Abstract
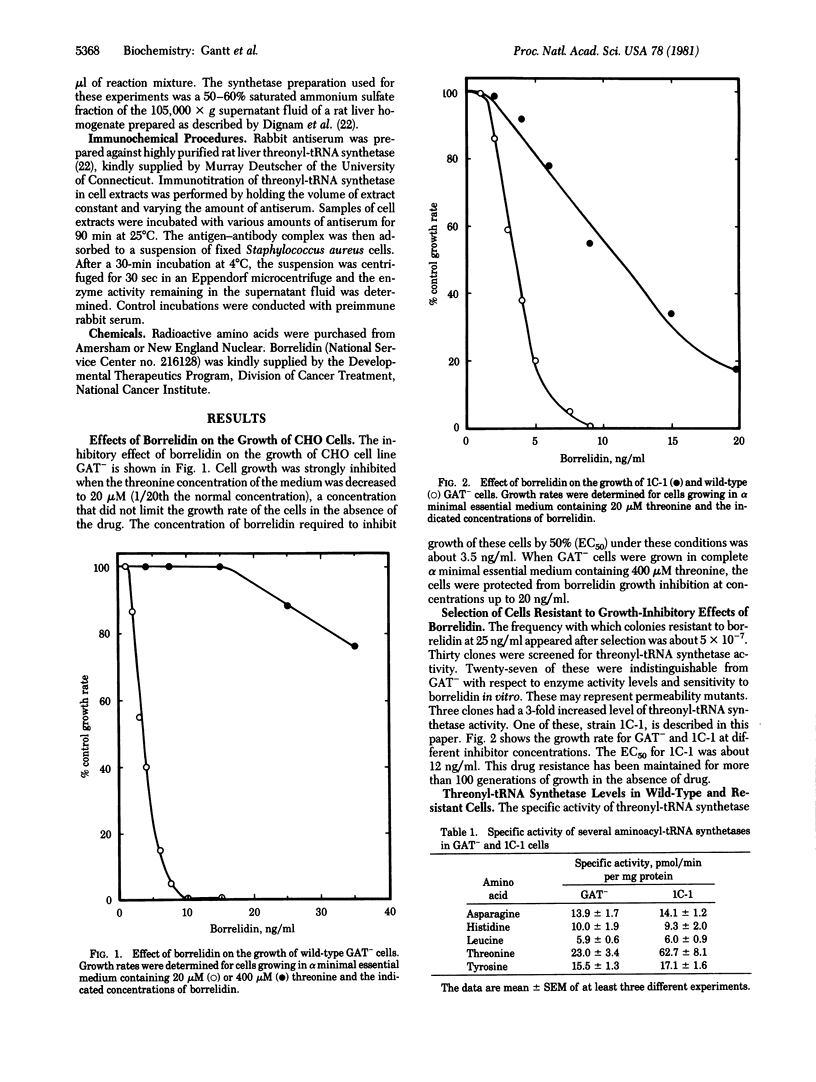
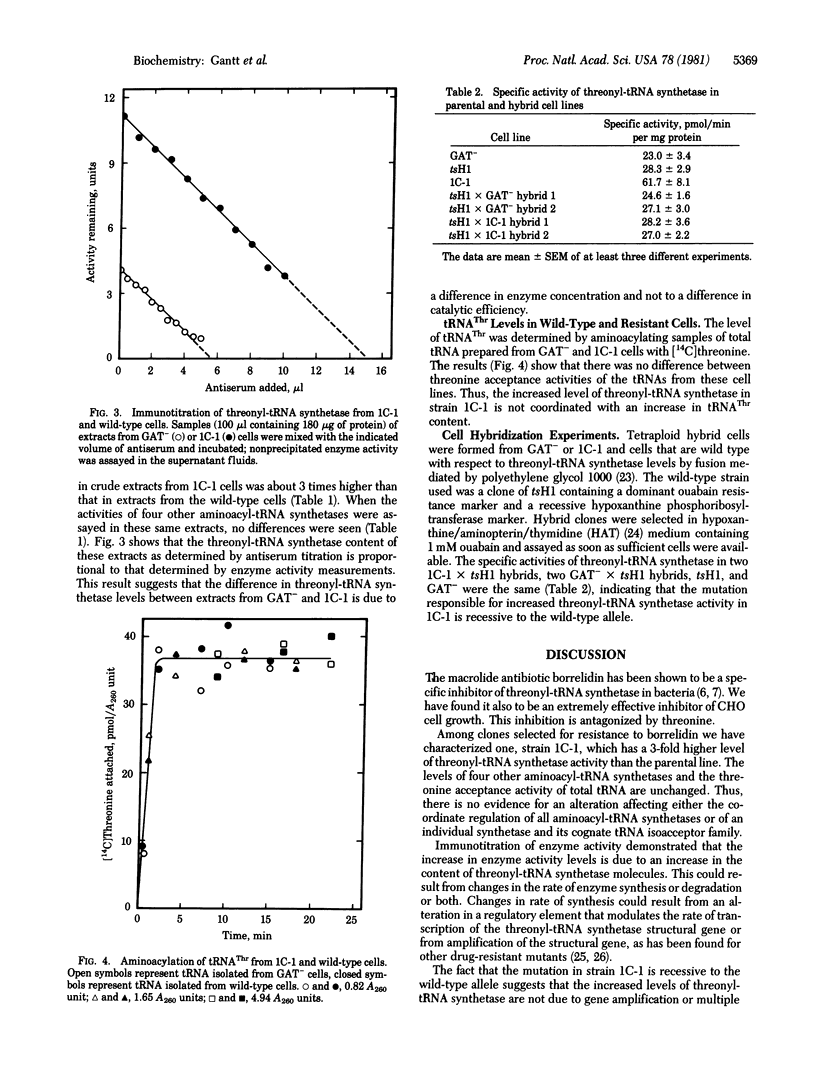
The growth of Chinese hamster ovary cells in medium containing reduced concentrations of threonine is inhibited by borrelidin, a macrolide antibiotic. Borrelidin-resistant clones have been isolated after ethyl methanesulfonate mutagenesis. One clone, 1C-1, has a 3-fold increased level of threonyl-tRNA synthetase [L-threonine:tRNAThr ligase (AMP-forming), EC 6.1.1.3] as determined by both activity measurements and antiserum titrations. The levels of four other aminoacyl-tRNA synthetases and of tRNAThr are the same in strain 1C-1 and in the wild-type parent. The phenotype of increased threonyl-tRNA synthetase activity is recessive to wild type in cell hybrids.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrulis I. L., Arfin S. M. Methods for determining the extent of tRNA aminoacylation in vivo in cultured mammalian cells. Methods Enzymol. 1979;59:268–271. doi: 10.1016/0076-6879(79)59089-2. [DOI] [PubMed] [Google Scholar]
- Andrulis I. L., Chiang C. S., Arfin S. M., Miner T. A., Hatfield G. W. Biochemical characterization of a mutant asparaginyl-tRNA synthetase from Chinese hamster ovary cells. J Biol Chem. 1978 Jan 10;253(1):58–62. [PubMed] [Google Scholar]
- Baer M., Low K. B., Söll D. Regulation of the biosynthesis of aminoacyl-transfer ribonucleic acid synthetases and of transfer ribonucleic acid in Escherichia coli. V. Mutants with increased levels of valyl-transfer ribonucleic acid synthetase. J Bacteriol. 1979 Jul;139(1):165–175. doi: 10.1128/jb.139.1.165-175.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A., Morgan S., Low K. B., Söll D. Regulation of the biosynthesis of aminoacyl-transfer ribonucleic acid synthetases and of transfer ribonucleic acid in Escherichia coli. VI. Mutants with increased levels of glutaminyl-transfer ribonucleic acid synthetase and of glutamine transfer ribonucleic acid. J Bacteriol. 1979 Jul;139(1):176–184. doi: 10.1128/jb.139.1.176-184.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Rhodes D. G., Deutscher M. P. Purification and structural characterization of rat liver threonyl transfer ribonucleic acid synthetase. Biochemistry. 1980 Oct 28;19(22):4978–4984. doi: 10.1021/bi00563a007. [DOI] [PubMed] [Google Scholar]
- Fröhler J., Rechenmacher A., Thomale J., Nass G., Böck A. Genetic analysis of mutations causing borrelidin resistance by overproduction of threonyl-transfer ribonucleic acid synthetase. J Bacteriol. 1980 Sep;143(3):1135–1141. doi: 10.1128/jb.143.3.1135-1141.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillin F. D., Roufa D. J., Beaudet A. L., Caskey C. T. 8-Azaguanine resistance in mammalian cells. I. Hypoxanthine-guanine phosphoribosyltransferase. Genetics. 1972 Oct;72(2):239–252. doi: 10.1093/genetics/72.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Vanatta P. R., Fresco J. R. Metabolic regulation of aminoacyl-tRNA synthetase biosynthesis in bakers' yeast. J Biol Chem. 1977 Feb 10;252(3):878–882. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaRossa R. A., Mao J. I., Low K. B., Söll D. Regulation of biosynthesis of aminoacyl-tRNA synthetases and of tRNA in Escherichia coli. III. Biochemical characterization of regulatory mutants affecting leucyl-tRNA synthetase levels. J Mol Biol. 1977 Dec 25;117(4):1049–1059. doi: 10.1016/s0022-2836(77)80012-0. [DOI] [PubMed] [Google Scholar]
- LaRossa R., Vögell G., Low K. B., Söll D. Regulation of biosynthesis of aminoacyl-tRNA synthetases and of tRNA in Escherichia coli. II. Isolation of regulatory mutants affecting leucyl-tRNA synthetase levels. J Mol Biol. 1977 Dec 25;117(4):1033–1048. doi: 10.1016/s0022-2836(77)80011-9. [DOI] [PubMed] [Google Scholar]
- McBurney M. W., Whitmore G. F. Isolation and biochemical characterization of folate deficient mutants of Chinese hamster cells. Cell. 1974 Jul;2(3):173–182. doi: 10.1016/0092-8674(74)90091-9. [DOI] [PubMed] [Google Scholar]
- Molnar S. J., Thompson L. H., Lofgren D. J., Rauth A. M. Isolation and characterization of revertants of the mammalian temperature sensitive leucyl-tRNA synthetase mutant tsHl. J Cell Physiol. 1979 Feb;98(2):327–339. doi: 10.1002/jcp.1040980209. [DOI] [PubMed] [Google Scholar]
- Neihardt F. C., Parker J., McKeever W. G. Function and regulation of aminoacyl-tRNA synthetases in prokaryotic and eukaryotic cells. Annu Rev Microbiol. 1975;29:215–250. doi: 10.1146/annurev.mi.29.100175.001243. [DOI] [PubMed] [Google Scholar]
- Paetz W., Nass G. Biochemical and immunological characterization of threonyl-tRNA synthetase of two borrelidin-resistant mutants of Escherichia coli K12. Eur J Biochem. 1973 Jun;35(2):331–337. doi: 10.1111/j.1432-1033.1973.tb02843.x. [DOI] [PubMed] [Google Scholar]
- Palatnik C. M., Katz E. R. Isolation and characterization of transfer RNAs from Dictyostelium discoideum during growth and development. J Biol Chem. 1977 Jan 25;252(2):694–703. [PubMed] [Google Scholar]
- Parker J., Neidhardt F. C. Metabolic regulation of aminoacyl-tRNA synthetase formation in bacteria. Biochem Biophys Res Commun. 1972 Oct 17;49(2):495–501. doi: 10.1016/0006-291x(72)90438-x. [DOI] [PubMed] [Google Scholar]
- Pizer L. I., McKitrick J., Tosa T. Characterization of a mutant of E. coli with elevated levels of seryl-tRNA synthetase. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1351–1357. doi: 10.1016/0006-291x(72)90615-8. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Kaufman R. J., Alt F. W., Kellems R. F. Gene amplification and drug resistance in cultured murine cells. Science. 1978 Dec 8;202(4372):1051–1055. doi: 10.1126/science.715457. [DOI] [PubMed] [Google Scholar]
- Siminovitch L. On the nature of hereditable variation in cultured somatic cells. Cell. 1976 Jan;7(1):1–11. doi: 10.1016/0092-8674(76)90249-x. [DOI] [PubMed] [Google Scholar]
- Stanners C. P., Wightman T. M., Harkins J. L. Effect of extreme amino acid starvation on the protein synthetic machinery of CHO cells. J Cell Physiol. 1978 May;95(2):125–137. doi: 10.1002/jcp.1040950202. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Harkins J. L., Stanners C. P. A mammalian cell mutant with a temperature-sensitive leucyl-transfer RNA synthetase. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3094–3098. doi: 10.1073/pnas.70.11.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Padgett R. A., Stark G. R. Gene amplification causes overproduction of the first three enzymes of UMP synthesis in N-(phosphonacetyl)-L-aspartate-resistant hamster cells. J Biol Chem. 1979 Sep 10;254(17):8679–8689. [PubMed] [Google Scholar]
- Wasmuth J. J., Hill J. M., Vock L. S. Biochemical and genetic evidence for a new class of emetine-resistant Chinese hamster cells with alterations in the protein biosynthetic machinery. Somatic Cell Genet. 1980 Jul;6(4):495–516. doi: 10.1007/BF01539152. [DOI] [PubMed] [Google Scholar]


