Abstract
Background
Extramedullary disease is an uncommon manifestation in multiple myeloma and can either accompany newly diagnosed disease or develop with disease progression or relapse. We evaluated the impact of this disease feature on patients' outcome in the context of novel agents.
Design and Methods
We analyzed clinical and biological features of extramedullary disease in 936 patients with multiple myeloma enrolled in Total Therapy protocols, 240 patients in non-Total Therapy protocols, and 789 non-protocol patients, all of whom had baseline positron emission tomography scans to document extramedullary disease at diagnosis and its subsequent development at the time of disease progression or relapse.
Results
The most common sites for extramedullary disease at diagnosis were skin and soft tissue whereas liver involvement was the striking feature in extramedullary disease at disease relapse or progression. Regardless of therapy, extramedullary disease was associated with shorter progression-free and overall survival, as well as the presence of anemia, thrombocytopenia, elevated serum lactate dehydrogenase, cytogenetic abnormalities, and high-risk features in 70-and 80-gene risk models in univariate analysis. Multivariate analysis with logistic regression revealed that this disease feature was more prevalent in patients with an elevated centrosome index, as determined by gene expression profiling, as well as in myeloma molecular subtypes that are more prone to relapse. These include the MF subtype (also called the “MAF” subtype, associated with over-expression of the MAF gene seen with chromosome translocation 14;16 or 14;20) and the PR subtype (also called the “Proliferation” subtype, associated with overexpression of pro-proliferative genes).
Conclusions
These data show that extramedullary disease is more prevalent in genomically defined high-risk multiple myeloma and is associated with shorter progression-free survival and overall survival, even in the era of novel agents. All clinical trials included in the analyses were registered with www.clinicaltrials.gov (NCT00083551, NCT00083876, NCT00081939, NCT00572169, NCT00644228,NCT00002548,NCT00734877).
Key words: extramedullary disease, transplant, myeloma, survival
Introduction
Therapeutic advances over the last decade have nearly doubled the overall survival of patients with multiple myeloma when compared to that of patients treated before 2000, primarily due to the availability of effective, novel agents.1 This survival benefit will likely translate into an increase in the prevalence of MM patients who survive for more than 10 years from the time of their original diagnosis.2 Imaging techniques, such as positron emission tomography (PET) scans and magnetic resonance imaging, are emerging as prognostic instruments that can predict response earlier than conventional response criteria can.3,4 However, as with other malignancies that have been successfully treated with increasingly effective regimens, marked prolongation of overall survival has led to previously uncommon clinical presentations. In the case of MM, these include relapses in extramedullary sites, such as visceral organs, lymph nodes, and the central nervous system (mainly as meningeal myelomatosis) and secondary plasma-cell leukemia.
In the case of extramedullary disease (EMD), this can be present at the time of initial diagnosis, as evidenced on baseline imaging studies such as PET and magnetic resonance imaging, or develop at the time of disease relapse. It usually resembles a transformed malignant lymphoma both clinically and morphologically as well as in terms of laboratory features, such as high serum levels of lactate dehydrogenase. In addition, the majority of patients presenting with EMD have highly complex cytogenetic abnormalities and, as found most recently, high-risk features on gene expression profiling (GEP).5-8 In a classic monoclonal immunoglobulin-secreting tumor, EMD may present as light chain-secretory, hypo-secretory, or nonsecretory disease as a sign of disease de-differentiation and transformation.9 In this setting, modern imaging techniques, especially 18F-fluorodeoxyglucose (FDG) PET, have become extremely helpful in documenting suspected EMD.10
In our clinical practice, EMD is a rare primary disease manifestation; rather, it appears to evolve with repeated relapses. Here we describe our findings regarding the clinical and biological risk factors associated with EMD in our population of patients with MM and the impact of EMD on treatment outcomes.
Design and Methods
We searched our MM database for patients treated at the Myeloma Institute for Research and Therapy (University of Arkansas for Medical Sciences, Little Rock, Arkansas, USA) between 2000 and 2010 (inclusive) who either presented with EMD at diagnosis (EMD-1) or developed EMD at disease progression or relapse (EMD-2). The analysis was limited to this period since our institution started to perform baseline PET scanning in January 2000. All patients had signed written informed consent, in keeping with institutional, federal, and Helsinki Declaration requirements. The protocols had been approved by the Institutional Review Board which also reviewed annual progress reports of the clinical protocols. In addition, an independent, federally accredited investigator team had audited charts of more than 80% of patients for protocol eligibility, compliance with required tests and intended therapies, and for accuracy of toxicity and efficacy reporting.
We focused on 936 patients in Total Therapy (TT) protocols, 240 patients in non-TT protocols, and 789 non-protocol patients (total n=1965), all of whom had baseline PET scans at initial presentation at the Myeloma Institute for Research and Therapy in order to document whether primary EMD (EMD-1) was present, prior to therapy. We also documented which of these patients developed EMD (EMD-2) at the time of disease progression or relapse. Chi-square and Fisher's exact tests were used to compare baseline characteristics between patients receiving different treatment protocols. Logistic regression was used for multivariate analyses to model associations between baseline covariates and EMD-1. The Kaplan and Meier method was used to calculate progression-free survival and overall survival from the initial transplant at the Myeloma Institute for Research and Therapy. The cumulative incidence of EMD was calculated as described by Gooley.10
GEP was performed with the Affymetrix U133Plus2.0 microarray platform (Santa Clara, CA, USA) using methods previously described.11 Plasma cells were enriched by anti-CD138 immunomagnetic bead selection of mononuclear cell fractions of bone marrow aspirates and peripheral blood samples in a central laboratory. All samples applied to microarrays contained more than 85% plasma cells as determined by two-color flow cytometry (CD38+ and CD45-/dim) performed after selection. To maintain consistency and ensure faithful assessment of the MM transcriptome, we eliminated samples with a high degree of contamination of either myeloid cells or normal plasma cells, as assessed by gene expression signatures.
Results
EMD-1 was documented in 2.41% of TT protocol patients, 4.35% of non-TT protocol patients, and 4.50% of non-protocol patients. The incidence of EMD-2 in patients 5 years after autologous stem cell transplantation was 3.43% in TT protocol patients, 5.2% in non-TT protocol patients, and 7.24% in non-protocol patients. The most common sites of EMD-1 included the chest wall, liver, lymph nodes, skin/soft tissue, and paraspinal area whereas there was a preponderance of liver involvement in EMD-2 (Table 1).
Table 1.
Sites of extramedullary disease.
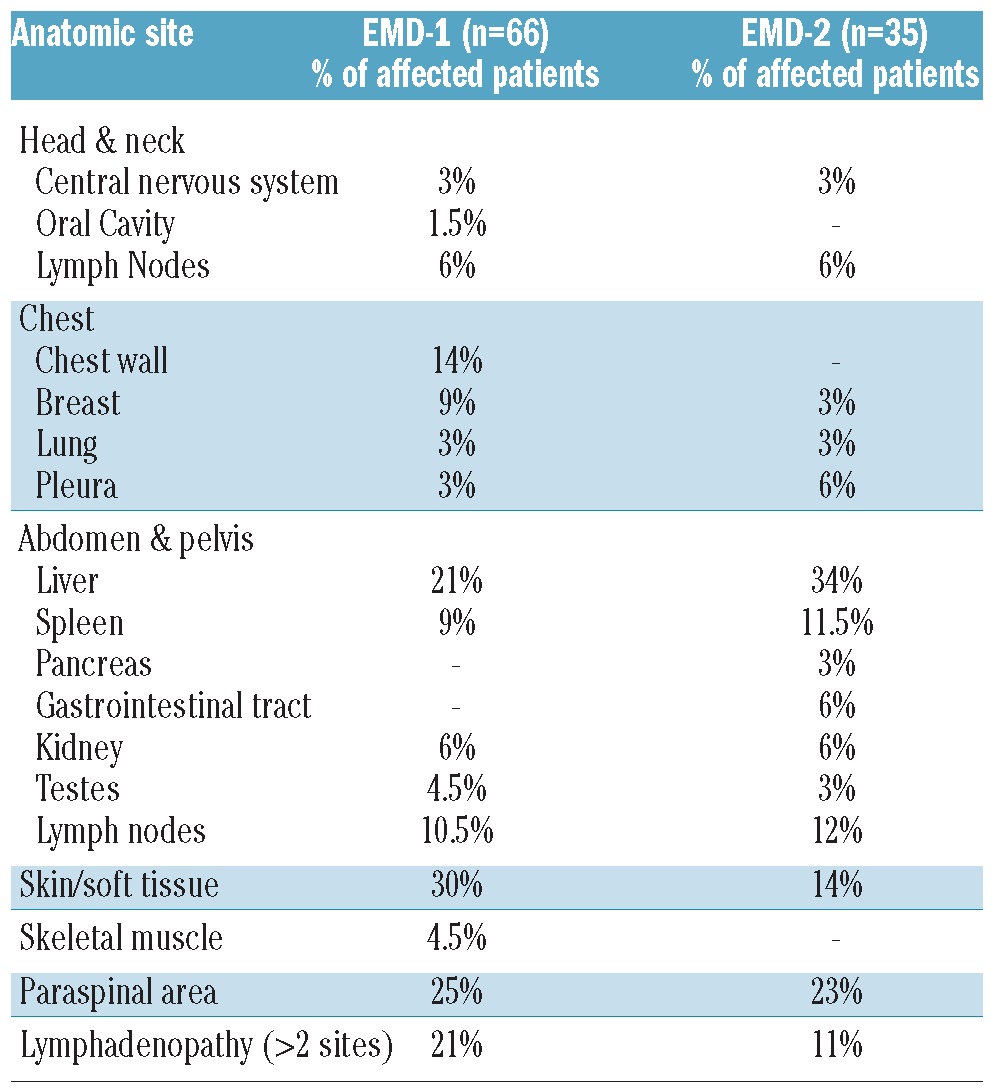
Baseline characteristics were compared between EMD-1 patients and non-EMD patients using Fisher's exact or χ2 tests, depending on group sizes. In univariate analysis, EMD-1 was significantly linked to GEP-defined risk, pretransplant cytogenetic abnormalities, low levels of hemoglobin, low platelet counts and, only marginally, to elevated lactate dehydrogenase (Table 2). Employing logistic regression for multivariate analysis including GEP analyses, EMD-1 was linked to the MF molecular subtype of myeloma (representing MAF and/or MAFB gene overexpression usually associated with translocation 14;16 and 14;20, respectively) and the PR molecular subtype (PR stands for ‘Proliferation’, representing highly proliferative disease),12 which are more common in GEP-70 defined high-risk myeloma.11 An elevated Centrosome Index, previously reported as a high-risk feature in myeloma,13 was associated with the presence of EMD-1.
Table 2.
Analysis of factors related to extramedullary disease at baseline.*
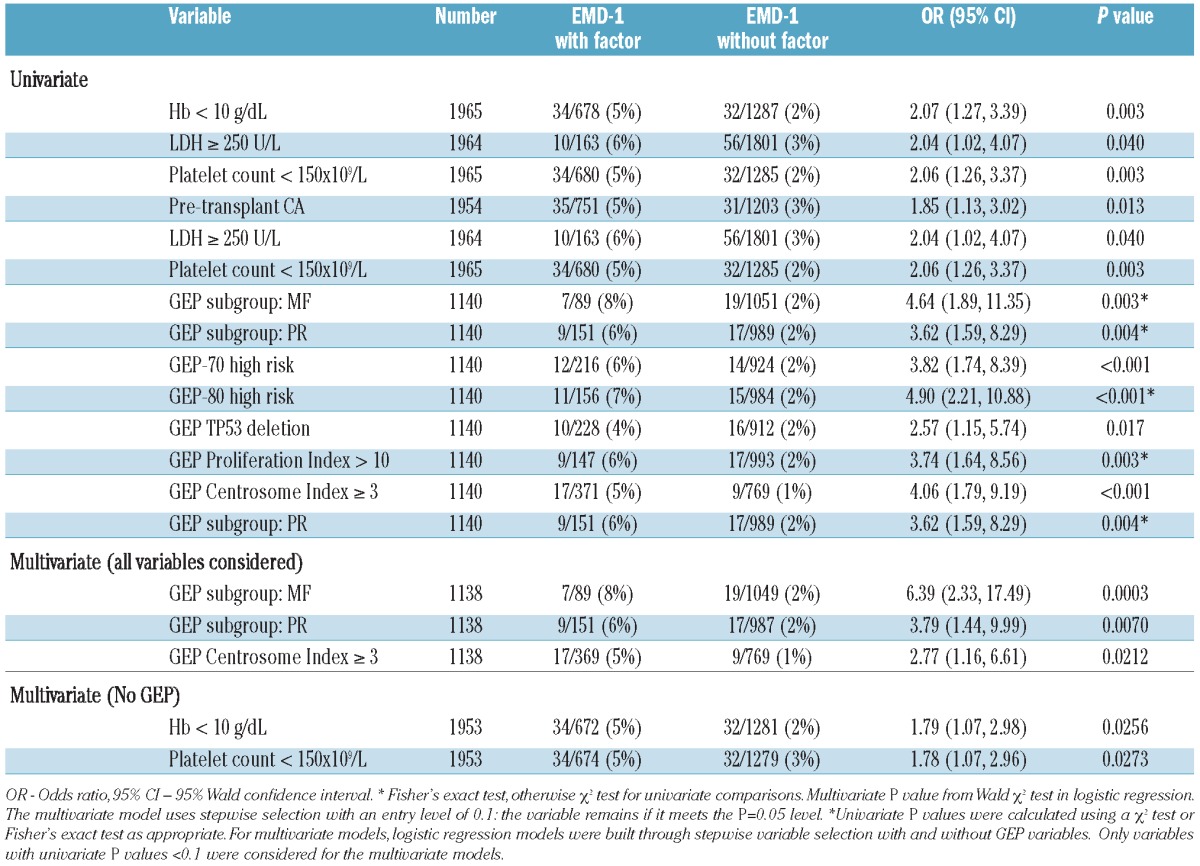
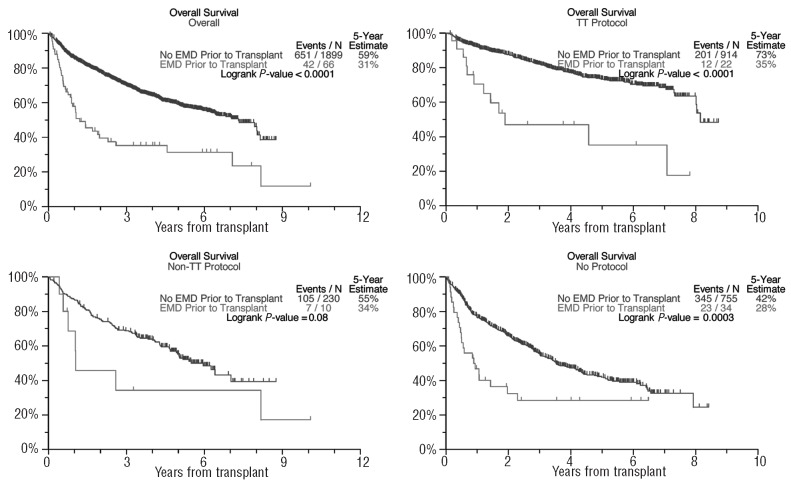
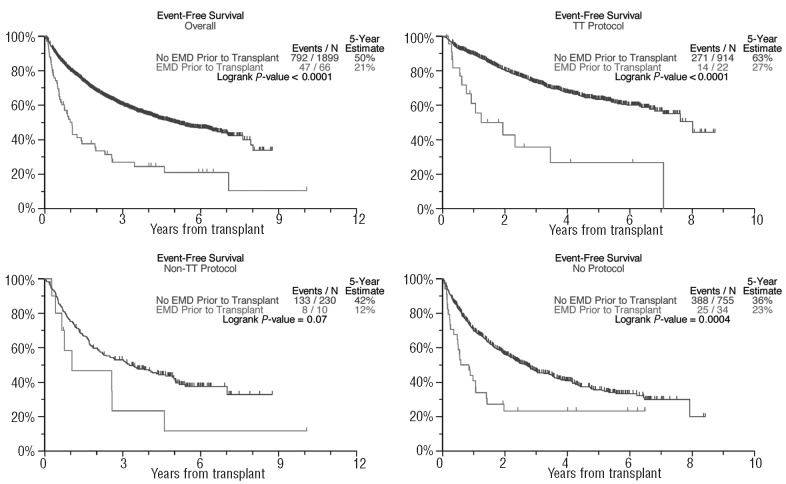
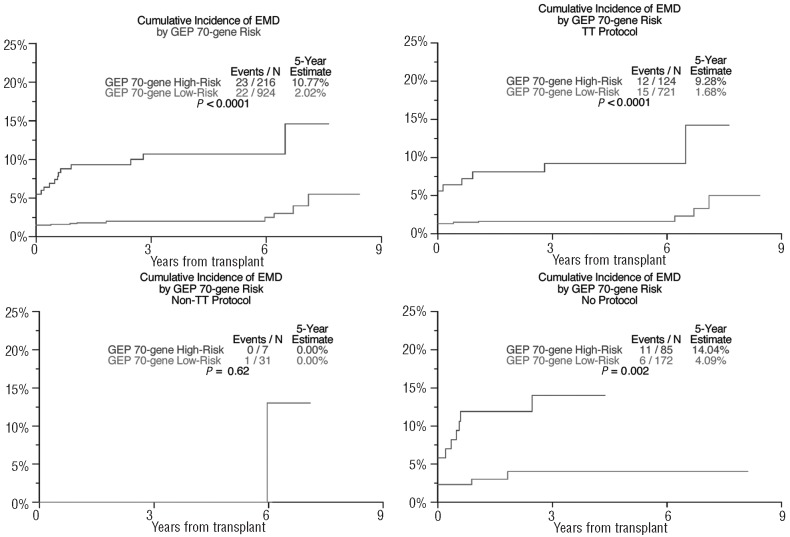
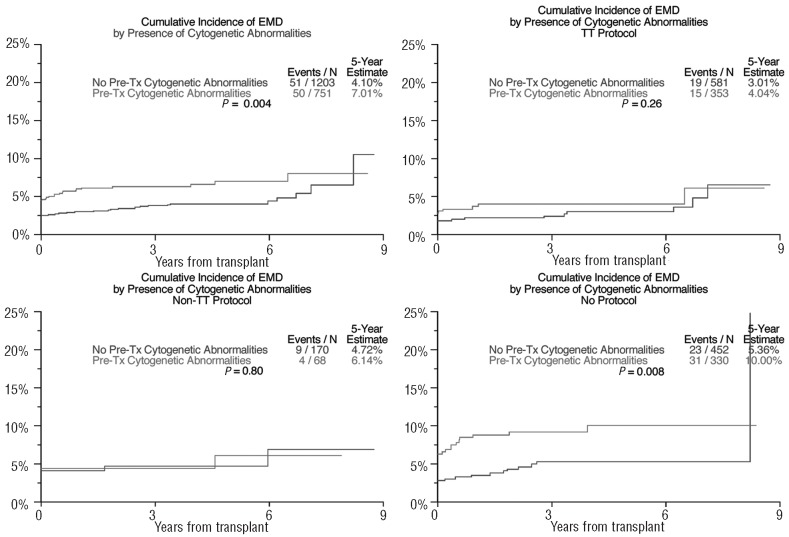
In the absence of differences in complete response frequency, the overall survival was shorter in patients with EMD-1 than in non-EMD patients (31% versus 59% after 5 years, P<0.001; Figure 1), as was progression-free survival in all three treatment groups (50% versus 21% after 5 years, P<0.001; Figure 2). The cumulative incidence of EMD (EMD-1 and EMD-2 combined) at 5 years posttransplant was higher among the GEP-defined high-risk patients (10.8% versus 2.0%, P<0.001, Figure 3) as well as among patients with pre-transplant cytogenetic abnormalities (7.0% versus 4.1%, P=0.004; Figure 4). Low hemoglobin levels and low platelet counts prior to transplant were also associated with increased incidences of EMD in all three treatment groups (8.9% versus 3.4%, P<0.001, and 8.6% versus 3.4%, P<0.001, respectively).
Figure 1.
Overall survival curves based on extramedullary disease status.
Figure 2.
Progression-free survival curves based on extramedullary disease status.
Figure 3.
Cumulative incidence of extramedullary disease by baseline GEP-70-defined risk. (Includes both EMD-1 and EMD-2: the initial incidence at transplant represents patients with EMD-1 while the cumulative incidence following transplant indicates both EMD-1 and EMD-2.)
Figure 4.
Cumulative Incidence of extramedullary disease by baseline cytogenetic abnormalities. (Includes both EMD-1 and EMD-2: the initial incidence at transplant represents patients with EMD-1 while the cumulative incidence following transplant indicates both EMD-1 and EMD-2.)
Discussion
MM usually evolves from a benign precursor condition referred to as monoclonal gammopathy of undetermined significance,14 the documentation of which has recently been further validated.15 Advances in therapy have markedly prolonged the median survival of patients with MM, which was less than 3 years with standard melphalan-prednisone–based therapy, 5 to 7 years with high-dose melphalan-based autologous hematopoietic progenitor cell-supported transplantation, and is now more than 10 years with combinations of high-dose melphalan-based autologous hematopoietic progenitor cell-supported transplantation and novel anti-angiogenic and immunomodulatory agents, as in the Arkansas TT protocols.16 With increasing awareness of this rare malignancy, more patients present with smoldering or asymptomatic MM.17 Nearly 80% of patients are anticipated to achieve some response to therapy, but the duration of disease control varies with the treatment applied and, importantly, with biological and genetic features. Among the latter, metaphase-derived cytogenetic abnormalities,18 critical subgroups detected by interphase fluorescence in situ hybridization19 and, most discriminatory, global GEP-based molecular subgroup designation and prognostic risk annotation can define MM entities with vastly different clinical outcomes.11 With the recent encouraging data suggesting an important role of novel imaging techniques3,4,8 as prognostic instruments at the time of diagnosis, the inclusion of such investigations in initial disease work-up is likely to uncover uncommon manifestations of MM such as EMD.
There are limited data on the true incidence and biology of EMD in MM. Two previous publications reported the incidence of EMD-1 and EMD-2 to be 15% and 20%, respectively.5,6 In a large study of 1003 consecutive MM patients,7 it was observed that EMD was present in 13% of cases (7% EMD-1 and 6% EMD-2). Bladè et al.20 recently reviewed the available data on extramedullary myeloma, distinguishing the EMD that may occur from direct skeletal extension into soft tissue from the EMD resulting from hematogenous spread. It may be postulated that EMD develops as a result of “bone marrow escape” of a MM subclone with either decreased cell adhesion or that acquires characteristics of the granulocytic lineage as observed in primary plasma cell leukemia.21 There is further acquisition of mutations such as k-ras22 and deletion 17p23 in EMD-2 when comparing biological features with concomitant bone marrow at the time of disease relapse.
Although published literature reports no differences in initial response to therapy in patients with EMD when treated with melphalan-based autologous stem cell transplants, 7,24 these patients appear to have a shorter progression-free survival even when treated with novel agents. In a recent report from the Spanish PETHEMA group, an upfront comparison was made of patients treated with three induction regimens: (i) thalidomide/dexamethasone, (ii) bortezomib/thalidomide/dexamethasone and (iii) vincristine/carmustine/melphalan/cyclophosphamide plus prednisone/vincristine/carmustine/adriamycin/bortezomib. EMD was reported in 18% of patients across the protocol with higher progressive disease (34% versus 12%, P=0.0002), with the lowest rate of progressive disease being observed in the bortezomib/thalidomide/dexamethasone arm.25
Our data show that EMD-1 is associated with poor progression-free and overall survival regardless of whether or not the patients were treated on TT protocols. EMD-1 was more frequent among patients with high-risk features, including both 70-gene and 80-gene risk models, the MF molecular subgroup (representing MAF and/or MAFB gene over-expression usually associated with translocation 14;16 and 14;20, respectively) and the PR molecular subgroup (representing highly proliferative disease). The cumulative incidence of EMD was significantly increased in patients who had GEP-defined high-risk disease at baseline and baseline cytogenetic abnormalities and was associated with a grave prognosis. Data published from the Mayo Clinic13 showed that poor prognostic genetic markers are associated with centrosome amplification. We found centrosome amplification was associated with a higher incidence of EMD. While uncommon at disease progression or relapse, EMD-2 represents a common terminal pathway in MM and occurs preponderantly in the liver.
Based on the published literature and the data presented here, EMD is a poor prognostic marker in both newly diagnosed and relapsed MM patients and, therefore, is a therapeutic challenge even in the era of novel agents. The authors have collected GEP data from both EMD-1 and EMD-2 sites. Additional studies comparing baseline GEP in EMD and non-EMD patients and comparing baseline GEP and EMD-GEP in patients who develop EMD are underway to understand the distinctive biology of the subset of patients with EMD. As the field moves towards more individualized therapies even within a specific cancer, GEP studies may help to identify EMD-unique gene(s) that could be amenable to targeted agent development.
Acknowledgments
we would like to thank Mr. Nathan Petty, Ms. Susan Panozzo, Mr. Doug Steward, Mr. Clyde Bailey, the UAMS-MIRT data management team, the UAMS-MIRT nursing staff, referring physicians and our patients without whom this body of work would not have been possible. The manuscript was edited by Mary Dornhoffer, Office of Grants and Scientific Publications, University of Arkansas for Medical Sciences.
Funding: supported by a grant from the National Cancer Institute, National Institutes of Health (grant number CA 55813).
Footnotes
Authorship and Disclosures: The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Kumar S, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008; 111(5):2516-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez-Lopez J, Blade J, Mateos MV, Grande C, Alegre A, García-Laraña J, et al. Long-term prognostic significance of response in multiple myeloma after stem cell transplantation. Blood. 2011;118(3):529-34 [DOI] [PubMed] [Google Scholar]
- 3.Usmani SZ, Mitchell A, Sexton R, Panozzo S, Nair B, Waheed Y, et al. Implications of serial magnetic resonance imaging and positron emission tomography scanning for survival of untreated myeloma patients treated with total therapy 3. Blood. 2011;118(21): abstract 3082. [Google Scholar]
- 4.Zamagni E, Patriarca F, Nanni C, Zannetti B, Englaro E, Pezzi A, et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood. 2011;118(23):5989-95 [DOI] [PubMed] [Google Scholar]
- 5.Blade J, Lust JA, Kyle RA. Immunoglobulin D multiple myeloma: presenting features, response to therapy, and survival in a series of 53 cases. J Clin Oncol. 1994;12(11):2398-404 [DOI] [PubMed] [Google Scholar]
- 6.Blade J, Kyle RA, Greipp PR. Presenting features and prognosis in 72 patients with multiple myeloma who were younger than 40 years. Br J Haematol. 1996;93(2):345-51 [DOI] [PubMed] [Google Scholar]
- 7.Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010; 21(2):325-30 [DOI] [PubMed] [Google Scholar]
- 8.Bartel TB, Haessler J, Brown TL, Shaughnessy JD, Jr, van Rhee F, Anaissie E, et al. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood. 2009;114(10):2068-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kühnemund A, Liebisch P, Bauchmüller K, zur Hausen A, Veelken H, Wäsch R, et al. ‘Light-chain escape-multiple myeloma’-an escape phenomenon from plateau phase: report of the largest patient series using LCmonitoring. J Cancer Res Clin Oncol. 2009;135(3):477-84 [DOI] [PubMed] [Google Scholar]
- 10.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695-706 [DOI] [PubMed] [Google Scholar]
- 11.Zhan F, Hardin J, Kordsmeier B, Bumm K, Zheng M, Tian E, et al. Global gene expression profiling of multiple myeloma, monoclonal gammopathy of undetermined significance, and normal bone marrow plasma cells. Blood. 2002; 99(5):1745-57 [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, Barlogie B, Shaughnessy JD., Jr The molecular characterization and clinical management of multiple myeloma in the post-genome era. Leukemia. 2009;23(11):1941-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chng WJ, Ahmann GJ, Henderson K, Santana-Davila R, Greipp PR, Gertz MA, et al. Clinical implication of centrosome amplification in plasma cell neoplasm. Blood. 2006;107(9):3669-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002. 21;346(8):564-9 [DOI] [PubMed] [Google Scholar]
- 15.Landgren O, Kyle RA, Pfeiffer RM. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113(22):5412-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barlogie B, Attal M, Crowley J, van Rhee F, Szymonifka J, Moreau P, et al. Long-term follow-up of autotransplantation trials for multiple myeloma: update of protocols conducted by the Intergroupe Francophone du Myelome, Southwest Oncology Group, and University of Arkansas for Medical Sciences. J Clin Oncol. 2010;28(7):1209-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyle RA, Durie BG, Rajkumar SV, Landgren O, Blade J, Merlini G, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24(6):1121-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avet-Loiseau H, Soulier J, Fermand JP, Yakoub-Agha I, Attal M, Hulin C, et al. Impact of high-risk cytogenetics and prior therapy on outcomes in patients with advanced relapsed or refractory multiple myeloma treated with lenalidomide plus dexaméthasone. Leukemia. 2010;24(3):623-8 [DOI] [PubMed] [Google Scholar]
- 19.Dewald GW, Therneau T, Larson D, Lee YK, Fink S, Smoley S, et al. Relationship of patient survival and chromosome anomalies detected in metaphase and/or interphase cells at diagnosis of myeloma. Blood. 2005;106(10):3553-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bladé J, Fernández de Larrea C, Rosiñol L, Cibeira MT, Jiménez R, Powles R. Soft-tissue plasmacytomas in multiple myeloma: incidence, mechanisms of extramedullary spread, and treatment approach. J Clin Oncol. 2011;29(28):3805-12 [DOI] [PubMed] [Google Scholar]
- 21.Usmani SZ, Nair B, Qu P, Hansen E, Zhang Q, Petty N, et al. Primary plasma cell leukemia: clinical and laboratory presentation, gene-expression profiling, and clinical outcome with Total Therapy protocols. Leukemia. 2012. April 17 10.1038/leu.2012.107 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasmussen T, Kuehl M, Lodahl M, Johnsen HE, Dahl IM. Possible roles for activating RAS mutations in the MGUS to MM transition and in the intramedullary to extramedullary transition in some plasma cell tumors. Blood. 2005;105(1):317-23 [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Anglada L, Gutierrez NC, Garcia JL, Mateos MV, Flores T, San Miguel JF. P53 deletion may drive the clinical evolution and treatment response in multiple myeloma. Eur J Haematol. 2010;84(4):359-61 [DOI] [PubMed] [Google Scholar]
- 24.Wu P, Davies FE, Boyd K, Thomas K, Dines S, Saso RM, et al. The impact of extramedullary disease at presentation on the outcome of myeloma. Leuk Lymphoma. 2009;50(2):230-5 [DOI] [PubMed] [Google Scholar]
- 25.Rosinol L, Cibeira MT, Martinez J, Esteve J, Aymerich M, Rozman M, et al. Thalidomide/dexamethasone (TD) vs. bortezomib (Velcade)/thalidomide/dexamethasone (VTD) vs. VBMCP/VBAD/Velcade as induction regimens prior to autologous stem cell transplantation (ASCT) in multiple myeloma (MM): results of a phase III PETHEMA/GEM trial. Blood. 2009;114(26):abstract 130. [Google Scholar]