Abstract
Background
The prognosis of patients with relapses of ETV6/RUNX1-positive acute lymphoblastic leukemia remains to be evaluated, particularly with regards to the frequency of late relapses. We performed a long-term, follow-up retrospective study to address the outcome of patients with ETV6/RUNX1-positive leukemia relapses.
Design and Methods
Among the 713 children tested for ETV6/RUNX1 enrolled into the FRALLE 93 protocol, 43 ETV6/RUNX1-positive patients relapsed (19.4%). Most were initially stratified in the low or intermediate risk groups. The median follow-up after relapse was 54.2 months. All but three received second-line salvage therapy and 16 underwent allogeneic transplantation.
Results
ETV6/RUNX1 had a strong effect on overall survival after relapse (3-year survival= 64.7% for positive cases versus 46.5% for negative cases) (P=0.007). The 5-year cumulative incidence of relapse was 19.4% and testes were more frequently involved in ETV6/RUNX1-positive relapses (P=0.04). In 81.4% of cases the relapses were late, early combined or isolated extramedullary relapses. The 5-year survival rate of patients with ETV6-RUNX1-positive acute lymphoblastic leukemia relapses reached 80.8% when the relapse occurred after 36 months (versus 31.2% when the relapse occurred earlier). In univariate analysis, female gender was associated with a poor survival, whereas site of relapse, age at diagnosis, leukocytosis and consolidation strategy had no effect. In multivariate analysis, only the duration of first remission remained associated with outcome.
Conclusions
We found an excellent outcome for patients with ETV6/RUNX1-positive leukemia relapses that occurred more than 36 months after diagnosis. The duration of first complete remission may, therefore, be a guide to define the treatment strategy for patients with relapsed ETV6/RUNX1- positive leukemia. Key words: ETV6/RUNX1, childhood leukemia, acute lymphoblastic, prognosis, relapse.
Key words: ETV6/RUNX1, childhood leukemia, acute lymphoblastic, prognosis, relapse
Introduction
Despite significant improvements in treatment, about 20% of children with acute lymphoblastic leukemia (ALL) still suffer from relapse.1-5 Unfortunately the cure rate remains low with event-free survival reaching only 30% at 10 years after relapse.6-9 Few prognostic factors have been identified.10 Blast phenotype (T versus B), site of relapse, and duration of the first remission are the main variables affecting outcome. The current relapse risk group stratifications are based on the system first described by the Berlin–Frankfurt–Munster (BFM) relapse group, which takes into account the time of relapse, the site of relapse (bone marrow and/or extramedullary), and the subsequent immunophenotype to categorize patients into one of four risk categories (S1 to S4).11 This stratification has been adapted by the Medical Research Council (MRC, UK) and is also used by the Children's Oncology Group which divided marrow relapse into early relapse occurring up to 36 months after diagnosis and late relapse which occurs 36 months or more after the initial diagnosis. 7,8 The Children's Cancer Group has also divided relapses into early (<18 months after diagnosis), intermediate (18-35 months) and late (≥36 months).12
In this context, ETV6/RUNX1-positive ALL has a separate status. Indeed, this subgroup of ALL is characterized by a good prognosis, which is better than for B-ALL without this fusion transcript.2-5,13 Nevertheless, some recent long-term results revealed late relapses among patients with ETV6/RUNX1-positive ALL.14,15 It has been hypothesized that a relapse is essentially a de novo ALL originating from a preleukemic stem cell.16-19 This notion should be taken into account when considering the treatment options. The increased proportion of extramedullary relapses, including testicular or ovarian relapses, has also been discussed by a few authors.20,21 To investigate the outcome of patients with relapses of ETV6/RUNX1-positive ALL, we studied the long-term results of the ETV6/RUNX1-positive ALL relapsed patients initially enrolled into the FRALLE93 protocol and identified some prognostic factors which call into question the current therapeutic approach for treating late relapses.
Design and Methods
The FRALLE (FRench group for childhood ALL) 93 trial was open to children aged 0 to 20 years with untreated ALL, excluding those with L3 ALL or Down's syndrome. Between June 1, 1993, and December 31, 1999, 1395 children were enrolled into the FRALLE 93 trial in 18 French pediatric centers and one Belgian pediatric center. This study was approved by the ethics committee of the Hôpital Saint Louis, France. All patients, or their parents, gave informed consent in accordance with the Declaration of Helsinki. The diagnosis of ALL was based upon morphological, immunophenotypic, and cytogenetic analyses of bone marrow samples. From 1995, children were systematically screened for four fusion transcripts (ETV6-RUNX1, BCRABL, E2A-PBX1, MLL-AF4). The FRALLE 93 study population was stratified into three groups (low-risk, intermediate-risk, and high-risk) based on the following risk factors: age, whitecell count at diagnosis, hemoglobin level, immunophenotype, karyotype, and response to steroids. The treatment, which has already been published,22 is described in the Online Supplementary Data.
Statistical analysis
Summary statistics were calculated, namely the median (with interquartile range, IQR) for continuous variables and frequency (with percentages) for qualitative variables.
The distribution of qualitative variables was compared with the χ2 test or Fisher's exact test, as appropriate; comparisons of continuous variables were based on the non-parametric Wilcoxon's rank-sum test.
We first compared the characteristics of tested and non-tested patients, to check for potential selection bias. We then focused on the ETV6/RUNX1-positive children who relapsed. We compared the cumulative incidence of relapse in ETV6/RUNX1-positive versus -negative patients. Relapses were divided into isolated central nervous system (CNS) relapse, isolated bone-marrow relapse, and others. We also considered bone-marrow relapses (whether isolated or combined), as well as testicular relapses, with these latter only assessed in boys.
Finally, we focused on the outcome of ETV6/RUNX1-positive patients compared with negative patients. Relapsed patients were classified according to the risk groups defined in the REZ-BFM 95/96 study.11 This distinguished S1/S2 patients (isolated bone-marrow relapses occurring ≥6 months after completion of primary therapy, isolated extramedullary relapses, and combined bone marrow relapses occurring ≥18 months after completion of primary therapy) from S3/S4 patients (isolated bone marrow relapses occurring <6 months and combined bone marrow relapses occurring <18 months after the primary diagnosis).
Patients' follow-up data were updated in October, 2010. The cumulative incidence of relapse was estimated from the date of complete remission, with death prior to relapse considered as a competing risk, and subgroup comparisons were based on Gray's test. The Kaplan–Meier method was used to estimate the overall survival (OS) probabilities with 95% confidence intervals (95% CI) from the date of relapse until death, irrespective of cause. The log-rank test was used for comparisons of Kaplan–Meier curves. Multivariate Cox stepwise forward-regression analysis (including testicular and non-testicular relapses) was performed to determine the independent set of prognostic variables.
All statistical tests were two-sided, with P values of 0.05 or less denoting statistical significance. Statistical analyses were performed using SAS v9.2 (SAS Inc, Cary, NC, USA), and R 2.10.1 (http://www.R-project.org) software packages.
Results
Total cohort
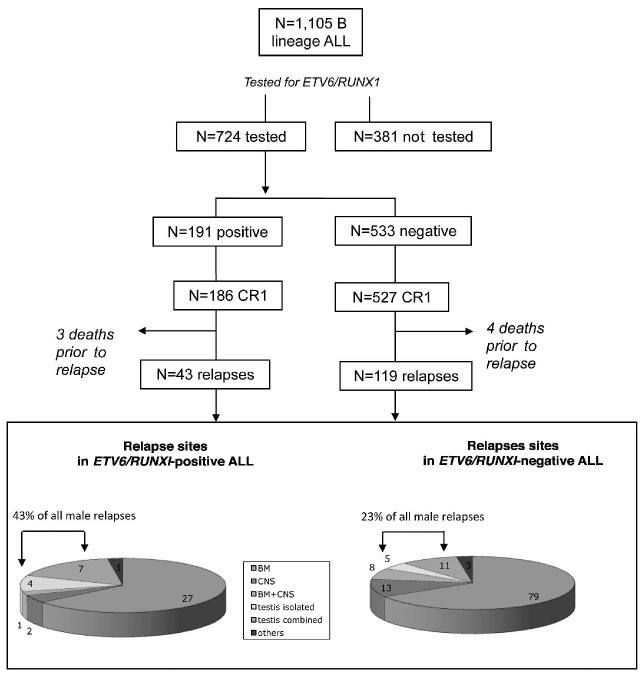
A flow chart illustrating the study design is shown in Figure 1. Among the 1,105 patients with B lineage ALL, excluding infants and patients with t(9;22) or t(4;11), ETV6/RUNX1 status was known for 724 patients (most of the other cases were diagnosed before systematic screening). In terms of age, gender, blood prednisone response, day 21 marrow status, complete remission, and survival, there were no differences between these 724 patients and the 381 who were not tested for ETV6/RUNX1. However, the initial median leukocyte count was higher in the tested group (10.109/L versus 6.4.109/L P=0.003), and more patients were enrolled in the low-risk group of treatment among those not tested for ETV6/RUNX1 ALL (22% versus 13%; P=0.001).
Figure 1.
Flow chart of the study design. Relapses occurred in 43 and 119 ETV6/RUNX1-positive and –negative cases of ALL, respectively. Testicular relapses were more frequent in ETV6/RUNX1-positive ALL (P=0.04) without any increase in other extramedullary sites of disease. Second complete remission was achieved in 98% of ETV6/RUNX1-positive ALL and in 82% of ETV6/RUNX1-negative ALL. CRI: first complete remission; BM: bone marrow; CNS: central nervous system.
Tested patients
Out of the 191 patients with t(12;21), 186 (97.4%) achieved a first complete remission (CR1), whereas six (1.1%) out of the 533 ETV6/RUNX1-negative patients did not achieve CR1 (P= 0.17). The median follow-up after CR was 9.9 years (IQR: 8.3-11.4). Overall, 162 (45% of boys) of the 713 children who reached CR1 relapsed, including 43 with t(12;21).
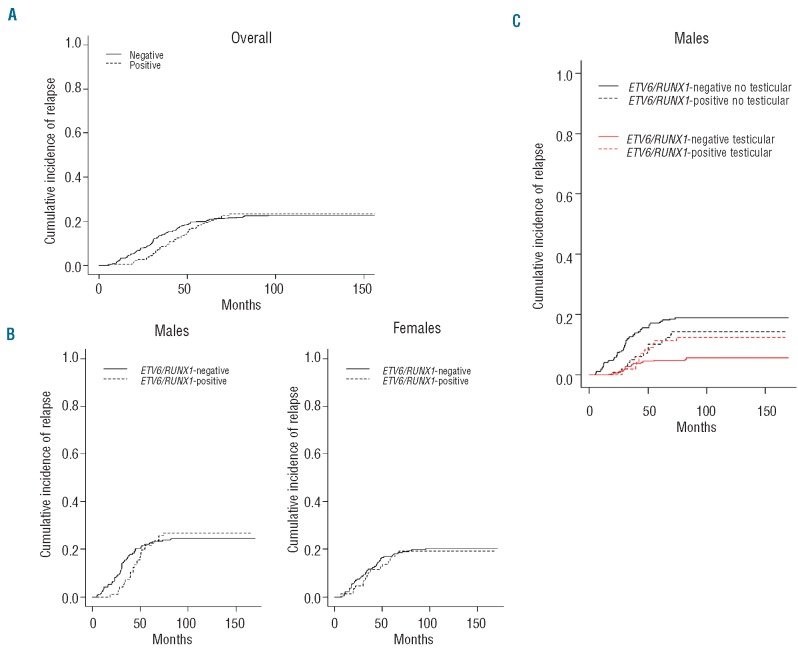
The cumulative incidence of relapse at 5 years did not differ significantly between ETV6/RUNX1-positive and - negative ALL patients (19.4% versus 19.9%, respectively; P=0.94) or according to gender (17% in females versus 22% in males; P=0.10) (Figures 2A, 2B). There was no difference in the type of relapse according to ETV6/RUNX1 positivity, with 106 (65.4%) isolated bone-marrow relapses (n=27 in ETV6/RUNX1-positive patients and n=79 in ETV6/RUNX1-negative patients), 24 (14.8%) isolated but extramedullary relapses (n=6 in ETV6/RUNX1-positive patients and n=18 in ETV6/RUNX1-negative patients), and 32 (19.8%) involving combined sites (P=0.80) (Figure 1). Among the 383 boys in CR1, 96 relapsed, with a 5-year cumulative incidence of relapse of 21.6% in ETV6/RUNX1-positive ALL and 22.4% in ETV6/RUNX1- negative ALL (P=0.90). Of note, testicular relapses accounted for 11 out of the 26 (43%) relapses in ETV6/RUNX1-positive ALL males versus 16 out of the 70 (23%) relapses in ETV6/RUNX1-negative ALL cases (P=0.04) (Table 2). As testicular relapses of ETV6/RUNX1- positive ALL occured mostly after 36 months (10 out of 11 cases), we considered the 5-year cumulative incidences of relapse in males and females separately. As shown in Figure 2B, the 5-year cumulative incidence of relapses did not differ significantly between ETV6/RUNX1-positive and ETV6/RUNX1-negative cases of ALL (21.6% versu 22.5% respectively) in either males (P=0.90 by Gray's test) or in females (P=0.81 by Gray's test) (Figure 2B). Nevertheless, in males, the cumulative incidence of testicular relapses appeared to be statistically increased in ETV6/RUNX1-positive patients (P=0.03, by Gray's test), with an estimated 5-year cumulative incidence of 11.3% compared to 4.9% in ETV6/RUNX1-negative patients. By contrast, non-testicular relapses were slightly decreased, albeit not statistically significantly so (P=0.24), in the ETV6/RUNX1-positive patients compared to in the ETV6/RUNX1-negativite patients (Figure 2C).
Figure 2.
Cumulative incidence of relapse according to ETV6/RUNX1 positivity (negative versus positive): (A) overall, (B) according to gender, and (C) according to site of relapse (testicular or not testicular) for males.
Table 2.
Univariate analyses of factors influencing survival of patients after a first relapse of ETV6/RUNX1-positive ALL.
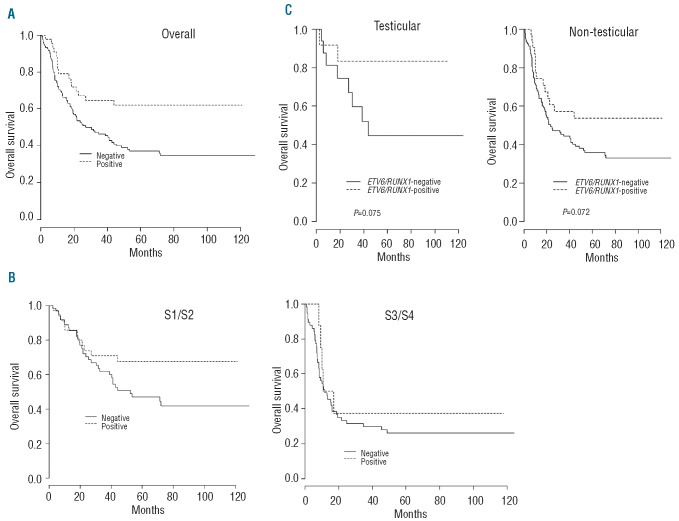
The median follow-up after relapse was 6.9 years (IQR: 5.7-8.6). The presence of the ETV6/RUNX1 fusion transcript greatly affected the outcome of relapses with the 5- year OS rate being 62.1% (95% CI: 49.0-78.7%) in ETV6/RUNX1-positive ALL patients compared to 37.2% (95% CI: 29.3-47.2%) in ETV6-RUNX1-negative ones (P=0.007) (Figure 3A). The difference in survival estimates between ETV6/RUNX1-positive and negative ALL patients persisted, although it was no longer statistically significant, in the S1/S2 subgroup with a 5-year OS rate of 67.7% (95% CI: 53.6-85.5%) in ETV6/RUNX1-positive patients and 47.0% (95% CI: 35.6-61.9%) in ETV6/RUNX1-negative ones (P=0.07) (Figure 3B). Patients with S3/S4 relapses had a poor outcome, with the 5-year OS rate for the whole cohort being 27.6% (95% CI: 18.6- 41.0%): the difference between ETV6/RUNX1-positive and –negative cases [(37.5% (95% CI: 15.3-91.7%) and 26.2% (95% CI: 16.9-40.6%), respectively] was not statistically significant (P=0.45 by the log-rank test) (Figure 3B). As the incidence of testicular relapses was high and could contribute to the favorable outcome of ETV6/RUNX1-positive ALL, we investigated the outcomes according to testicular versus other sites of relapse. The relapsed ETV6/RUNX1-positive ALL patients had a better OS than their transcript-negative counterparts whatever the site of relapse (although the differences were not statistically significant) (Figure 3C).
Figure 3.
Kaplan-Meier curves of overall survival of FRALLE 93 relapsed patients according to ETV6/RUNX1 positivity (negative versus positive) (A) overall, (B) in REZ-BFM risk groups, and (C) according to the site of relapse (testicular or non-testicular). ALL REZBFM classification: S1/S2 = isolated bone-marrow relapses occurring ≥6 months after completion of primary therapy, isolated extramedullary relapses, combined bone marrow relapses occurring ≥18 months after completion of primary therapy; S3/S4 = isolated bone marrow relapses <6 months or combined bone marrow relapses <18 months after primary diagnosis.
Patients with relapsed ETV6/RUNX1-positive acute lymphocytic leukemia
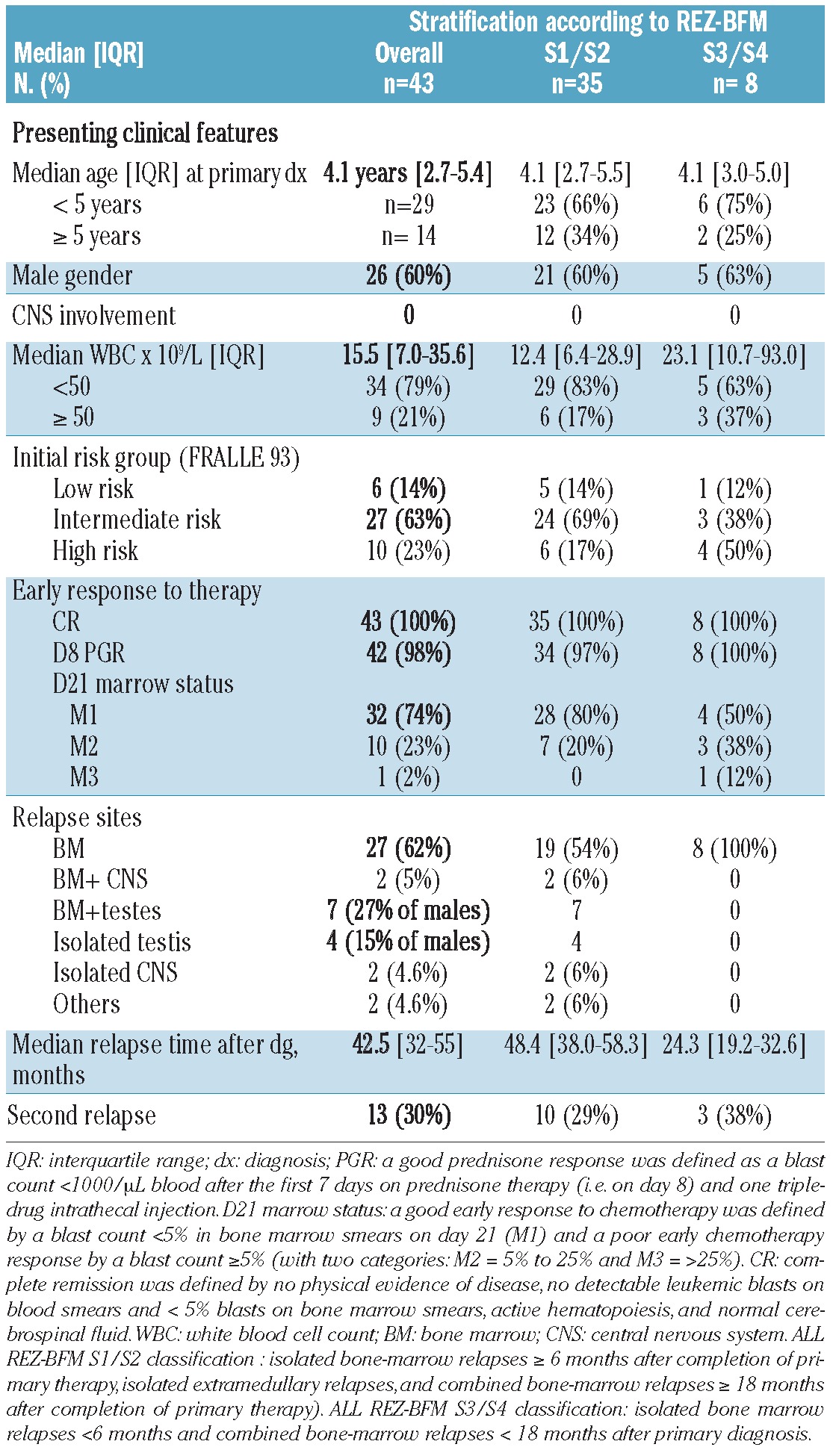
The initial clinical features of the 43 ETV6/RUNX1-positive ALL patients who relapsed are shown in Table 1 (further details are provided in Online Supplementary Table S1). There were 26 (60.5%) males, with a median age at diagnosis of 4.1 years (IQR: 2.7-5.4), a median leukocyte count of 15.5x109/L (IQR: 7.0-35.6) and no cases of CNS involvement. Thirty-three (77%) patients were classified as being at low or intermediate risk and had good early responses to therapy.
Table 1.
Initial features of the 43 patients with ETV6/RUNX1-positive ALL who relapsed.
Salvage therapy after relapse
According to the risk groups defined by the REZ-BFM 95/96 study, 35 (81.4%) patients were classified as S1/S2 and eight (18.6%) patients were classified as S3/S4.
All but three patients received a second-line therapy protocol (three patients were treated according to a first-line protocol after relapses occurring 68, 39, and 32 months after diagnosis). Thirty-seven patients were included in the COOPRALL-97 study23 which recommended a VANDA (VP-16, cytarabine, mitoxantrone, dexamethasone, asparaginase) induction regimen (except for late extramedullary relapses) followed by successive blocks (B1, B2, B3). If an HLA-identical donor was available, stem cell transplantation was performed after two or three blocks. Otherwise, alternative treatment consisted of three successive blocks repeated three times followed by autologous transplantation or - for late combined/isolated medullary or isolated extramedullary relapses - maintenance and CNS radiotherapy. Two patients were treated according to a Capizzi scheme24 and the latest one according to the UKALL R2.8
Outcomes
The median follow-up after relapse was 7.7 years (IQR: 6.2-9), with only one child lost to follow-up at 12 months after first relapse. All but one patient (due to early death) achieved a second complete remission (n=42, 98%). Eighteen children received consolidation chemotherapy and 24 were transplanted (Online Supplementary Figure S1). The 16 allogeneic transplants were performed at 4.2±1.2 months after relapse (10 in S1/S2 patients and 6 in S3/S4 patients), while the eight autologous transplants were performed 7.6±1.2 months after relapse.
A second relapse occurred in 13 patients, including 10 (77%) initially stratified as S1/S2. At 5 years, the cumulative incidence of second relapse was 28.8% (95% CI: 14.8- 42.8%) overall: 18.8% in allogeneic transplant recipients, 37.5% in autografted patients, and 36.2% in those who received chemotherapy. Of these 13 patients, four were still alive in a third complete remission, three having been treated with chemotherapy in second complete remission and one having received an allogeneic transplant.
Finally, a total of 16 deaths were observed. The 5-year OS rate after first relapse was 62.1% (95% CI: 49.0- 78.7%), with differences according to the type of relapse: 70.9% (95% CI: 57.3-87.9%) in S1/S2 versus 37.5% (95% CI: 15.3-91.7%) in the S3/S4 risk group (P=0.049).
Prognostic factors
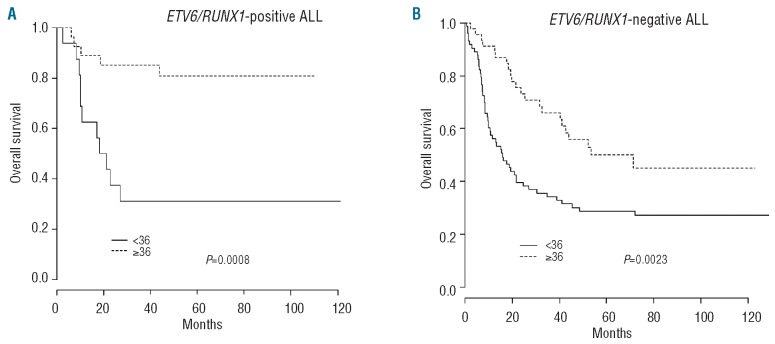
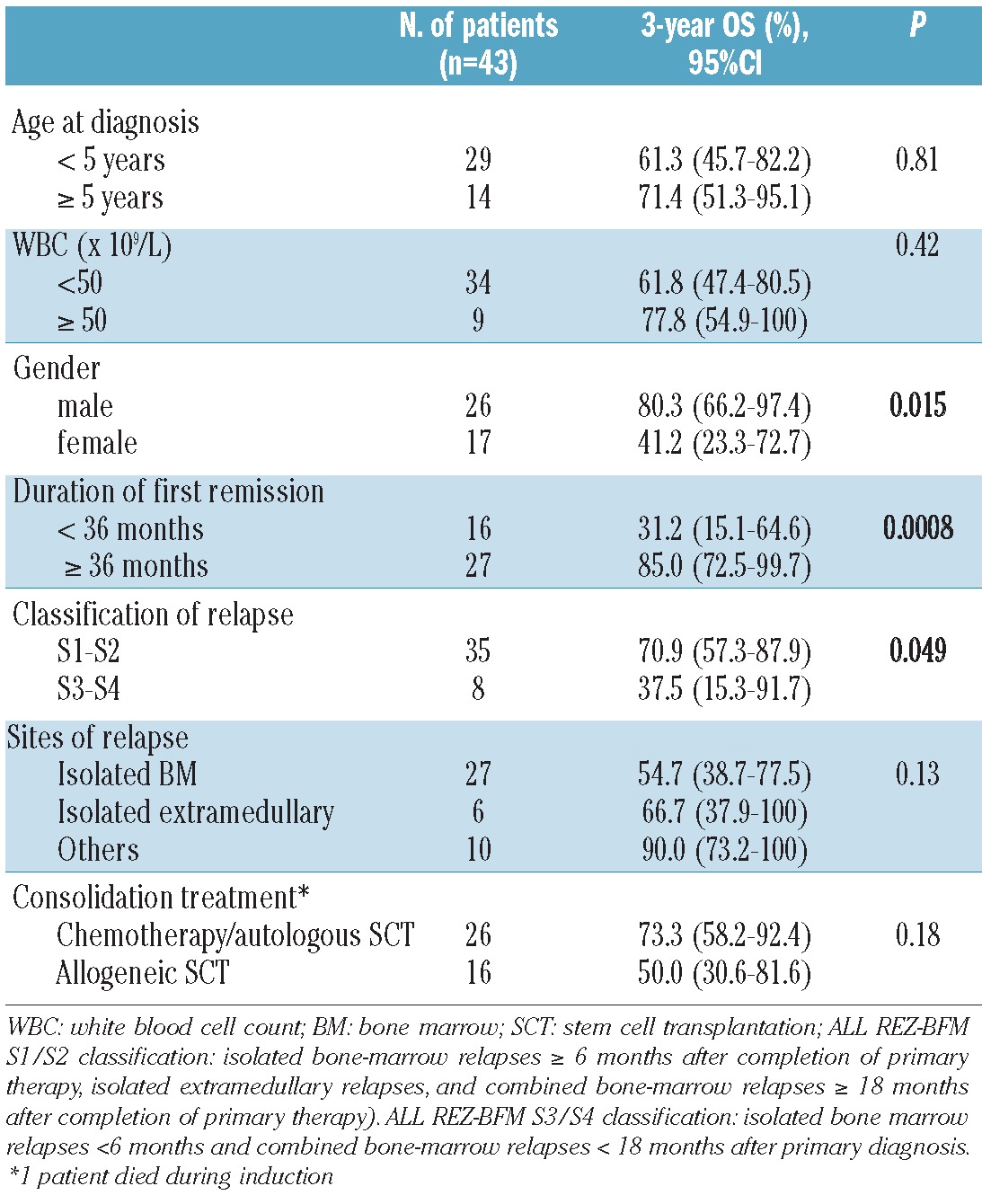
Based on univariate analyses, the overall survival of ETV6/RUNX1-positive ALL patients after relapse was significantly affected by the duration of the first remission with a 5-year OS rate that was significantly better when relapse occurred after 36 months than when it occurred before this time (80.8%, 95% CI: 66.9-97.5% versus 31.2%, 95% CI: 15.1-64.6%, respectively; P=0.0008). Female gender was also associated with a poor survival (P=0.015), whereas the site of relapse (P=0.13), age at initial diagnosis (P=0.81), and leukocytosis (P=0.42) were all found not to be of any prognostic value (Table 2). Similarly, the consolidation strategy (allografting or chemotherapy ± autografting) had no effect on survival (P=0.18).
In multivariate Cox-regression analysis, only the duration of first remission remained associated with the outcome (Figure 4A). In particular, the site of relapse (testicular versus non-testicular relapse) did not provide any additional prognostic information (P=0.79). Although timing of relapse in ETV6/RUNX1-negative patients also significantly affected prognosis (Figure 4B), among the 73 tested patients who relapsed after 36 months, the 5-year OS rates appeared to be significantly different in the 27 ETV6/RUNX1-positive patients (4 extramedullary, 9 combined and 14 isolated bone marrow relapses) compared to the 46 ETV6/RUNX1-negative ones (81% versus 50%, respectively; P=0.015 by the log-rank test).
Figure 4.
Prognostic value of time elapsed (<36 months versus ≥ 36 months) since diagnosis in terms of survival after relapse in (A) ETV6/RUNX1-positive ALL and (B) ETV6/RUNX1-negative ALL.
Discussion
We have reported here the results of the long-term follow- up of relapsed patients with ETV6/RUNX1-positive ALL initially enrolled in the FRALLE 93 protocol. It is now essential to analyze long-term results because very late relapses have been reported.20,21 In agreement with previous studies, we found that relapses occurred in about 20% of cases of ETV6/RUNX1-positive ALL, and for males after a longer remission period than in other B-lineage ALL.13,15,25-27 Indeed, the cumulative incidence of relapses among males was influenced by the frequency of testicular relapses, which occurred later than relapses in other sites in boys. More recent protocols based on intensified chemotherapy schemes, especially with intensive use of L-asparaginase,28 report lower frequencies of relapse for ETV6/RUNX1-positive ALL but should be examined with longer follow-up.3,5,13,29 We also highlight that boys were slightly over-represented in our series, an imbalance which has been rarely reported except by Seeger et al. and, more recently, by the NOPHO group.15,27 Moreover, we observed a specific increase of testicular relapses in ETV6/RUNX1-positive ALL without any increase of relapses in other extramedullary sites (mainly CNS). Whatever gender, when pooling all sites of relapses, we did not find any difference in cumulative incidence of relapses according to ETV6/RUNX1-positivity. To our knowledge, this finding has not been previously reported and highlights the importance of a primary treatment including drugs able to cross the testicular barrier, such as high-dose methotrexate. Most of the testicular relapses occurred late and special attention should be given to the testes, and may also the ovaries, in the long-term follow-up of ETV6/RUNX1-positive ALL patients. According to the responsiveness of the disease, ETV6/RUNX1-positive relapses are generally considered to be well treatable,13,15 and indeed we found a good overall outcome of 62.1% in this group, which is significantly better than that in ETV6/RUNX1-negative relapses. We also found that almost 98% of patients achieved a second complete remission, a rate significantly higher than that in the ALL-REZ BFM 90 and the MRC UKALL-R6,9 and also higher than that in FRALLE 93 ETV6/RUNX1-negative relapsed patients. Unfortunately, in our retrospective study, we were unable to explore IgH/TCR rearrangements or sequence the ETV6/RUNX1 genome to understand treatment failures better.
The prognostic roles of age, site, and time of relapse in the outcome of all types of relapses have often been debated. 9,12 Furthermore, the definition of extramedullary relapses could change considering the prognostic relevance of detection of submicroscopic bone marrow involvement based on sensitive in vitro amplification methods. 30 Age did not affect prognosis in our exclusively pediatric study but it should be noted that ETV6/RUNX1-positive ALL can have a different clinical profile in young adults.14 In multivariate analysis we found that only the time of relapse had prognostic significance for outcome. The OS rate of patients relapsing after ≥36 months was 80.8% which is excellent and similar to that of the prognosis of primary ALL. We could not show that the high incidence of testicular relapses contributed to this very favorable outcome, possibly because of some lack of statistical power. In contrast, the prognosis of patients with early recurrences (stratified as S3/S4 according to the ALL REZ-BFM classification) remained poor despite positivity for ETV6/RUNX1. The timing of relapse was also significant for ETV6/RUNX1-negative relapsed patients, but in these cases the OS rate was not so high among the patients who had late relapses. This finding may support the hypothesis that the majority of ETV6/RUNX1 “late” relapses could be due to a novel leukemic clone arising from a persistent initial ETV6/RUNX1-positive preleukemic clone.16,18,31 Indeed, ETV6/RUNX1 is an early event which is insufficient for leukemic development32,33 and requires the occurrence of additional events conferring proliferative advantages for full malignant transformation. 19,34 Bhojwani et al. have even suggested that this model could be operating in non-ETV6/RUNX1 subtypes of late relapses.35 Staal et al., in a genome-wide expression analysis of paired diagnosis-relapse samples, also found that relapses could result from therapy involving selection of minor clones present at diagnosis and from genetic alteration of the original tumor cells.36
Although the mechanisms governing relapses remain unclear, the particular natural history of ETV6/RUNX1- positive ALL may lead to the use of intensive but first-line therapeutic approaches for late relapses. The impact of glucocorticoid resistance at relapse should probably also be explored in the future in the light of recent data published by Kuster et al. who have implicated glucocorticoid signaling in relapses in ETV6/RUNX1-positive ALL.37 In this small study sample we failed to show any impact of the use of allogeneic transplantation, possibly because of an excess of transplantation-related mortality. However, the poor survival rate of patients with early relapses encourages the use of a second-line treatment with cell therapy according to the REZ-BFM. In both types of relapse,38 clinical decisions could also be helped by assessing the dynamics of treatment response using minimal residual disease quantification, which is a promising strategy being validated in ongoing relapse trials.25,39 Finally, our data on ETV6/RUNX1-positive ALL from the FRALLE 93 study can probably be extended to other first-line protocols because Freyer et al. recently showed that post-relapse survival in relapsed childhood ALL was independent of initial treatment intensity.38
In conclusion, the prognosis of patients with relapses of ETV6/RUNX1-positive ALL occurring more than 36 months after diagnosis is excellent. Testicular relapses are particularly common which suggests that a primary treatment able to cross the testicular barrier should be used and that follow-up should be prolonged with careful screening of the gonads. While early relapses need common relapse therapeutic approaches, the physiopathology of ETV6/RUNX1-positive ALL could allow the postponement of high-dose therapy for late relapses under the cover of monitoring biological markers of responsiveness.
Acknowledgments
we thank all members of the FRALLE group and are grateful to the children and parents who agreed to participate in the study.
Funding: this work was supported by the Délégation à la Recherche Clinique (Assistance Publique- Hôpitaux de Paris), a public, non-profit organization.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures: The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Kamps WA, van der Pal-de Bruin KM, Veerman AJ, Fiocco M, Bierings M, Pieters R. Long-term results of Dutch Childhood Oncology Group studies for children with acute lymphoblastic leukemia from 1984 to 2004. Leukemia. 2010;24(2):309-19 [DOI] [PubMed] [Google Scholar]
- 2.Moricke A, Zimmermann M, Reiter A, Henze G, Schrauder A, Gadner H, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia. 2010;24(2):265-84 [DOI] [PubMed] [Google Scholar]
- 3.Pui CH, Pei D, Sandlund JT, Ribeiro RC, Rubnitz JE, Raimondi SC, et al. Long-term results of St Jude Total Therapy Studies 11, 12, 13A, 13B, and 14 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24(2):371-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salzer WL, Devidas M, Carroll WL, Winick N, Pullen J, Hunger SP, et al. Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984-2001: a report from the Children's Oncology Group. Leukemia. 2010;24(2):355-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverman LB, Stevenson KE, O'Brien JE, Asselin BL, Barr RD, Clavell L, et al. Longterm results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985-2000). Leukemia. 2010;24(2):320-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chessells JM, Veys P, Kempski H, Henley P, Leiper A, Webb D, et al. Long-term follow-up of relapsed childhood acute lymphoblastic leukaemia. Br J Haematol. 2003;123(3):396-405 [DOI] [PubMed] [Google Scholar]
- 7.Raetz EA, Borowitz MJ, Devidas M, Linda SB, Hunger SP, Winick NJ, et al. Reinduction platform for children with first marrow relapse of acute lymphoblastic leukemia: a Children's Oncology Group Study[corrected]. J Clin Oncol. 2008;26(24):3971-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roy A, Cargill A, Love S, Moorman AV, Stoneham S, Lim A, et al. Outcome after first relapse in childhood acute lymphoblastic leukaemia - lessons from the United Kingdom R2 trial. Br J Haematol. 2005;130(1):67-75 [DOI] [PubMed] [Google Scholar]
- 9.Tallen G, Ratei R, Mann G, Kaspers G, Niggli F, Karachunsky A, et al. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J Clin Oncol. 2010;28(14):2339-47 [DOI] [PubMed] [Google Scholar]
- 10.Gaynon PS. Childhood acute lymphoblastic leukaemia and relapse. Br J Haematol. 2005;131(5):579-87 [DOI] [PubMed] [Google Scholar]
- 11.Borgmann A, von Stackelberg A, Hartmann R, Ebell W, Klingebiel T, Peters C, et al. Unrelated donor stem cell transplantation compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission: a matched-pair analysis. Blood. 2003;101(10):3835-9 [DOI] [PubMed] [Google Scholar]
- 12.Gaynon PS, Qu RP, Chappell RJ, Willoughby ML, Tubergen DG, Steinherz PG, et al. Survival after relapse in childhood acute lymphoblastic leukemia: impact of site and time to first relapse--the Children's Cancer Group experience. Cancer. 1998;82(7):1387-95 [DOI] [PubMed] [Google Scholar]
- 13.Loh ML, Goldwasser MA, Silverman LB, Poon WM, Vattikuti S, Cardoso A, et al. Prospective analysis of TEL/AML1-positive patients treated on Dana-Farber Cancer Institute Consortium Protocol 95-01. Blood. 2006;107(11):4508-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burmeister T, Gokbuget N, Schwartz S, Fischer L, Hubert D, Sindram A, et al. Clinical features and prognostic implications of TCF3-PBX1 and ETV6-RUNX1 in adult acute lymphoblastic leukemia. Haematologica. 2009;95(2):241-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forestier E, Heyman M, Andersen MK, Autio K, Blennow E, Borgstrom G, et al. Outcome of ETV6/RUNX1-positive childhood acute lymphoblastic leukaemia in the NOPHO-ALL-1992 protocol: frequent late relapses but good overall survival. Br J Haematol. 2008;140(6):665-72 [DOI] [PubMed] [Google Scholar]
- 16.Ford AM, Fasching K, Panzer-Grumayer ER, Koenig M, Haas OA, Greaves MF. Origins of “late” relapse in childhood acute lymphoblastic leukemia with TEL-AML1 fusion genes. Blood. 2001;98(3):558-64 [DOI] [PubMed] [Google Scholar]
- 17.Hong D, Gupta R, Ancliff P, Atzberger A, Brown J, Soneji S, et al. Initiating and cancerpropagating cells in TEL-AML1-associated childhood leukemia. Science. 2008;319(5861):336-9 [DOI] [PubMed] [Google Scholar]
- 18.Pine SR, Wiemels JL, Jayabose S, Sandoval C. TEL-AML1 fusion precedes differentiation to pre-B cells in childhood acute lymphoblastic leukemia. Leuk Res. 2003;27(2):155-64 [DOI] [PubMed] [Google Scholar]
- 19.Zuna J, Ford AM, Peham M, Patel N, Saha V, Eckert C, et al. TEL deletion analysis supports a novel view of relapse in childhood acute lymphoblastic leukemia. Clin Cancer Res. 2004;10(16):5355-60 [DOI] [PubMed] [Google Scholar]
- 20.Chow CD, Dalla-Pozza L, Gottlieb DJ, Hertzberg MS. Two cases of very late relapsing ALL carrying the TEL:AML1 fusion gene. Leukemia. 1999;13(11):1893-4 [DOI] [PubMed] [Google Scholar]
- 21.Ly-Sunnaram B, Henry C, Gandemer V, Mee FL, Burtin F, Blayau M, et al. Late ovarian relapse of TEL/AML1 positive ALL confirming that TEL deletion is a secondary event in leukemogenesis. Leuk Res. 2005;29(9):1089-94 [DOI] [PubMed] [Google Scholar]
- 22.Dufourg MN, Landman-Parker J, Auclerc MF, Schmitt C, Perel Y, Michel G, et al. Age and high-dose methotrexate are associated to clinical acute encephalopathy in FRALLE 93 trial for acute lymphoblastic leukemia in children. Leukemia. 2007;21(2):238-47 [DOI] [PubMed] [Google Scholar]
- 23.Domenech C, Mercier M, Plouvier E, Puraveau M, Bordigoni P, Michel G, et al. First isolated extramedullary relapse in children with B-cell precursor acute lymphoblastic leukaemia: results of the Cooprall-97 study. Eur J Cancer. 2008;44(16):2461-9 [DOI] [PubMed] [Google Scholar]
- 24.Capizzi RL, Poole M, Cooper MR, Richards F, 2nd, Stuart JJ, Jackson DV, Jr, et al. Treatment of poor risk acute leukemia with sequential high-dose ARA-C and asparaginase. Blood. 1984;63(3):694-700 [PubMed] [Google Scholar]
- 25.Bader P, Kreyenberg H, Henze GH, Eckert C, Reising M, Willasch A, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. J Clin Oncol. 2009;27(3):377-84 [DOI] [PubMed] [Google Scholar]
- 26.Harbott J, Viehmann S, Borkhardt A, Henze G, Lampert F. Incidence of TEL/AML1 fusion gene analyzed consecutively in children with acute lymphoblastic leukemia in relapse. Blood. 1997;90(12):4933-7 [PubMed] [Google Scholar]
- 27.Seeger K, Buchwald D, Taube T, Peter A, von Stackelberg A, Schmitt G, et al. TELAML1 positivity in relapsed B cell precursor acute lymphoblastic leukemia in childhood. Berlin-Frankfurt-Munster Study Group. Leukemia. 1999;13(9):1469-70 [DOI] [PubMed] [Google Scholar]
- 28.Ramakers-van Woerden NL, Pieters R, Loonen AH, Hubeek I, van Drunen E, Beverloo HB, et al. TEL/AML1 gene fusion is related to in vitro drug sensitivity for Lasparaginase in childhood acute lymphoblastic leukemia. Blood. 2000;96(3):1094-9 [PubMed] [Google Scholar]
- 29.Bhojwani D, Pei D, Sandlund JT, Jeha S, Ribeiro RC, Rubnitz JE, et al. ETV6-RUNX1- positive childhood acute lymphoblastic leukemia: improved outcome with contemporary therapy. Leukemia. 2012;26(2):265-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagedorn N, Acquaviva C, Fronkova E, von Stackelberg A, Barth A, zur Stadt U, et al. Submicroscopic bone marrow involvement in isolated extramedullary relapses in childhood acute lymphoblastic leukemia: a more precise definition of “isolated” and its possible clinical implications, a collaborative study of the Resistant Disease Committee of the International BFM study group. Blood. 2007;110(12):4022-9 [DOI] [PubMed] [Google Scholar]
- 31.Konrad M, Metzler M, Panzer S, Ostreicher I, Peham M, Repp R, et al. Late relapses evolve from slow-responding subclones in t(12;21)-positive acute lymphoblastic leukemia: evidence for the persistence of a preleukemic clone. Blood. 2003;101(9):3635-40 [DOI] [PubMed] [Google Scholar]
- 32.Ford AM, Bennett CA, Price CM, Bruin MC, Van Wering ER, Greaves M. Fetal origins of the TEL-AML1 fusion gene in identical twins with leukemia. Proc Natl Acad Sci USA. 1998;95(8):4584-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiemels JL, Cazzaniga G, Daniotti M, Eden OB, Addison GM, Masera G, et al. Prenatal origin of acute lymphoblastic leukaemia in children. Lancet. 1999;354(9189):1499-503 [DOI] [PubMed] [Google Scholar]
- 34.Mullighan CG, Phillips LA, Su X, Ma J, Miller CB, Shurtleff SA, et al. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science. 2008;322(5906):1377-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhojwani D, Kang H, Moskowitz NP, Min DJ, Lee H, Potter JW, et al. Biologic pathways associated with relapse in childhood acute lymphoblastic leukemia: a Children's Oncology Group study. Blood. 2006;108(2):711-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staal FJ, de Ridder D, Szczepanski T, Schonewille T, van der Linden EC, van Wering ER, et al. Genome-wide expression analysis of paired diagnosis-relapse samples in ALL indicates involvement of pathways related to DNA replication, cell cycle and DNA repair, independent of immune phenotype. Leukemia. 2010;24(3):491-9 [DOI] [PubMed] [Google Scholar]
- 37.Kuster L, Grausenburger R, Fuka G, Kaindl U, Krapf G, Inthal A, et al. ETV6/RUNX1- positive relapses evolve from an ancestral clone and frequently acquire deletions of genes implicated in glucocorticoid signaling. Blood. 2011;117(9):2658-67 [DOI] [PubMed] [Google Scholar]
- 38.Freyer DR, Devidas M, La M, Carroll WL, Gaynon PS, Hunger SP, et al. Postrelapse survival in childhood acute lymphoblastic leukemia is independent of initial treatment intensity: a report from the Children's Oncology Group. Blood. 2011;117(11):3010-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eckert C, Biondi A, Seeger K, Cazzaniga G, Hartmann R, Beyermann B, et al. Prognostic value of minimal residual disease in relapsed childhood acute lymphoblastic leukaemia. Lancet. 2001;358(9289):1239-41 [DOI] [PubMed] [Google Scholar]