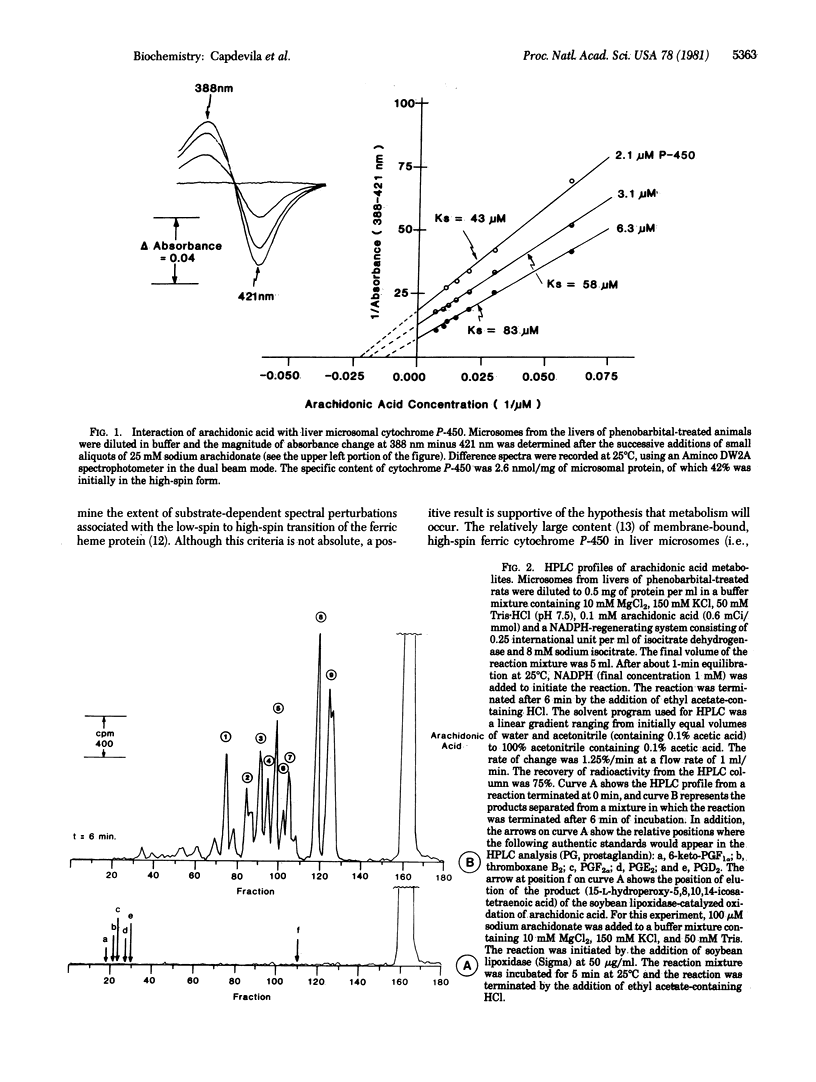
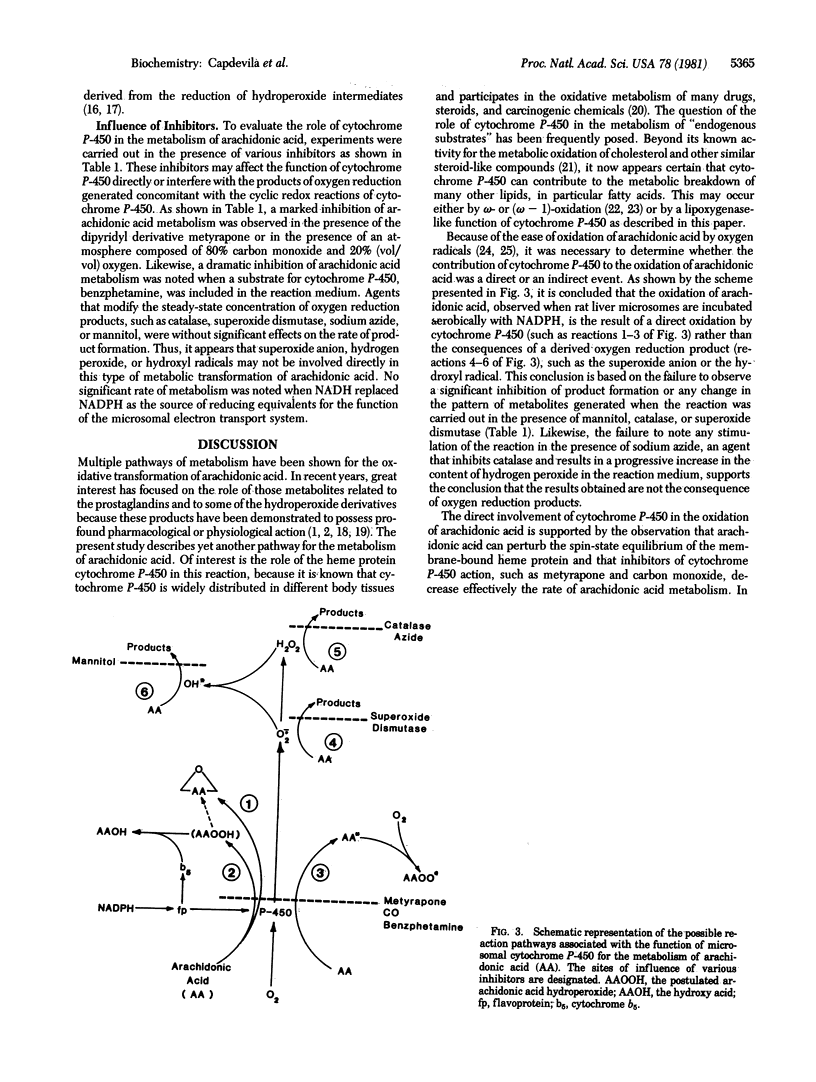
Abstract
Arachidonic acid is oxidatively metabolized by rat liver microsomes at a rate of approximately 5 nmol per min per mg of protein at 25 degrees C. This reaction is dependent on the presence of NADPH and oxygen. Studies with various inhibitors indicate a role for membrane-bound cytochrome P-450 in the transformation of arachidonic acid to a mixture of hydroxy acid derivatives. The stoichiometry of the reaction conforms to that of a monooxygenase reaction--i.e., one mole of NADPH is oxidized per mole of oxygen utilized--suggesting a reaction mechanism different from that proposed for lipid peroxidation reactions. No evidence for the formation of prostaglandin-like metabolites was obtained. The diene character of some of the metabolites formed suggests another role for cytochrome P-450--i.e., participation in hydrogen abstraction reactions for the activation of various substrates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Capdevila J., Estabrook R. W., Prough R. A. Differences in the mechanism of NADPH- and cumene hydroperoxide-supported reactions of cytochrome P-450. Arch Biochem Biophys. 1980 Mar;200(1):186–195. doi: 10.1016/0003-9861(80)90345-8. [DOI] [PubMed] [Google Scholar]
- Dahlén S. E., Hedqvist P., Hammarström S., Samuelsson B. Leukotrienes are potent constrictors of human bronchi. Nature. 1980 Dec 4;288(5790):484–486. doi: 10.1038/288484a0. [DOI] [PubMed] [Google Scholar]
- Di Augustine R. P., Fouts J. R. The effects of unsaturated fatty acids on hepatic microsomal drug metabolism and cytochrome P-450. Biochem J. 1969 Nov;115(3):547–554. doi: 10.1042/bj1150547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellin A., Jakobsson S. V., Schenkman J. B., Orrenius S. Cytochrome P 450K of rat kidney cortex microsomes: its involvement in fatty acid - and ( -1)-hydroxylation. Arch Biochem Biophys. 1972 May;150(1):64–71. doi: 10.1016/0003-9861(72)90010-0. [DOI] [PubMed] [Google Scholar]
- Fridovich S. E., Porter N. A. Oxidation of arachidonic acid in micelles by superoxide and hydrogen peroxide. J Biol Chem. 1981 Jan 10;256(1):260–265. [PubMed] [Google Scholar]
- Gibson G. G., Cinti D. L., Sligar S. G., Schenkman J. B. The effect of microsomal fatty acids and other lipids on the spin state of partially purified cytochrome P-450. J Biol Chem. 1980 Mar 10;255(5):1867–1873. [PubMed] [Google Scholar]
- Hildebraunt A. G., Roots I. Reduced nicotinamide adenine dinucleotide phosphate (NADPH)-dependent formation and breakdown of hydrogen peroxide during mixed function oxidation reactions in liver microsomes. Arch Biochem Biophys. 1975 Dec;171(2):385–397. doi: 10.1016/0003-9861(75)90047-8. [DOI] [PubMed] [Google Scholar]
- Hochstein P., Nordenbrand K., Ernster L. Evidence for the involvement of iron in the ADP-activated peroxidation of lipids in microsomes and mitochondria. Biochem Biophys Res Commun. 1964;14:323–328. doi: 10.1016/s0006-291x(64)80004-8. [DOI] [PubMed] [Google Scholar]
- Hrycay E. G., O'Brien P. J. Microsomal electron transport. I. Reduced nicotinamide adenine dinucleotide phosphate-cytochrome c reductase and cytochrome P-450 as electron carriers in microsomal NADPH-peroxidase activity. Arch Biochem Biophys. 1973 Jul;157(1):7–22. doi: 10.1016/0003-9861(73)90383-4. [DOI] [PubMed] [Google Scholar]
- Kupfer D., Navarro J., Piccolo D. E. Hydroxylation of prostaglandins A1 and E1 by liver microsomal monooxygenase. Characteristics of the enzyme system in the guinea pig. J Biol Chem. 1978 Apr 25;253(8):2804–2811. [PubMed] [Google Scholar]
- Lee T. C., Snyder F. Phospholipid metabolism in rat liver endoplasmic reticulum. Structural analyses, turnover studies and enzymic activities. Biochim Biophys Acta. 1973 Jan 2;291(1):71–82. doi: 10.1016/0005-2736(73)90061-8. [DOI] [PubMed] [Google Scholar]
- May H. E., McCay P. B. Reduced triphosphopyridine nucleotide oxidase-catalyzed alterations of membrane phospholipids. II. Enzymic properties and stoichiometry. J Biol Chem. 1968 May 10;243(9):2296–2305. [PubMed] [Google Scholar]
- Pessayre D., Mazel P., Descatoire V., Rogier E., Feldmann G., Benhamou J. P. Inhibition of hepatic drug-metabolizing enzymes by arachidonic acid. Xenobiotica. 1979 May;9(5):301–310. doi: 10.3109/00498257909038733. [DOI] [PubMed] [Google Scholar]
- Porter N. A., Wolf R. A., Yarbro E. M., Weenen H. The autoxidation of arachidonic acid: formation of the proposed SRS-A intermediate. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1058–1064. doi: 10.1016/0006-291x(79)92115-6. [DOI] [PubMed] [Google Scholar]
- Prough R. A., Burke M. D. The role of NADPH-cytochrome c reductase in microsomal hydroxylation reactions. Arch Biochem Biophys. 1975 Sep;170(1):160–168. doi: 10.1016/0003-9861(75)90107-1. [DOI] [PubMed] [Google Scholar]
- Rådmark O., Malmsten C., Samuelsson B., Goto G., Marfat A., Corey E. J. Leukotriene A. Isolation from human polymorphonuclear leukocytes. J Biol Chem. 1980 Dec 25;255(24):11828–11831. [PubMed] [Google Scholar]
- Samuelsson B., Goldyne M., Granström E., Hamberg M., Hammarström S., Malmsten C. Prostaglandins and thromboxanes. Annu Rev Biochem. 1978;47:997–1029. doi: 10.1146/annurev.bi.47.070178.005025. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Kondo K., Hayaishi O. Role of prostaglandin endoperoxides in the serum thiobarbituric acid reaction. Arch Biochem Biophys. 1981 Feb;206(2):271–276. doi: 10.1016/0003-9861(81)90091-6. [DOI] [PubMed] [Google Scholar]
- Werringloer J., Kawano S., Estabrook R. W. Spin state transitions of liver microsomal cytochrome P-450. Acta Biol Med Ger. 1979;38(2-3):163–175. [PubMed] [Google Scholar]
- White R. E., Coon M. J. Oxygen activation by cytochrome P-450. Annu Rev Biochem. 1980;49:315–356. doi: 10.1146/annurev.bi.49.070180.001531. [DOI] [PubMed] [Google Scholar]



