytogenetics]. With conventional chemotherapy, CR is achieved in up to 50% of patients with long-term survival in less than 10%, while CR rates for patients without those features are 70% and over with long-term survival in 30-40%.1-3 CR rate and duration also decrease with increasing age (i.e. ≥ 55-60 years) to 50% and below and 3-5 year survival up to 10-15%, even without poor-risk features.2,4
The serine-threonine kinase inhibitor flavopiridol triggers cell death via multiple mechanisms. These include inhibition of multiple cyclin dependent kinases, thereby inducing cell cycle arrest and inhibiting RNA polymerase II phosphorylation with consequent decreased production of key growth and survival factors.5-15 We have conducted longitudinal clinical-laboratory studies of flavopiridol followed in a timed sequential manner by ara-C and mitoxantrone (FLAM).16-19 The hypothesis-driven design of FLAM was generated in an in vitro model where flavopiridol followed by ara-C enhanced ara-C related apoptosis in marrow leukemic blasts.20,21 Serial trials of FLAM in poor-risk AML16-18 have documented reproducible and durable CRs and low morbidity and mortality. For newly diagnosed, poor-risk patients, 67% achieved CR, 9% died in the first 60 days, and median overall survival (OS) and disease-free survival (DFS) for patients achieving CR were 12.6 and 13.3 months, respectively.18
Flavopiridol is highly protein-bound in human serum.22-25 To overcome binding, Byrd and Grever26 developed a pharmacologically-modeled 'hybrid' schedule of flavopiridol administration of a 30-min bolus of between one-third and half the total dose, followed by a 4-h infusion of the remainder. The 'hybrid' schedule yielded dramatic responses in more than 50% of refractory chronic lymphocytic leukemia (CLL) patients, accompanied by severe tumor lysis syndrome.26-28 Based on our phase II data using 1-h bolus flavopiridol in FLAM,16-18 and the results of single-agent hybrid flavopiridol in CLL, we conducted a phase I trial of ‘hybrid FLAM’ built on the template of previous trials.19 Dose-limiting toxicity occurred with 100 mg/m2 (30 mg/m2 bolus, 70 mg/m2 infusion), with tumor lysis, hyperbilirubinemia and mucositis. Toxicities and time to recovery with the hybrid schedule were similar to the bolus schedule. Death occurred in 9% of patients. CR occurred in 40% across all doses with CR in 90% and over of patients with relapsed AML, and 30% and over for primary refractory AML. OS and DFS for CR patients were 60% and over at two years and over.19
To determine whether the ‘hybrid’ schedule of flavopiridol administration would improve clinical results in adults with newly diagnosed, poor-risk AML, we conducted a randomized phase II trial of FLAM with flavopiridol given as a daily bolus for three days (Arm A) versus flavopiridol given as a 'hybrid' bolus-infusion for three days (Arm B), both followed in a timed sequence by ara-C and mitoxantrone.
Design and Methods
Patient eligibility and selection
Eligibility criteria were age 18 years and over with pathologically confirmed, previously untreated AML (excluding acute promyelocytic leukemia). Poor risk features included: 1) age 50 years or over; 2) secondary AML (MDS/AML, MPD/AML, treatment-related AML); and/or 3) adverse cytogenetics. Patients with peripheral blast count of 50,000 mL or over could receive hydroxyurea (HU) for up to 24 h before beginning flavopiridol. Patients who had received prior therapy for MDS or MPD were eligible. Eligibility criteria were similar to those for previous studies.16-19 All patients provided written informed consent according to The Johns Hopkins Medical Institutions and Fred Hutchinson Cancer Research Center (FHCRC) Institutional Review Boards and guidelines.
Treatment
Patients were randomized to receive bolus flavopiridol (Arm A) at 50 mg/m2 daily for three days (Days 1-3) or hybrid flavopiridol (Arm B) given as a 30-min bolus of 30 mg/m2 followed by a 4-h infusion of 40 mg/m2 daily (total daily dose 70 mg/m2) for three days (Days 1-3). The hybrid dose was selected during a phase I trial for ‘hybrid’ FLAM for tolerability and similar total dose as bolus FLAM.19 In order to mitigate imbalance in randomization, patients were stratified by presence or absence of secondary AML, antecedent MDS or MPD six months or more prior to AML transformation, and therapy for antecedent disorder.29,30
As in previous FLAM trials,16-19 a 72-h continuous infusion of ara-C 2 gms/m2 (667 mg/m2/24 h) began Day 6 and mitoxantrone 40 mg/m2 was administered as an intravenous bolus over 60-120 min on Day 9, 12 h after completing ara-C. Patients who achieved CR after cycle 1 were eligible to receive a second cycle of FLAM beginning 21±7 days following hospital discharge from the first cycle. Patients who achieved CR and had a suitable matched related or unrelated donor or a related haploidentical donor were eligible to undergo allogeneic hematopoietic cell transplant (HCT) following the first or second cycle of FLAM.
Supportive care
Prophylaxis measures against tumor lysis syndrome (TLS) and infection were similar to those used in previous studies,16-19 including measures developed by Byrd and Grever26-28 and Blum31 for management of TLS-related hyperkalemia. Use of growth factors was not permitted.
Response and toxicity
Bone marrow aspirates and biopsies were performed before treatment, on Day 14 of FLAM, and at hematologic recovery or when leukemia regrowth was suspected. Hematologic recovery was defined as ANC 0.5×109/L or over and transfusion-independent platelet count of 50×109/L. CR, CR with incomplete recovery (CRi) and no response (NR) were defined as according to Dohner.32 Adverse events were graded by NCI Common Toxicity Criteria, version 3.0.
Pharmacokinetic sampling and analysis
Flavopiridol pharmacokinetics were evaluated for both arms during cycle 1 at the following time points: prior to starting flavopiridol on each day of administration (Days 1-3), 30 min after completion of the bolus for each arm and before the start of the infusion (Arm B) on Days 1 and 3, after the end of infusion Days 1 and 3 (4.5 h from start of bolus in both arms), and 24 and 48 h after the final flavopiridol administration on Day 3. Flavopiridol concentrations in plasma were quantitated using a validated analytic assay consisting of high-performance liquid chromatography (HPLC) with mass spectrometric detection over the analytical range of 0.1-100 ng/mL (0.00025-0.25 μM).19 Plasma samples that were diluted 1:10 (v/v) or 1:100 (v/v) with pooled plasma were accurately quantified. A mciro-equilibrium dialysis method was utilized to determine unbound flavopiridol concentrations.
Statistical analysis
The study was designed as a randomized phase II with a “pick the winner” approach to evaluate two schedules of flavopiridol administration followed by ara-C and mitoxantrone for response and toxicities. The primary outcome was CR. Simon's two-stage designs were used in each arm which allowed an arm to stop early for strong evidence of futility (i.e. lack of efficacy).30 With both arms proceeding through to the second stage and rejecting the null hypothesis, the schedule with the higher response rate would be selected for further study.
The two-stage design parameters were based on our null hypothesis (per arm) that the response rate is 30% and our alternative hypothesis that the response rate is 55%. This range represents the results from other novel and standard approaches to poor-risk AML and accommodates the inherently heterogenous nature of patients entered on such trials. Furthermore, 55% is the lower range of the confidence intervals that we had documented in our prior FLAM studies in this patient population.17,18 At the first stage, 15 patients were enrolled in each arm. The arm would close to accrual if 4 or under responses were seen in that arm in the first stage. If 5 or more CRs were observed, then the arm would remain open for 20 additional patients. An arm would be considered promising if the CR rate is over 42% (i.e. at least 15 responses in 35 patients). This study has a (one-sided) type I error of 7% and power of 88%. Due to the possibility that some patients would not be evaluable, 39 patients were enrolled in each arm.
Flavopiridol concentrations were summarized by descriptive statistics. A Wilcoxon's rank sum test was used to compare concentrations between administration schedules (bolus vs. ‘hybrid’).
OS was calculated from Day 1 of FLAM to date of last known follow up or death. DFS was calculated from achievement of CR to date of last known follow up, relapse or death in CR. Survival data were analyzed as of 1st April 2011. The Kaplan-Meier method was used to estimate OS and DFS for the whole cohort and stratified by groups of interest. Log rank P values were used for descriptive comparison of survival outcomes among disease characteristics and treatments.
Results
Patients' characteristics
A total of 78 adults with newly diagnosed AML with poor-risk features were enrolled between 20th November 2008 and 20th July 2010. All patients have been followed for at least ten months with the longest follow up being 27 months. Clinical demographics and disease biological features are presented in Table 1. The majority of patients had secondary AML and/or adverse genetics (including 12% with FLT3-ITD positivity), with more than 90% having at least one poor-risk factor independent of age over 50 years and 45% having two or more age-independent risk factors. Only 5 (6%) had age over 50 years (range 51-78 years) as the sole eligibility criterion.
Table 1.
Demographic and biological characteristics of 78 adults with newly diagnosed, poor-risk acute myelogenous leukemia.

Patients' characteristics between the two centers (62 patients at Johns Hopkins, 16 patients at Fred Hutchinson) were comparable with respect to randomization and stratification (data not shown).
Interim analysis and evaluable patient population
At the time of interim analysis, 8 of 15 patients in each arm had achieved CR or CRi and both arms proceeded to stage two of Simon's two-stage design, with a goal of 35 evaluable patients in each arm. At the end of the study, 39 patients had been enrolled in each arm with 2 patients in each arm receiving incomplete therapy (2 TLS, one ara-C-induced adult respiratory distress syndrome, one hyperbilirubinemia). Nonetheless, all 78 patients were included in toxicity, response and survival analyses.
Pharmacokinetics
There was significant interindividual variation in both total and unbound flavopiridol concentrations regardless of the administration schedule (Figure 1). The bolus schedule (Arm A) resulted in higher maximum concentrations (Day 1 total P<0.0001; Day 3 total P=0.0003; Days 1 and 3 unbound P<0.0001), but there were no differences noted between the bolus and 'hybrid' (Arm B) schedules at trough concentrations and up to 48 h after completing the last infusion (total and unbound P>0.05).
Figure 1.
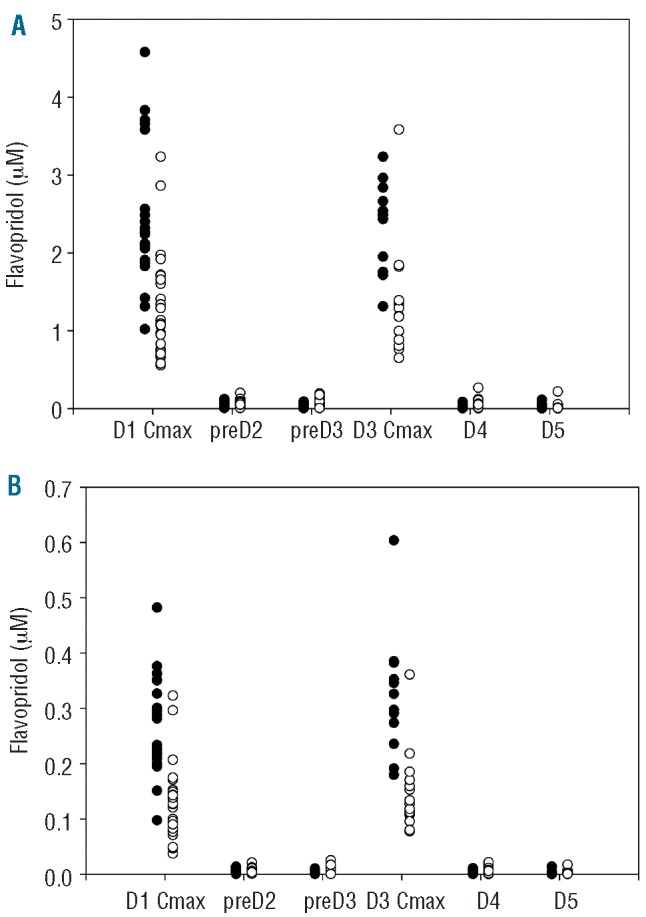
Total (A) and unbound (B) flavopiridol concentrations at various time points during and after administration by bolus (filled circles) and hybrid (open circles) schedules.
Comparative toxicities
The incidence of grade 3 or higher non-hematologic toxicities occurring during the induction cycle (cycle 1) of FLAM was equivalent for both arms with respect to TLS (9%), oral and/or gastrointestinal mucositis (6%), cardiac dysfunction (6%) and death from any cause (8%) within 60 days of starting FLAM. The overall toxicity profiles for both arms were similar to previous studies of bolus FLAM16-18 and ‘hybrid’ FLAM.19 In addition, the time from initiation of therapy to hematologic recovery was similar to our previous FLAM studies, with median time to ANC over 0.5×109/L being 33 days (range 22-71 days) and platelets over 50×109/L being 30 days (range 21-80 days) for both arms.
Comparative clinical outcomes
Response to FLAM was assessed initially by Day 14 bone marrow aspirates and/or biopsies obtained on 38 Arm A (bolus) patients and 37 Arm B ('hybrid') patients, with similar results in the two arms. Marked marrow hypocellularity (<20%) with complete tumor clearance (CTC) or less than 5% blasts was achieved in 28 (74%) Arm A and 28 (78%) Arm B patients, of whom 21 (75%) Arm A and 24 (86%) Arm B achieved CR. In contrast, for the 10 Arm A and 9 Arm B patients who had 20% or over cellularity and 5% or over blasts in the Day 14 marrow, CR was achieved in up to 20%. Three patients did not have Day 14 marrow aspirates: one patient with grade 5 tumor lysis died Day 2, 2 patients refused (one CR, one NR as assessed by marrow at time of recovery).
Median OS was 11.4 months (95% CI: 7.6, Inf) in Arm A and 13.0 months (95% CI: 11.2, Inf) in Arm B, without significant differences between the two arms (P=0.38). OS was associated with age: median OS in patients under the age of 60 years was not achieved (95% CI: 12.6, Inf) and for those over the age of 60 years, the median OS was only 9.2 months (95% CI: 6.6, 15.5) (P=0.02). Interestingly, the treatment effect appears to be more dramatic in patients over the age of 60 years: the estimated hazard ratio (HR) comparing OS for patients in Arm B versus Arm A among patients aged 60 years and older is 0.53 (P=0.13). The treatment effect favors Arm A in patients under the age of 60 years (HR=1.41); however, this was not significant (P=0.51).
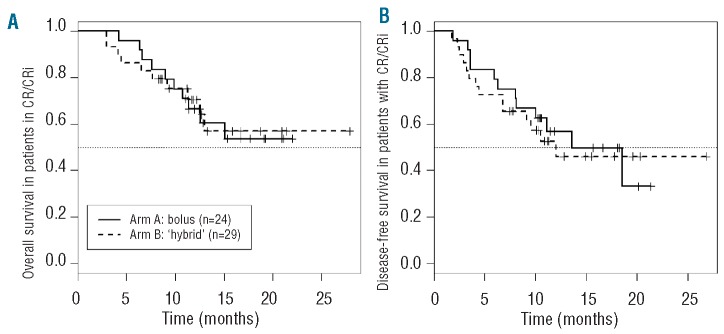
As detailed in Table 2, 53 (68%; 95% CI: 56%, 78%) of the 78 evaluable patients achieved CR (n=49) or CRi (n=4). All CR and CRi were accompanied by clearance of any pre-treatment cytogenetic or genetic abnormalities. CR+CRi occurred in 24 (62%; 95% CI 45%, 73%) Arm A patients and 29 (74%; 95% CI: 56%, 78%) Arm B patients.33 Remission rates were at least 65% in both arms in those cohorts with poor-risk disease biology, namely secondary AML and adverse genetics. Arm B adults aged 60 years and over appeared to achieve a higher CR/CRi rate than those in Arm A, although this was without statistical significance (78% Arm B vs. 48% Arm A; P=0.10). Median OS has not been reached in either arm for those achieving CR/CRi (Figure 2A). For Arm A, the estimated 12-month survival from CR is 58% (95% CI: 43%, 82%). For Arm B, the 12 month survival is estimated to be 66% (95% CI: 55%, 90%).
Table 2.
Response following FLAM induction: bolus versus hybrid flavopiridol administration.
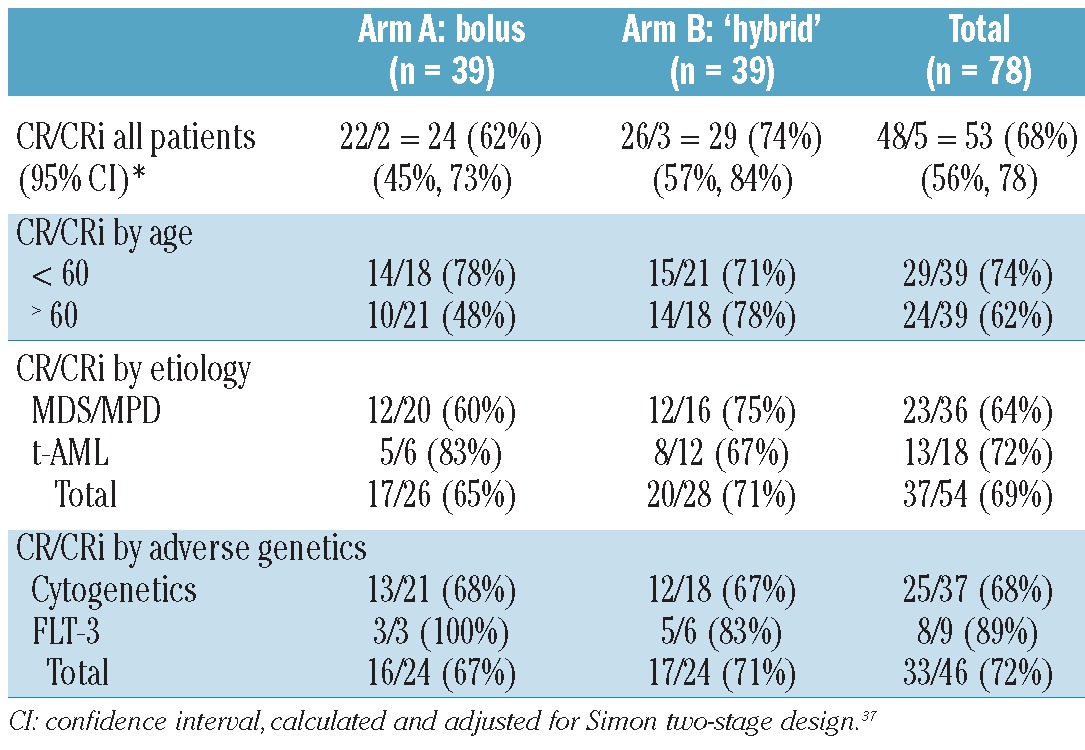
Figure 2.
(A) Overall survival for 24 CR/CRi patients treated in Arm A (—) and for 29 CR/CRi patients treated in Arm B (---). (B) Disease-free survival for 24 CR/CRi patients treated in Arm A (—) and for 29 CR/CRi patients treated in Arm B (---).
Table 3 shows clinical outcome in relation to therapy in CR for the 53 CR patients (24 Arm A, 29 Arm B). Two (8%) Arm A patients and 4 (14%) Arm B patients did not receive therapy in CR due to persistent grade 3-4 organ dysfunction (n=3, renal, cardiac, hepatic), fatal viral pneumonia (n=1) and patient refusal (n=2). Of these 6, only one has remained in CR for 8.4 months. The remaining 47 CR patients received chemotherapy (33% A, 52% B), allogeneic HCT (25% A, 14% B), or both (38% A, 21% B) in CR. OS for the CR patients is similar for both arms.
Table 3.
Comparative clinical outcomes according to therapy in complete remission.
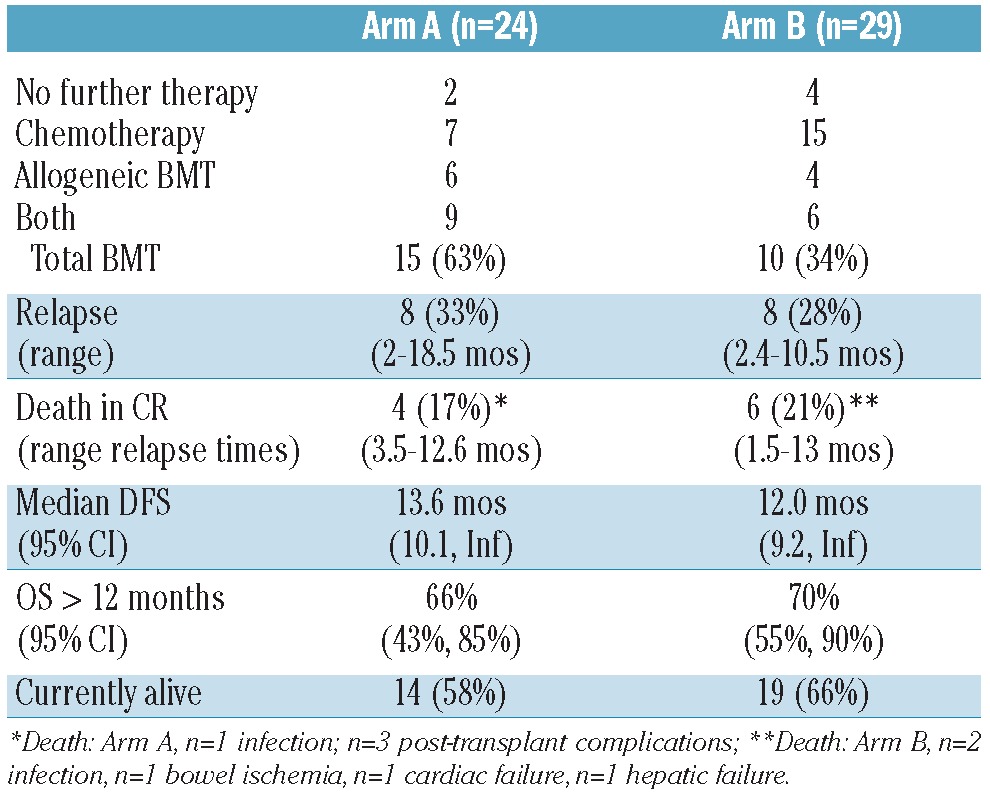
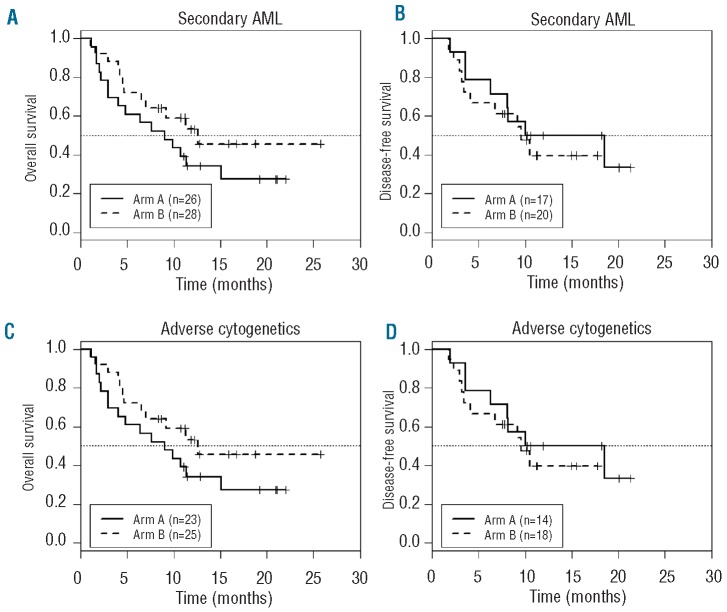
For CR/CRi patients, 12 (50%) Arm A and 15 (55%) Arm B patients remain in continuous CR with similar DFS and OS in both arms (Figure 2B). Outcomes were similar for patients with secondary AMLs (Figure 3A) and for those with adverse cytogenetics (Figure 3B). Median DFS for the 15 Arm A patients undergoing allogeneic HCT was 12 months, with 3 patients with treatment-related AML succumbing at 4-6 months post-HCT from transplantrelated complications, 4 relapsing 2-3 months post-HCT, and 8 continuing in CR for from (8.5 to18 months and over). For the 10 Arm B patients undergoing allogeneic HCT in CR, median DFS was not reached, with 2 relapsing 7-9 months post-HCT and 8 continuing in CR 8 to 19.5 months and over. For 7 Arm A patients receiving consolidation chemotherapy only, one died (CR 3.3 months), 2 relapsed (CR 3.6 and 14 months), and 4 (57%) continue in CR (10≥21 months). For 15 Arm B patients receiving consolidation chemotherapy only, 3 died (CR 2.5-6.8 months), 5 relapsed (CR 2.4-10.5 months), and 7 (47%) continue in CR (7≥27 months). Two additional patients who did not achieve CR/CRi (one in each arm) underwent allogeneic HCT without additional chemotherapy, with OS being over 21 months (Arm A) and over 26 months (Arm B). The Arm A patient achieved CRi with DFS over 17 months, and the Arm B patient achieved CR with DFS over 18 months. These 2 patients are not included in the calculations of OS or DFS for CR/CRi patients, but are included in OS for all patients.
Figure 3.
(A) and (B) Overall and disease-free survivals (OS, DFS) for patients with secondary AML. For Arm A (—), median OS (26 patients) = 10.7 months (95% CI: 6.64, Inf) and median DFS (17 patients) = 18.5 months (95% CI: 8.13, Inf). For Arm B (---), median OS (28 patients) = 13 months (95% CI: 7.0, Inf) and median DFS (20 patients) = 9.6 months (6.8-inf). (C) and (D) Overall and disease-free survivals (OS, DFS) for patients with adverse cytogenetics. For Arm A (—), median OS (23 patients) = 9 months (4.0, Inf) and median DFS (14 patients) = 14.3 months (95% CI: 8.1, Inf). For Arm B (---), median OS (25 patients) = 12.6 months (95% CI: 7.0, Inf) and median DFS (18 patients) = 9.6 months (4.1, Inf).
Discussion
Our randomized phase II trial comparing the clinical outcome of FLAM with flavopiridol given by bolus versus ‘hybrid’ bolus-infusion administration confirms our previous results in newly diagnosed, poor-risk adult AML17,18 and demonstrates the equivalency of the two schedules. The arms were relatively balanced for features of secondary AML by up-front stratification. Both had low rates of grade 3 or higher toxicities and death, including TLS. While the CR rate in Arm B was moderately higher than Arm A, OS and DFS were comparable between the 2 arms. There was modest imbalance in post-induction therapy, with fewer Arm B patients receiving chemotherapy alone in CR (48% Arm B vs. 33% Arm A) and more Arm A patients undergoing allogeneic HCT at any time during CR (58% Arm A vs. 38% Arm B). This imbalance may relate to more deaths during consolidation chemotherapy in Arm B (3 of 21, 14% Arm B vs. 1 of 16, 6% Arm A), but was balanced by more transplant-related death in Arm A (3 of 15, 20% Arm A vs. 0 of 11, 0% Arm B).
Both arms yielded high CR rates in patients with FLT-3 mutations and treatment-related AML. The 89% CR rate in 9 FLT3-mutant patients replicates our previous FLAM study.18 Furthermore, the current cohort of FLT3-mutant patients has achieved substantial DFS following consolidation chemotherapy and/or allogeneic BMT, with 6 of the 8 CR patients in continuous CR at from 5.5 to over 19.2 months. Likewise, 14 of 18 (78%) patients with treatment-related AML achieved CR, including the subset with adverse cytogenetics (9 of 13, 69%). Unfortunately, this cohort is relatively intolerant of allogeneic HCT. Of the 8 treatment-related AML patients undergoing allogeneic HCT in CR, 3 died from transplant-related causes, 2 relapsed, and only 3 remain in CR at five, seven, eight, and 18 months. Nonetheless, the overall mortality of BMT following FLAM therapy is consistent with expected rates and suggests that FLAM induction (with or without consolidation) does not increase transplant-related mortality. Of 6 patients who received consolidation FLAM without allogeneic HCT (all aged over 60 years), 3 remain in CR at 5.3, 18.1, and 25 months.
The comparability of bolus and 'hybrid' flavopiridol for newly diagnosed AML differs from the situation in refractory CLL, where tumor lysis and clinical efficacy were markedly increased with the 'hybrid' schedule.26-28 This discrepancy may reflect a different mechanism of action of flavopiridol in these disparate cell types. Nonetheless, it is possible that a higher dose of 'hybrid' flavopiridol could have yielded greater differences in overall clinical outcome. In our phase I trial of FLAM with escalating doses of 'hybrid' flavopiridol for adults with relapsed and refractory acute leukemias,19 the maximal tolerated dose was 90 mg/m2 (30 mg/m2 bolus followed by 60 mg/m2 infusion) daily for three days, which represents a 28% increase over the 70 mg/m2 total dose daily for three days used in the present study. However, pharmacokinetic studies of flavopiridol given by bolus,16,23,25 infusional,23,24,34 or ‘hybrid’19,26-29 schedules display significant inter-individual variability. This variability occurs for both total and unbound drug concentrations and, in the context of the ‘hybrid’ schedule, is independent of total serum protein, albumin, WBC or occurrence of flavopiridol-related toxicities. 19 Preliminary analysis of this study suggests that higher concentrations were achieved using the bolus schedule that dissipated in 24 h. A population pharmacokinetic analysis of the two flavopiridol schedules for total and unbound drug are under investigation.
The '7+3' approach of ara-C at doses of 100-200 mg/m2/day for 7-10 days by continuous infusion with three days of an anthracycline (idarubicin 12mg/m2 or daunorubicin 45-60 mg/m2) is widely used as induction therapy for AML in all age groups.35 Lowenberg36 demonstrated that doubling the daunorubicin dose during induction therapy for ‘fit’ AML patients aged 60 years and over improved the CR rate from 54% to 64%, with achievement of CR following a single induction cycle in 52% of the high-dose versus 35% of the conventional dose group. High-dose daunorubicin, defined as 90 mg/m2 daily × 3, improved 2-year OS and event free survival (EFS) in patients aged 65 years and under, but did not improve OS and EFS in patients with adverse cytogenetics, independently of age. In contrast, Fernandez's study of high-dose daunorubicin in adults under the age of 60 years yielded increases in CR rate (71% vs. 57%) and OS (23.7 vs. 15.3 months), but no benefit for patients aged 50-60 years or those with unfavorable cytogenetics or FLT-3 mutations.37 While these studies demonstrate an impact of high-dose daunorubicin on subcategories of AML, the beneficial effects are restricted to patients without poor-risk features.
In summary, FLAM produces encouraging results in adults with poor-risk AML. Although the study was not powered to detect subtle differences in the 2 arms, bolus and 'hybrid' administrations yielded comparable results in terms of overall efficacy and toxicity. Since the bolus schedule is easier to administer than the 'hybrid' schedule, we will use bolus flavopiridol in further studies for newly diagnosed AML patients. As a first step towards establishing the validity of FLAM, we conducted a multivariate analysis comparing CR rates in poor-risk patients given FLAM with unselected patients treated with various '7+3' regimens through SWOG or outside SWOG at the FHCRC (data not shown). The analysis accounted for age, secondary AML, and cytogenetics, and the results suggest that FLAM and '7+3' at FCHRC may have similar CR rates, with 69% for FLAM and 73% for FCHRC (adjusted odds ratio 1.16, range 0.51-2.64, P=0.72). It is worth noting, however, that these populations differed considerably in terms of adverse cytogenetics (48% FLAM vs. 22% FCHRC) and secondary AML (65% FLAM vs. 43% FCHRC). Furthermore, after accounting for covariates, there was no similarity in CR rates with '7+3' regimens in SWOG and at the FHCRC. This discrepancy suggests that unknown covariates that cannot be accounted for by multivariate analysis can confound such analyses, thereby making comparisons unreliable. This under-scores the need to randomize patients between FLAM and '7+3' contemporaneously, with careful up-front stratification based on host and disease biology to ensure comparability among arms. Toward this end, to address what role FLAM might play in induction therapy for AML without so-called 'good-risk' features, we have begun a randomized, stratified phase II trial to compare FLAM with '7+3' for adults aged 70 years and under with newly diagnosed AML, excluding core binding factor AMLs.
Acknowledgments
the authors thank the Johns Hopkins Sidney Kimmel Cancer Center and Fred Hutchinson Cancer Research Center nursing staffs for superb medical care, and the patients and their families, without whose partnership we could never have conducted the trial and from whom we have learned critical information that will help us to improve the treatment of these diseases. We also thank Christy Stewart for her expert administrative support.
Funding: this work was supported in part by NCI Cooperative Agreement U01 CA70095 (JEK and MAR), NCI Cancer Center Support Grant 2P30 CA06973-46, National Center for Research Resources Grant UL1 RR025005 (a component of the National Institutes of Health Roadmap for Medical Research), and philanthropic funds from Dr. Robert E. Fischell in memory of his late wife Marian (JEK). The contents of the project are solely the responsibility of the authors and do not necessarily represent the official view of NCI, NCRR, or NIH.
Footnotes
Authorship and Disclosures: The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Byrd JC, Mrózek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood. 2002;100(13):4325-35 [DOI] [PubMed] [Google Scholar]
- 2.Estey E. Acute myeloid leukemia and myelodysplastic syndromes in older patients. J Clin Oncol. 2007;25(14):1908-15 [DOI] [PubMed] [Google Scholar]
- 3.Schlenk RF, Dohner K, Krauter J, Frohling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358(18):1909-18 [DOI] [PubMed] [Google Scholar]
- 4.Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bible KC, Kaufmann SH. Flavopiridol: a cytotoxic flavone that induces cell death in noncycling A549 human lung carcinoma cells. Cancer Res. 1995;56(11):4856-61 [PubMed] [Google Scholar]
- 6.Carlson BA, Dubay MM, Sausville EA, Brizuela L, Worland PJ. Flavopiridol induces G1 arrest with inhibition of cyclin-dependent kinase (CDK)2 and CDK 4 in human breast carcinoma cells. Cancer Res. 1996;56(14):2973-8 [PubMed] [Google Scholar]
- 7.Carlson B, Lahusen T, Singh S, Loaiza-Perez A, Worland PJ, Pestell R, et al. Down-regulation of Cyclin D1 by transcriptional repression in MCF-7 human breast carcinoma cells induced by flavopiridol. Cancer Res. 1999;59(18):4634-43 [PubMed] [Google Scholar]
- 8.Chao SH, Price DH. Flavopiridol inactivates P-TEFb and blocks most RNA polymerase II transcription in vivo. J Biol Chem. 2001; 276(34):31793-9 [DOI] [PubMed] [Google Scholar]
- 9.Decker RH, Dai Y, Grant S. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in human leukemia cells (U937) through the mitochondrial rather than the receptor-mediated pathway. Cell Death Diff. 2001;8(7):715-24 [DOI] [PubMed] [Google Scholar]
- 10.Gojo I, Zhang B, Fenton RG. The cyclin-dependent kinase inhibitor Flavopiridol induces apoptosis in multiple myeloma cells through transcriptional repression and down-regulation of Mcl-1. Clin Cancer Res. 2002;8(8):3527-38 [PubMed] [Google Scholar]
- 11.Lam LT, Pickeral OK, Peng AC, Rosenwald A, Hurt EM, Giltnane JM, et al. Genomic-scale measurement of mRNA turnover and the mechanisms of action of the anti-cancer drug flavopiridol. Genome Biol. 2001;2(10):1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YK, Isham CR, Kaufmann SH, Bible KC. Flavopiridol disrupts STAT3/DNA interactions, attenuates STAT3-directed transcription, and combines with the Jak kinase inhibitor AG490 to achieve cytotoxic synergy. Mol Cancer Ther. 2006;5(1):138-48 [DOI] [PubMed] [Google Scholar]
- 13.Senderowicz AM, Sausville EA. Preclinical and clinical development of cyclin-dependent kinase modulators. J Natl Cancer Inst. 2000;92(5):376-87 [DOI] [PubMed] [Google Scholar]
- 14.Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006;24(11):1770-83 [DOI] [PubMed] [Google Scholar]
- 15.Yu C, Rahmani M, Dai Y, Conrad D, Krystal G, Dent P, Grant S. The lethal effects of pharmacological cyclin-dependent kinase inhibitors in human leukemia cells proceed through a phosphatidylinositol 3-kinase/Akt-dependent pathway. Cancer Res. 2003;63(8):1822-33 [PubMed] [Google Scholar]
- 16.Karp JE, Passaniti A, Gojo I, Kaufmann S, Bible K, Garimella TS, et al. Phase I and pharmacokinetic study of flavopiridol followed by 1-beta-D-arabinofuranosylcytosine and mitoxantrone in relapsed and refractory adult acute leukemias. Clin Cancer Res. 2005;11(23):8403-12 [DOI] [PubMed] [Google Scholar]
- 17.Karp JE, Smith BD, Levis MJ, Gore SD, Greer J, Hattenberg C, et al. Sequential flavopiridol, cytosine arabinoside, and mitoxantrone: a Phase II trial in adults with poor-risk acute myelogenous leukemia. Clin Cancer Res. 2007;13(15 Pt 1):4467-73 [DOI] [PubMed] [Google Scholar]
- 18.Karp JE, Blackford A, Smith BD, Alino K, Hatfield-Seung A, Bolanos-Meade J, et al. Clinical activity of sequential flavopiridol, cytosine arabinoside, and mitoxantrone for adults with newly diagnosed, poor risk acute myelogenous leukemia. Leuk Res. 2010;34:877-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karp JE, Smith BD, Resar LS, Greer JM, Blackford A, Zhao M, et al. Phase I and pharmacokinetic study of bolus-infusion flavopiridol followed by cytosine arabinoside and mitoxantrone for acute leukemias. Blood. 2011;117(12):3302-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bible KC, Kaufmann SH. Cytotoxic synergy between Flavopiridol (NSC 649890, L86-8278275) and various antineoplastic agents: the importance of sequence of administration. Cancer Res. 1997;57(16):3375-80 [PubMed] [Google Scholar]
- 21.Karp JE, Ross DD, Yang W, Tidwell ML, Wei Y, Greer J, et al. Timed sequential therapy of acute leukemia with flavopiridol: in vitro model for a Phase I clinical trial. Clin Cancer Res. 2003;9(1):307-15 [PubMed] [Google Scholar]
- 22.Byrd JC, Shinn C, Wasalenko JK, Fuchs EJ, Lehman TA, Nguyen PL, et al. Flavopiridol induces apoptosis in chronic lymphocytic leukemia cells via activation of caspase-3 without evidence of bcl-2 modulation or dependence on functional p53. Blood. 1998;92(10):3804-16 [PubMed] [Google Scholar]
- 23.Byrd JC, Peterson BL, Gabrilove J, Odenike OM, Grever M, Rai K, et al. Treatment of relapsed chronic lymphocytic leukemia by 72-hour continuous infusion or 1-hour bolus infusion of flavopiridol: results from Cancer and Leukemia Group B study 19805. Clin Cancer Res. 2005;11(11):4176-81 [DOI] [PubMed] [Google Scholar]
- 24.Flinn IW, Byrd JC, Bartlett N, Kipps TJ, Gribben D, Thomas RA, et al. Flavopiridol administered as a 24-hour continuous infusion in chronic lymphocytic leukemia lacks clinical activity. Leuk Res. 2005;29(11):1253-7 [DOI] [PubMed] [Google Scholar]
- 25.Tan AR, Headlee D, Messman R, Sausville EA, Arbuck SG, Murgo AJ, et al. Phase I clinical and pharmacokinetic study of flavopiridol administered as a daily 1-hour infusion in patients with advanced neoplasms. J Clin Oncol. 2002;20(19):4074-82 [DOI] [PubMed] [Google Scholar]
- 26.Byrd JC, Lin TS, Dalton JT, Wu D, Phelps MA, Fischer B, et al. Flavopiridol administered using a pharmacologically derived schedule of flavopiridol is associated with marked clinical activity in refractory, genetically high risk chronic lymphocytic leukemia. Blood. 2007;109(2):399-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin TS, Ruppert AS, Johnson AJ, Fischer B, Heerema NA, Andritsos LA, et al. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. J Clin Oncol. 2009;27(35):6012-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phelps MA, Lin TS, Johnson AJ, Hurh E, Rozewski DM, Farley KL, et al. Clinical response and pharmacokinetics from a phase I study of an active dosing schedule of flavopiridol in relapsed chronic lymphocytic leukemia. Blood. 2009;113(12):2637-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blum W, Phelps MA, Klisovic RB, Rozewski DM, Ni W, Albanese KA, et al. Phase I clinical and pharmacokinetic study of a novel schedule of flavopiridol in relapsed and refractory acute leukemias. Haematol. 2010;95(7):1098-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103-15 [PubMed] [Google Scholar]
- 31.Simon R, Wittes RE, Ellenberg SS. Randomized phase II clinical trials. Cancer Treat Rep. 1985;69(12):1375-81 [PubMed] [Google Scholar]
- 32.Dohner H, Estey EH, Amadori S, Applebaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010;115(3):453-74 [DOI] [PubMed] [Google Scholar]
- 33.Koyama T, Chen H. Proper inference form Simon's two-stage designs. Stat Med. 2008; 27(16):3145-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudek MA, Bauer JS, Lush RM, III, Stinson SF, Senderowicz AM, Headlee DJ, et al. Clinical pharmacology of flavopiridol following a 72-hour continuous infusion. Ann Pharmacother 2003;37(10):1369-74 [DOI] [PubMed] [Google Scholar]
- 35.Rai K, Holland J, Glidewell O, Weinberg V, Brunner K, Obrecht JP, et al. Treatment of acute myelocytic leukemia: a study by cancer and leukemia group B. Blood. 1981; 58(6):1203-12 [PubMed] [Google Scholar]
- 36.Lowenberg B, Ossenkoppele GJ, van Putten W, Schouten HC, Graux C, Ferrant A, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. New Engl J Med. 2009;361(13):1235-48 [DOI] [PubMed] [Google Scholar]
- 37.Fernandez HF, Sun Z, Yao X, Litzow MR, Luger SM, Paietta EM, et al. Anthracycline dose intensification in acute myeloid leukemia. New Engl J Med. 2009;361(13):1249-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Estey EH, Thall PF. New designs for phase 2 clinical trials. Blood. 2003;102(2):442-8 [DOI] [PubMed] [Google Scholar]