Marker assisted backcross breeding for combining three resistance genes (xa13 and Xa21 for Bacterial Blight, Pi54 for blast) and a major QTL (qSBR11-1 for resistance to Sheath blight) in Basmati rice.
Abstract
Background and aims
Basmati rice grown in the Indian subcontinent is highly valued for its unique culinary qualities. Production is, however, often constrained by diseases such as bacterial blight (BB), blast and sheath blight (ShB). The present study developed Basmati rice with inbuilt resistance to BB, blast and ShB using molecular marker-assisted selection.
Methodology
The rice cultivar ‘Improved Pusa Basmati 1’ (carrying the BB resistance genes xa13 and Xa21) was used as the recurrent parent and cultivar ‘Tetep’ (carrying the blast resistance gene Pi54 and ShB resistance quality trait loci (QTL), qSBR11-1) was the donor. Marker-assisted foreground selection was employed to identify plants possessing resistance alleles in the segregating generations along with stringent phenotypic selection for faster recovery of the recurrent parent genome (RPG) and phenome (RPP). Background analysis with molecular markers was used to estimate the recovery of RPG in improved lines.
Principal results
Foreground selection coupled with stringent phenotypic selection identified plants homozygous for xa13, Xa21 and Pi54, which were advanced to BC2F5 through pedigree selection. Marker-assisted selection for qSBR11-1 in BC2F5 using flanking markers identified seven homozygous families. Background analysis revealed that RPG recovery was up to 89.5%. Screening with highly virulent isolates of BB, blast and ShB showed that the improved lines were resistant to all three diseases and were on a par with ‘Improved Pusa Basmati 1’ for yield, duration and Basmati grain quality.
Conclusions
This is the first report of marker-assisted transfer of genes conferring resistance to three different diseases in rice wherein genes xa13 and Xa21 for BB resistance, Pi54 for blast resistance, and a major QTL qSBR11-1 have been combined through marker-assisted backcross breeding. In addition to offering the potential for release as cultivars, the pyramided lines will serve as useful donors of gene(s) for BB, blast and ShB in future Basmati rice breeding programmes.
Introduction
Basmati rice cultivated in the north-western part of the Indo-Gangetic plains of the Indian subcontinent is highly valued in the international market due to its unique combination of aroma, grain, cooking and eating qualities (Singh et al. 1988, 2000). Basmati rice worth $2 billion was exported from India during 2009–10 (Website 1). Basmati rice germplasm including improved cultivars are highly susceptible to biotic and abiotic stresses. Among the biotic stresses, bacterial blight (BB) caused by Xanthomonas oryzae pv. oryzae, blast caused by Magnoporthae oryzae and sheath blight (ShB) caused by Rhizoctonia solani not only cause severe yield losses but also impair the quality of the rice grain (Singh et al. 2011).
Globally, rice blast causes annual yield losses of up to 50 % (Scardaci et al. 1997). Sheath blight also causes major crop loss worldwide (Ou 1985), and in India yield loss of up to 54.3 % has been reported (Chahal et al. 2003). In the case of Basmati rice, economic losses from reduced yield are amplified by a severe deterioration in quality. Although these diseases can be managed by fungicides, breeding resistant varieties with durable resistance is a more ecologically sound and sustainable approach.
A large number of genes conferring resistance have been reported. These include a major gene Pi54. This encodes a protein containing a nucleotide-binding leucine-rich repeat domain that provides resistance to the predominant races of the pathogen in India (Sharma et al. 2005). Resistance to the ShB pathogen is quantitative in nature (Pinson et al. 2005) and 16 quality trait loci (QTLs) providing ShB resistance across different genetic backgrounds have been identified in different QTL mapping studies (Srinivasachary et al. 2011). qSBR11-1 is a major QTL that has been found to be effective against the ShB pathogen consistently over time and at different locations (Channamallikarjuna et al. 2010). ‘Tetep’, an indica rice cultivar from Vietnam, is the source of resistance to both blast (Pi54) and ShB (qSBR11-1) diseases. In addition, ‘Tetep’ is an invaluable donor of several other resistance genes, including Xa1, Xa2, Xa12 and Xa16 for BB resistance (Nelson et al. 1994; Goel et al. 1998); Pita (Jia et al. 2003), Pi1(t), Pi4a(t), Pi4b(t), Pi3(t) (Inukai et al. 1994), Pi-kh (Xu et al. 2008) and Pi54 (Rai et al. 2011) for blast resistance; and 10 other QTLs for ShB resistance (Channamallikarjuna et al. 2010).
The long history of rice cultivation and favourable climatic factors in tropical Asia are conducive to many diseases (including BB, blast and ShB) that pose a constant threat to rice production (Khush 1989). Consequently, breeding cultivars with multiple disease resistance would help sustain rice productivity. Marker-assisted selection (MAS) is an approach that has enabled efficient and precise transfer of genes/QTL(s) in many crop species, and offers a fast and efficient alternative to conventional breeding and selection methods. Marker-assisted selection has been shown to be highly efficient for the precise transfer of major genes for BB resistance (Huang et al. 1997; Joseph et al. 2004; Gopalakrishnan et al. 2008; Basavaraj et al. 2010), blast resistance (Singh et al. 2012) and QTLs for ShB resistance (Tan et al. 2005; Pinson et al. 2008; Zuo et al. 2008; Wang et al. 2012) in rice.
Marker-assisted backcross breeding (MABB) coupled with phenotypic selection for agronomic, grain and cooking quality traits has been used to incorporate BB resistance genes xa13 and Xa21 into ‘Pusa Basmati 1’ (Joseph et al. 2004). One of the improved lines was released as ‘Improved Pusa Basmati 1’ for commercial cultivation in 2007 (Gopalakrishnan et al. 2008), and this is one of the first products of MAS to be used in India. However, the susceptibility of ‘Improved Pusa Basmati 1’ and other Basmati rice varieties to rice blast and ShB disease remains a major concern. These problems are further accentuated by the non-availability of resistant sources for these diseases in the Basmati germplasm.
In the current study, the cultivar ‘Tetep’ was used as donor mainly for the transfer of the blast resistance gene, Pi54, through marker-assisted foreground selection in combination with stringent phenotypic selection for recovery of agro-morphological, grain and cooking quality traits of the recurrent parent ‘Improved Pusa Basmati 1’. However, ‘Tetep’, the donor for the blast resistance gene, Pi54, also possesses a major QTL for ShB resistance, qSBR11-1, on the arm of chromosome 11, in which Pi54 is located. Therefore, the superior advanced lines possessing both BB- and blast-resistant genes were also analysed for the possible introgression of this major QTL for ShB resistance through MAS. Here we report the use of MAS for combining resistance to three diseases, namely BB, blast and ShB, in Basmati rice.
Materials and methods
Plant materials and development of improved lines
The Basmati rice cultivar ‘Improved Pusa Basmati 1’, resistant to BB, was used as the recurrent parent, while the cultivar ‘Tetep’ was used as donor for the blast resistance gene, Pi54, and ShB resistance QTL, qSBR11-1. A single F1 plant was backcrossed with ‘Improved Pusa Basmati 1’ to generate the BC1F1s (the backcross series was designated as ‘Pusa1608’). Marker-assisted foreground selection was employed using gene-linked/gene-based markers for xa13, Xa21 and Pi54 to identify the plant homozygous for the genes xa13 and Xa21, and heterozygous for the gene Pi54. Further, the selected plants were subjected to stringent phenotypic selection for agro-morphological, grain and cooking quality traits. The desirable BC1F1 plant with maximum recovery of the recurrent parent phenome (RPP) was backcrossed to develop the BC2F1 generation. The BC2F1 plants were also subjected to foreground selection followed by phenotypic selection to identify five plants homozygous for xa13 and Xa21, heterozygous for Pi54 and with maximum recovery for RPP. These plants were then selfed to generate BC2F2 populations. In the BC2F2 generation, plants homozygous for all three genes were identified and then advanced to the BC2F5 generation through the pedigree method of selection.
DNA extraction and polymerase chain reaction amplification
Total genomic DNA was extracted by the micro-extraction protocol of Prabhu et al. (1998). Polymerase chain reaction (PCR) was performed in a thermal cycler (G-Storm, Somerset, UK) using a 10 μL total reaction volume as described previously (Basavaraj et al. 2010). This contained 30 ng μL−1 of template DNA, 5 pmol of each primer (synthesized from Sigma Inc., St. Louis, MO, USA), 1.5 mM MgCl2, 0.2 mM dNTPs (MBI, Fermentas, Vilnius, Lithuania) and 0.5 U of Taq polymerase (Bangalore Genei, Bangalore, Karnataka, India). Polymerase chain reaction comprised one cycle of denaturation at 95 °C for 5 min, followed by 35 cycles at 95 °C for 30 s, 55 °C for 30 s and 72 °C for 1 min, with a final extension of 72 °C for 7 min. The amplified products were resolved on 3.5 % Metaphor™ gel (Lonza, Rockland, ME, USA) containing 0.1 µg mL−1 of ethidium bromide (Amresco, Solon, OH, USA) along with a DNA size standard ladder (MBI, Fermentas) and documented in a Gel Documentation System (Biorad, Hercules, CA, USA).
Molecular marker analysis
Foreground selection
The gene-based markers for BB resistance genes xa13 (xa-13 prom) and Xa21 (pTA248), and the gene-linked marker for blast resistance gene Pi54 (RM206) were used in the foreground selection. The markers RM224, sbq1, sbq11, K39516 and sbq33 (Channamallikarjuna et al. 2010) flanking the ShB resistance QTL qSBR11-1 were analysed for parental polymorphism between ‘Improved Pusa Basmati 1’ and ‘Tetep’, and only one marker, RM224 (27.2 Mb), was found to be polymorphic. Therefore, 10 additional SSR markers from the region flanking (Website 2) qSBR11-1 were subjected to parental polymorphism, of which the marker RM7443 (physical position—28.3 Mb, same as that of the flanking marker, sbq33) was found to be polymorphic between ‘Tetep’ and ‘Improved Pusa Basmati 1’. The foreground selection for ShB resistance QTL qSBR11-1 was carried out using the flanking markers RM224 and RM7443 in the segregating generations. Details of the primer sequence, chromosomal location, fragment size, annealing temperature and physical position are presented in Table 1.
Table 1.
Molecular markers used for foreground selection for BB, blast and ShB resistance genes.
| Trait | Gene/QTL | Marker | LG | MD (cM) | Forward sequence | Reverse sequence | Reference |
|---|---|---|---|---|---|---|---|
| Bacterial blight | xa13 | xa13-prom | 8 | 0.0 | GGCCATGGCTCAGTGTTTAT | GAGCTCCAGCTCTCCAAATG | Singh et al. (2011) |
| Xa21 | pTA248 | 11 | 0.0 | AGACGCGGAAGGGTGGTTCCCGGA | AGACGCGGTAATCGAAGATGAAA | Ronald et al. (1992) | |
| Blast | Pi54 | RM206 | 11 | 0.6 | CCCATGCGTTTAACTATTC | CGTTCCATCGATCCGTATGG | Sharma et al. (2005) |
| Sheath blight | qSBR11-1 | RM224 | 11 | FM | ATCGATCGATCTTCACGAGG | TGCTATAAAAGGCATTCGGG | Channamallikarjuna et al. (2010) |
| RM7443 | 11 | FM | ACACTGTACACCACACTTCAGC | CAGGGAAATGACACTGTCCC |
LG, linkage group; MD, map distance; AT, annealing temperature; FM, flanking marker.
Background analysis
A set of 435 SSR primer pairs distributed uniformly across the 12 chromosomes of the rice genome was selected for a parental polymorphism survey between the donor ‘Tetep’ and the recurrent parent ‘Improved Pusa Basmati 1’ (Website 2). The SSR markers, which were polymorphic, were then used to identify plants with maximum recovery of recurrent parent genome (RPG) in BC2F5 generation. Additionally, markers were added in the 5 Mb region on either side of the genes on the respective carrier chromosomes (chromosomes 8 and 11) in order to identify BC2F5 recombinants with minimum donor segments as well as the target loci. The PCR products were amplified and resolved in 3.5 % Metaphor™ gel. The genomic contribution of the parents in the elite selections was analysed and represented using the software Graphical Geno Types (GGT) Version 2.0 (Van Berloo 1999).
Screening for BB, blast and ShB resistance
Artificial inoculation
A set of 10 plants raised in two replications was screened for BB resistance through artificial inoculation during Kharif (rainy season) 2010 under field conditions at the experimental farm of the Division of Genetics, Indian Agricultural Research Institute (IARI), New Delhi, India. Plants were inoculated with the bacterial suspension at a density of 109 cells/mL at maximum tillering stage using the most virulent isolate ‘Kaul’ of X. oryzae pv. oryzae (Joseph et al. 2004). Five young leaves in each plant were inoculated through the clip inoculation method (Kauffman et al. 1973) and the disease reaction was scored 21 days after inoculation (DAI). The weather during Kharif 2010 was conducive for BB infection with the temperature ranging from 21.8 to 31.9 °C and relative humidity in the range 88.0–100 %. Plants with an average lesion length of up to 6 cm were considered resistant and those with lesion lengths >6 cm were scored as susceptible.
Screening for blast resistance used the four most virulent strains (Singh et al. 2012) of M. oryzae from Basmati-growing regions using standard protocol (Bonman et al. 1986). About 30–40 mL of the spore suspension containing gelatin (0.1 %) and Tween-20 (0.02 %) were sprayed onto seedlings using a glass atomizer. Inoculated seedlings were kept in a humid chamber with the temperature maintained at 25 ± 1 °C. Distilled water was sprinkled three to four times a day to maintain high humidity. The disease reaction was recorded 7 DAI using a 0–5 disease scoring scale (Bonman et al. 1986). The plants exhibiting reactions that scored 0–3 were considered resistant while those showing reactions that scored 4–5 were categorized as susceptible.
The screening for ShB resistance in the advanced breeding lines was carried out on a set of three hills in two replications in separate plots through artificial inoculation under field conditions at the experimental farm of the Division of Genetics, IARI, New Delhi, India. A virulent isolate of R. solani from Kaparthula (Rs-K), which was used for mapping QTLs for ShB resistance in ‘Tetep’ by Channamallikarjuna et al. (2010), was mass multiplied on shoots of the water sedge, Typha angustata. The inoculation was achieved by placing the shoot pieces having mycelium and sclerotia between the tillers in the central region of the rice hills, 5–10 cm above the waterline (Bhaktavatsalam et al. 1978). The weather in New Delhi during Kharif 2010 (as indicated above) was conducive for the development of ShB disease. The phenotypic observations on vertical spread were recorded 10 and 25 DAI. The relative lesion height (RLH) was calculated using the formula: relative lesion height (RLH) = lesion height/plant height × 100.
The average lesion height over two replications (six lesions) consisting of three tillers in each replication after 25 DAI was used for calculating RLH. Based on the RLH, ShB disease reactions of test entries were grouped into six categories according to Ahn et al. (1986). The lines with a disease grade score of 0 were considered as highly resistant, 1 as resistant, 3 as moderately resistant, 5 as moderately susceptible, 7 as susceptible and 9 as highly susceptible.
Field screening for blast resistance at the Uniform Blast Nursery
All the promising lines were evaluated for their reaction to leaf and neck blast at the Uniform Blast Screening Nursery (UBN) at the Agricultural Research Station (ARS), Mugad, in Karnataka (Southwest India) using standard protocol during Kharif 2010 (Singh et al. 2012). A 50-cm-long row of each entry was planted in an upland nursery bed with a row spacing of 10 cm. A row of susceptible check was planted after every five entries and also on the borders to ensure uniform spread of the disease. The weather during Kharif 2010 was conducive for blast infection with a mean temperature of 23.1 °C and a mean relative humidity of 75.1 %, and a maximum humidity of 100 % during the growth period. Data on the blast reaction of the entries were recorded three times using a scale of 0–9 (SES 1996) at 10-day intervals starting 30 days after sowing. The lines with scores of 0–3 were considered resistant, 4–5 as moderately resistant, 6 as moderately susceptible and 7–9 as susceptible.
Evaluation of agronomic performance and grain quality
The advanced lines along with parental lines were planted at a spacing of 15 × 20 cm in a randomized complete block design with three replications, and were evaluated for agronomic traits during Khairf 2010 at the experimental farm of the Division of Genetics, IARI, New Delhi, India. Data on five plants were recorded for various agronomic traits: days to 50 % flowering (DFF), plant height (PH), number of tillers (NT), panicle length (PL), filled grains per panicle (FG/P), spikelet fertility (SF), 1000-grain weight (TW) and yield per plant (Y/P). The analysis of grain quality traits such as grain size, kernel length before cooking (KLBC), kernel length after cooking (KLAC), kernel breadth before cooking (KBBC), kernel breadth after cooking (KBAC), length/breadth ratio (L/B), elongation ratio (ER), alkali spreading value (ASV) and aroma was carried out as described in Basavaraj et al. (2010).
Results
Marker-assisted selection for BB and blast resistance gene(s)
Marker-assisted foreground selection for BB resistance genes xa13 and Xa21, and blast resistance gene Pi54 was carried out in the BC1F1 generation using gene-based/linked markers xa13-prom, pTA248 and RM206, respectively. Among the 188 BC1F1 plants analysed for the presence of the Pi54 gene using RM206, 80 plants were heterozygous for the resistance allele. All 80 plants heterozygous for the Pi54 gene-linked marker were then subjected to foreground selection for the genes xa13 and Xa21 using the respective gene-based markers, which led to the identification of 18 plants homozygous for the two BB resistance genes. The plant exhibiting maximum similarity to the recurrent parent was then backcrossed with the recurrent parent to generate 145 BC2F1 seeds. Based on foreground selection, five BC2F1 plants found to be heterozygous for Pi54 and homozygous for xa13, Xa21 as well as having desirable agro-morphological and grain quality characteristics were advanced to generate BC2F2 populations. Twenty-two plants homozygous for the three genes xa13, Xa21 and Pi54 were identified in BC2F2 populations and advanced to BC2F5 generation through pedigree breeding with selection for superior plant phenotype and grain and cooking quality characters of Basmati rice.
Marker-assisted selection for the major QTL qSBR11-1 for ShB resistance
A total of 22 BC2F5 families homozygous for all three genes were further evaluated for a major QTL for ShB resistance, qSBR11-1, using flanking markers RM224 and RM443. A total of seven families were found to amplify ‘Tetep’-specific alleles for both flanking markers, while one plant was found to be heterozygous for both marker alleles. These seven families possessing the major QTL qSBR11-1 for ShB resistance were also re-confirmed for the presence of Pi54, xa13 and Xa21, and advanced to BC2F6 generation.
Marker-assisted background analysis
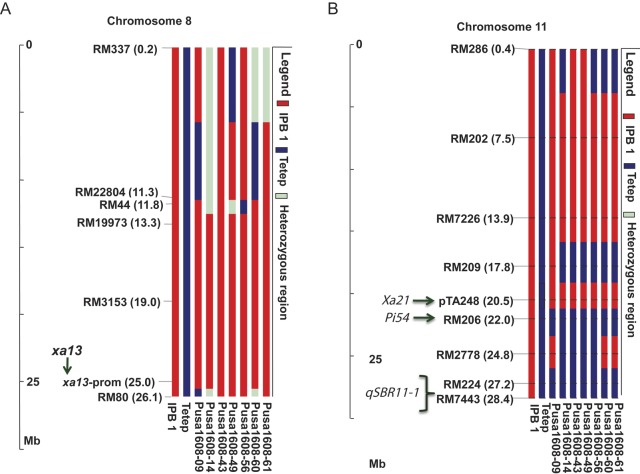
Sixty of the 435 sequence tagged microsatellite site (STMS) markers were found to be polymorphic between the parental lines and were used for background analysis of seven selected BC2F5 families (Table 2). The background analysis of the improved lines indicated RPG recovery ranging from 76.25 % in ‘Pusa1608-06-16-3-60’ to 89.50 % in ‘Pusa1608-06-7-10-14’. To identify the recombinants with least donor segment introgression, 22 markers were identified from the flanking regions (∼5 Mb on either side) of the gene xa13 (25.01 Mb) on chromosome 8. However, only one marker RM80, flanking the gene xa13 at a distance of 1.1 Mb, was found to be polymorphic and this was used to identify the recombinant line with minimum donor parent segment (Fig. 1A). Similarly, 43 markers in the genomic region spanning across the genes Xa21 (20.54 Mb), Pi54 (21.97 Mb) and qSBR11-1 (27.2-28.4 Mb) on chromosome 11 were analysed for polymorphism between the parental lines, of which only two markers, RM209 and RM2778, were found to be polymorphic (Fig. 1B). The advanced breeding line ‘Pusa1608-06-7-5-9’ had the least donor segment in the carrier chromosome 11 (<4.25 Mb donor segment in the genomic region of Xa21 and Pi54; <1.8 Mb donor segment around ShB QTL qSBR11-1), while the rest of the lines showed higher donor parent genome introgression. The polymorphic marker RM2778, located between Pi54 and qSBR11-1, also identified two other advanced breeding lines (‘Pusa1608-06-16-3-60’ and ‘Pusa1608-06-16-3-61’) with minimum donor segments around ShB QTL qSBR11-1, but they had a larger donor segment in the genomic region of Xa21 and Pi54 (<6.2 Mb) compared with ‘Pusa1608-06-7-5-9’.
Table 2.
Agronomic performance of the improved lines of Pusa1608.
| Designation | DFF (days) | PH (cm) | NT | PL (cm) | FG/P | SF (%) | TW (g) | Y/P (g) | RPG (%) |
|---|---|---|---|---|---|---|---|---|---|
| Pusa1608-06-7-5-9 | 107 | 93.20 | 15 | 28.60 | 148 | 90.58 | 24.16 | 21.10 | 78.33 |
| Pusa1608-06-7-10-14 | 115 | 90.80 | 12 | 32.20 | 155 | 88.88 | 21.50 | 19.70 | 89.50 |
| Pusa1608-06-13-1-43 | 103 | 89.60 | 10 | 27.00 | 121 | 86.88 | 21.95 | 17.80 | 89.17 |
| Pusa1608-06-14-2-49 | 100 | 94.60 | 11 | 24.20 | 135 | 79.89 | 22.20 | 18.70 | 85.00 |
| Pusa1608-06-15-4-56 | 108 | 95.20 | 12 | 27.80 | 155 | 89.86 | 24.20 | 21.20 | 85.00 |
| Pusa1608-06-16-3-60 | 108 | 98.20 | 14 | 33.80 | 141 | 86.73 | 23.21 | 19.70 | 76.25 |
| Pusa1608-06-16-4-61 | 110 | 91.40 | 14 | 30.20 | 140 | 88.25 | 23.61 | 21.10 | 80.83 |
| Improved Pusa Basmati 1 | 105 | 93.40 | 13 | 32.20 | 146 | 89.50 | 22.79 | 19.20 | – |
| CD (0.05) | 2.63 | 0.44 | 2.78 | 3.01 | 7.91 | 1.43 | 0.31 | 3.12 | – |
DFF, days to 50% flowering; PH, plant height; NT, no. of tillers; PL, panicle length; FG/P, filled grains/panicle; SF, spikelet fertility; TW, thousand grain weight; Y/P, yield per plant; RPG, per cent recurrent parent genome recovery.
Fig. 1.
Analysis of genome introgression associated with resistance genes/QTL. (A) xa13 on chromosome 8 and Xa21, Pi54 and qSBR11-1 on chromosome 11 (B) in ‘Pusa1608’ families.
Agronomic performance
The agronomic performance of advanced breeding lines was evaluated during Kharif (autumn) 2010 (Table 2). There were no significant differences between the improved lines and the recurrent parent ‘Improved Pusa Basmati 1’ for important agronomic traits such as (i) NT and (ii) PL. Days to 50 % flowering of advanced lines ranged from 100 to 115 days with a mean of 107.8 days, which was similar to ‘Improved Pusa Basmati 1’ (105 days). The PL was as low as 24.20 cm in ‘Pusa1608-06-14-2-49’ to 33.8 cm in ‘Pusa1608-06-16-3-60’. This compares with 32.20 cm in ‘Improved Pusa Basmati 1’. Spikelet fertility and TW of some of the advance breeding lines were better than those of the recurrent parent. The Y/P in the advanced lines was on a par with the recipient parent ‘Improved Pusa Basmati 1’. All seven selections were characterized by semi-dwarf plant height, sturdy stems, and dark and green flag leaves. These are the prominent traits distinguishing the recurrent parent ‘Improved Pusa Basmati 1’ from the donor ‘Tetep’.
Evaluation of grain and cooking quality
The grain and cooking quality of the Pusa1608 lines was evaluated and is presented in Table 3. All the improved lines possessed extra long, slender grains similar to those of the recurrent parent ‘Improved Pusa Basmati 1’ (Fig. 2). The KLBC of the advanced breeding lines ranged from 7.80 mm in ‘Pusa1608-06-7-5-9’ to 8.73 mm in ‘Pusa1608-06-16-3-60’. The L/B ratio of the improved lines was in the range 4.03–5.46 as compared with 5.0 for ‘Improved Pusa Basmati 1’. One of the improved lines (‘Pusa1608-06-7-10-14’) showed a significantly superior elongation ratio (1.83) compared with the recurrent parent ‘Improved Pusa Basmati 1’. Although this line had significantly lower KLBC, it was able to make up the KLAC on a par with the recurrent parent because of its significantly superior elongation ratio. The alkali spreading value and aroma of all the advanced breeding lines were similar to those of ‘Improved Pusa Basmati 1’.
Table 3.
Grain and cooking quality characteristics of the improved lines of Pusa1608.
| Designation | Grain shape | KLBC (mm) | KBBC (mm) | L/B ratio | KLAC (mm) | KBAC (mm) | ER | ASV | Aroma |
|---|---|---|---|---|---|---|---|---|---|
| Pusa1608-06-7-5-9 | Extra long | 7.80 | 1.93 | 4.03 | 12.40 | 2.80 | 1.59 | 7 | 2 |
| Pusa1608-06-7-10-14 | Extra long | 8.07 | 1.67 | 4.84 | 14.80 | 2.60 | 1.83 | 7 | 2 |
| Pusa1608-06-13-1-43 | Extra long | 8.33 | 1.93 | 4.31 | 14.00 | 2.53 | 1.68 | 7 | 2 |
| Pusa1608-06-14-2-49 | Extra long | 8.00 | 1.87 | 4.29 | 13.67 | 2.60 | 1.71 | 7 | 2 |
| Pusa1608-06-15-4-56 | Extra long | 8.20 | 1.73 | 4.73 | 14.40 | 2.47 | 1.76 | 7 | 2 |
| Pusa1608-06-16-3-60 | Extra long | 8.73 | 1.60 | 5.46 | 13.47 | 2.33 | 1.54 | 7 | 2 |
| Pusa1608-06-16-4-61 | Extra long | 7.93 | 1.67 | 4.76 | 13.13 | 2.33 | 1.66 | 7 | 2 |
| Improved Pusa Basmati 1 | Extra long | 8.33 | 1.67 | 5.00 | 14.73 | 2.53 | 1.77 | 7 | 2 |
| Tetep | Medium | 5.58 | 1.93 | 2.89 | 8.40 | 2.87 | 1.50 | 3 | 0 |
| CD (0.05) | – | 0.13 | 0.01 | 0.09 | 0.24 | 0.19 | 0.03 | – | – |
Grain shape, extra long: >7.50 mm, medium: 5.51–6.60 mm; iKLBC, kernel length before cooking; KBBC, kernel breadth before cooking; L/B ratio, length/breadth ratio; KLAC, kernel length after cooking; KBAC, kernel breadth after cooking; ER, elongation ratio; ASV, alkali spreading value (1–2 high and 6–7 low); aroma, 0 absent and 2 strong.
Fig. 2.

Rough, milled and cooked rice of ‘Improved Pusa Basmati 1’ and ‘Pusa1608’ lines.
Disease reaction for BB, blast and ShB
The advanced lines in the background of ‘Improved Pusa Basmati 1’ were screened for resistance to BB, blast and ShB with ‘Improved Pusa Basmati 1’ and ‘Tetep’ as checks using standard procedures (Table 4). The donor parent ‘Tetep’ was found to be resistant to the most virulent BB isolate ‘Kaul’ (lesion length 2.20 cm), all four isolates of blast under artificial inoculation (score of 0 for all four isolates) and in UBN (score of 0) when tested in the UBN of the Agricultural Research Station at Mugad for leaf and neck blast. It was also resistant to the highly virulent ‘Kapurthala’ isolate (Rs-K) of ShB. In contrast, the recurrent parent ‘Improved Pusa Basmati 1’ was resistant to BB (lesion length of 1.80 cm) and highly susceptible to both blast (both under artificial conditions—a score of 5 for all the isolates and in the UBN a score of 7 for leaf and neck blast) and ShB (score of 7).
Table 4.
Reaction of the improved lines of Pusa1608 to BB, blast and ShB.
| Details | BB | Blast |
Sheath blight | |||||
|---|---|---|---|---|---|---|---|---|
| Disease reaction (lesion length) | Disease reaction under artificial inoculation (0–5 scale) |
Disease reaction in UBN trials (0–9 scale) ARS, Mugad |
Disease reaction (0–9 scale) | |||||
| Kaul isolate | Mo-ni-007 | Mo-ni-012 | Mo-ni-018 | Mo-ni-019 | Leaf blast | Neck blast | Rs-K isolate | |
| Pusa1608-06-7-5-9 | 3.56 | 1 | 2 | 0 | 0 | 2 | 3 | 3 |
| Pusa1608-06-7-10-14 | 2.88 | 2 | 1 | 2 | 0 | 3 | 3 | 3 |
| Pusa1608-06-13-1-43 | 2.50 | 0 | 0 | 1 | 3 | 2 | 7 | 3 |
| Pusa1608-06-14-2-49 | 2.25 | 1 | 0 | 0 | 1 | 2 | 5 | 3 |
| Pusa1608-06-15-4-56 | 1.88 | 1 | 2 | 1 | 0 | 2 | 3 | 3 |
| Pusa1608-06-16-3-60 | 2.33 | 2 | 3 | 0 | 1 | 4 | 5 | 3 |
| Pusa1608-06-16-4-61 | 1.40 | 1 | 2 | 0 | 1 | 2 | 3 | 3 |
| Improved Pusa Basmati 1 | 1.80 | 5 | 5 | 5 | 5 | 9 | 9 | 7 |
| Tetep | 2.20 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
UBN, Uniform Blast Nursery; DAI, days after inoculation; RLH, relative lesion height.
Resistance to BB was evaluated on the basis of lesion length. A lesion length <6 cm was considered as resistant, 6–10 cm as moderately resistant, 10–15 cm as moderately susceptible and >15cm as susceptible. For evaluation of blast resistance under artificial inoculation a 0–5 scale was adopted, where scores of 0–2 were considered as resistant, 3 as moderately resistant and 4–5 as susceptible, while in UBN a 0–9 scale was used, wherein scores of 0–3 were considered as resistant, 4–5 as moderately resistant, 6 as moderately susceptible and 7–9 as susceptible. For evaluation of ShB resistance, a 0–9 scale based on RLH was used, where 0 was considered as highly resistant (RLH = 0), 1 as resistant (RLH < 20), 3 as moderately resistant (RLH: 20–30), 5 as moderately susceptible (RLH: 31–45), 7 as susceptible (RLH: 45–65) and 9 (>65) as highly susceptible.
All seven improved lines were resistant to BB and the lesion lengths ranged from 1.40 cm in ‘Pusa1608-06-16-4-61’ to 3.56 cm in ‘Pusa1608-06-7-5-9’, in comparison with 1.80 and 2.20 cm in ‘Improved Pusa Basmati 1’ and ‘Tetep’, respectively. Evaluation of all these advanced lines for blast resistance showed a differential reaction pattern to different isolates, with five out of seven entries showing resistant reactions and two showing moderately resistant reactions with different isolates. Four out of seven advanced breeding lines (‘Pusa1608-06-7-5-9’, ‘Pusa1608-06-7-10-14’, ‘Pusa 1608-06-15-4-56’ and ‘Pusa1608-06-16-4-61’) were found to be resistant to blast both under artificial inoculation and under natural epiphytotic conditions in a hotspot location. All the advanced lines (‘Pusa1608’ lines) were moderately resistant (score of 3) under artificial screening with the virulent isolate of ShB (Fig. 3) when compared with the susceptible reaction shown by ‘Improved Pusa Basmati 1’ (score of 7).
Fig. 3.

Sheath blight symptoms 25 days after inoculation in the parents and an improved line.
Discussion
Basmati rice production is often constrained by several biotic stresses, among which diseases such as BB, blast, and ShB impose both severe yield and quality losses. The development of improved ‘Pusa Basmati 1’ with BB resistance through MAS of two BB resistance genes xa13 and Xa21 (Gopalakrishnan et al. 2008) helped address the problem of BB. However, the severe incidence of blast disease, especially neck blast, is a major constraint in Basmati rice production. Therefore, the present study was undertaken with the objective of transferring a gene, Pi54, providing broad-spectrum resistance to blast disease using the cultivar ‘Tetep’ as the multiple disease-resistant donor with resistance to blast, BB and ShB.
Marker-assisted selection offers a simpler and more efficient and accurate way to breed improved cultivars. It is especially helpful for breeding disease resistance compared with selection-only based on phenotype screening. Marker-assisted pyramiding of major genes/QTLs has helped in the past to reduce susceptibility to major diseases such as BB (Huang et al. 1997; Singh et al. 2001; Joseph et al. 2004; Zhang et al. 2006; Gopalakrishnan et al. 2008; Sundaram et al. 2008, 2009; Basavaraj et al. 2009, 2010), blast (Hittalmani et al. 2000; Zhou et al. 2011) and ShB (Wang et al. 2011). However, there are no reports of the transfer of genes/QTLs conferring resistance to multiple diseases in rice. Despite many advances made in rice genomics and breeding, we are only aware of two reports that combined resistance to multiple diseases in rice using a transgenic approach (Datta et al. 2002; Narayanan et al. 2002), and there is no previous report on the development of multiple disease-resistant rice cultivars through MAS.
With no pre-existing sources of resistance against blast, BB and ShB in the Basmati rice germplasm, there was a need to transfer the genes from non-Basmati sources. One of the most important limitations in transferring disease resistance from other sources is the impairment of grain and cooking quality traits, which are unique to Basmati rice. Therefore, we adopted an MABB strategy involving marker-assisted foreground selection for gene(s)/QTLs of interest along with stringent phenotypic selection for grain and cooking quality traits (Singh et al. 2011).
The disease reaction for BB resistance in some of the advanced breeding lines showed greater resistance than the recurrent parent ‘Improved Pusa Basmati 1’. This could be due to complementarity of genes from the donor parent ‘Tetep’, which have been reported to carry other BB-resistant genes, namely Xa1, Xa2, Xa12 and Xa16 (Nelson et al. 1994; Goel et al. 1998). However, the markers linked to the Xa2 gene (He et al. 2006) were found to be monomorphic and the presence of these genes could not be ascertained in the improved lines. However, because ‘Tetep’ is a highly valuable source of genes for resistance to multiple diseases, the contribution from this donor parent cannot be ruled out.
The differential response of the improved lines for blast resistance with different isolates and in the hotspot location could be attributed to the differences in the genetic make-up of the improved lines (Singh et al. 2012). Although screening of the backcross-derived lines under artificial inoculation is time consuming and laborious, it does offer scope for selecting genotypes with genetic backgrounds offering higher levels of resistance to disease in the presence of one major gene such as Pi54. Therefore, meticulous screening of the advanced/elite lines for traits of interest (such as disease resistance in this case) in the advanced stages of the breeding programmes can help in identifying genotypes with superior levels of trait expression. All the improved lines were moderately resistant to ShB compared with the donor parent ‘Tetep’, which exhibited a higher level of resistance (score 1). The complete resistance of ‘Tetep’ can be attributed to the presence of 12 different QTLs spanning the genome (Channamallikarjuna et al. 2010). Although MAS for pyramiding multiple minor resistant QTLs is a possible contributor to achieving a higher level of resistance in the advanced lines, it risks being accompanied by the possibility of bringing undesirable traits into the advanced breeding lines due to linkage drag (Kou and Wang 2012).
Marker-assisted background selection in early backcross generations has been advocated for quick recovery of the RPG (Chen et al. 2001; Joseph et al. 2004). Parallel to this study, Channamallikarjuna et al. (2010) independently reported markers linked to as many as 12 QTLs for ShB resistance in Tetep, the donor for the blast resistance gene Pi54, which included one major QTL, qSBR11-1, for ShB resistance in the genomic region of Pi54 in chromosome 11. Therefore, we used the flanking markers RM224 and RM7228 linked to qSBR11-1 to successfully identify the superior breeding lines integrating the blast resistance gene Pi54 also at BC2F5 stage. A strict adoption of marker-assisted background selection in the carrier chromosome for precise transfer of the blast resistance gene Pi54 would have meant the removal of possible linkage drag associated with Pi54 in chromosome 11. This would have eliminated the ShB resistance QTL qSBR11-1 from the advanced breeding lines. However, the delayed application of marker-assisted background selection has been favourable in retaining the recombinants possessing the ShB resistance QTL qSBR11-1 without compromising either agronomic performance or Basmati-type grain and cooking quality characteristics. This has been made possible by combining stringent phenotypic selection with a major focus on agronomic and quality traits for selection of the plants and MAS for gene(s) of interest (Gopalakrishnan et al. 2008). Background analysis of the advanced lines using 60 polymorphic STMS markers across the genome revealed that up to 89.50 % of the RPG had been recovered in only two backcross generations. Among these markers, four have been reported to be linked with important grain and cooking quality traits, viz. RM276 for gelatinization temperature and ASV (Tian et al. 2005; Wang et al. 2007), RM80 for aroma (Amarawathi et al. 2008), RM202 associated with KL (Agrama et al. 2007) and RM247 linked to the QTL, qRLW12, determining L/B ratio (Li et al. 2010) (data not presented). It was observed that at all these marker loci, all seven advanced elite genotypes had recovered the recurrent parent-specific alleles, possibly due to stringent phenotypic selection for grain and cooking quality characters. Although four of the improved lines had KBBC significantly different from ‘Improved Pusa Basmati 1’, the KBBC values of all the lines were ≤1.93 mm. As per the notified standards for Basmati rice, the KBBC should be <2 mm; therefore, slightly thicker grains but within the standard norm is a desirable attribute, as this would help in reducing the percentage of broken grains and increasing the head rice recovery. The elite genotype ‘Pusa1608-06-7-10-14’ was found to be resistant to BB and blast, moderately resistant to ShB, while possessing better grain and cooking quality with a yield on a par with the recurrent parent ‘Improved Pusa Basmati 1’.
Although marker-assisted background selection is very useful in rapid reconstitution of the RPG, it can be very demanding in terms of application in a breeding programme due to several constraints, including identifying useful polymorphisms, its cost and timely execution (Singh et al. 2012). Therefore, a more pragmatic approach involving marker-assisted foreground selection for trait(s) of interest, in combination with phenotypic selection for specific characters unique to the recurrent parent, would help not only in transferring the trait of interest into the recurrent parent but also in developing superior lines with additional desirable traits from the donor parent. This would be particularly relevant when the donor parent has many desirable traits in addition to the target trait being transferred, e.g. ShB resistance QTL qSBR11-1 in addition to Pi54 for blast resistance from ‘Tetep’. Altogether, MAS has been effective in combining three genes (xa13, Xa21, Pi54) and one QTL (qSBR11-1) conferring resistance to BB, blast and ShB diseases in an elite Basmati rice cultivar, ‘Improved Pusa Basmati 1’.
Conclusions and forward look
This is the first report of marker-assisted transfer of genes conferring resistance to three different diseases in rice. The genes involved are xa13 and Xa21 for BB resistance; Pi54 for blast resistance; and a major QTL qSBR11-1 for ShB resistance. These have been combined through MABB to lead to the development of improved lines that are highly resistant to BB and blast, and moderately resistant to ShB. The improved lines have desirable Basmati grain and cooking quality characteristics, in tandem with inbuilt resistance to BB, blast and ShB, and yield on a par with ‘Improved Pusa Basmati 1’. These multiple biotic stress-resistant lines will now be evaluated under multilocation trials for release to farmers as improved Basmati cultivars. They will also be a unique source for BB, blast and ShB resistance genes in future Basmati breeding programmes.
Sources of funding
The research was funded by the Indian Council of Agriculture Research, New Delhi, India, under the ICAR-Network Project on Gene Pyramiding (2005–2008).
Contributions by the authors
All the authors contributed to a similar extent overall.
Conflicts of interest statement
None declared.
Acknowledgements
The help extended for screening the improved lines by staff of the Uniform Blast Screening Nursery of the Agricultural Research Station, Mugad, Karnataka (Southwest India) is gratefully acknowledged. The virulent isolates of the pathogen Magnaporthae oryzae used for assessing the reaction of improved lines to blast disease were collected under the Indian Council of Agricultural Research—National Agricultural Innovation Project ‘Allele mining and expression profiling of resistance and avirulence genes in rice-blast pathosystem for development of race non-specific disease resistance’.
Literature cited
- Agrama H, Eizenga G, Yan W. Association mapping of yield and its components in rice cultivars. Molecular Breeding. 2007;19:341–356. [Google Scholar]
- Ahn SW, DenaDela RC, Candole BL, Mew TW. A new scale for rice sheath blight disease assessment. International Rice Research Newsletter. 1986;11:17. [Google Scholar]
- Amarawathi Y, Singh R, Singh AK, Singh VP, Mohapatra T, Sharma TR, Singh NK. Mapping of quantitative trait loci for Basmati quality traits in rice (Oryza sativa L.) Molecular Breeding. 2008;21:49–65. [Google Scholar]
- Basavaraj SH, Singh VK, Singh A, Singh D, Nagarajan M, Mohapatra T, Prabhu KV, Singh AK. Marker aided improvement of Pusa6B, the maintainer parent of hybrid Pusa RH10, for resistance to bacterial blight. Indian Journal of Genetics and Plant Breeding. 2009;69:10–16. [Google Scholar]
- Basavaraj SH, Singh VK, Singh A, Singh A, Singh A, Yadav S, Ellur RK, Singh D, Gopalakrishnan S, Nagarajan M, Mohapatra T, Prabhu KV, Singh AK. Marker-assisted improvement of bacterial blight resistance in parental lines of Pusa RH10, a superfine grain aromatic rice hybrid. Molecular Breeding. 2010;26:293–305. [Google Scholar]
- Bhaktavatsalam G, Satyanarayana K, Reddy APK, John VT. Evaluation of sheath blight resistance in rice. International Rice Research Newsletter. 1978;3:9–10. [Google Scholar]
- Bonman JM, Dedios TIV, Khin MM. Physiologic specialization of Pyricularia oryzae in the Philippines. Plant Disease. 1986;70:767–769. [Google Scholar]
- Chahal SS, Sokhi SS, Ratan GS. Investigation on sheath blight of rice in Punjab. Indian Journal of Phytopathology. 2003;56:22–26. [Google Scholar]
- Channamallikarjuna V, Sonah H, Prasad M, Rao GJN, Chand S, Upreti HC, Singh NK, Sharma TR. Identification of major quantitative trait loci qSBR11-1 for sheath blight resistance in rice. Molecular Breeding. 2010;25:155–166. [Google Scholar]
- Chen S, Lin XH, Xu CG, Zhang Q. Improving bacterial blight resistance of ‘6078’, an elite restorer line of hybrid rice, by molecular marker-aided selection. Plant Breeding. 2001;120:133–137. [Google Scholar]
- Datta K, Baisakh N, Thet MK, Tu J, Datta SK. Pyramiding transgenes for multiple resistance in rice against bacterial blight, yellow stem borer and sheath blight. Theoretical and Applied Genetics. 2002;106:1–8. doi: 10.1007/s00122-002-1014-1. [DOI] [PubMed] [Google Scholar]
- Goel RK, Kaur L, Saini RG. Effectiveness of different Xa genes against Xanthomonas oryzae pv. oryzae population causing bacterial blight of rice in Punjab (India) Rice Genetics Newsletter. 1998;15:131. [Google Scholar]
- Gopalakrishnan S, Sharma RK, Rajkumar KA, Joseph M, Singh VP, Singh AK, Bhat KV, Singh NK, Mohapatra T. Integrating marker assisted background analysis with foreground selection for identification of superior bacterial blight resistant recombinants in Basmati rice. Plant Breeding. 2008;127:131–139. [Google Scholar]
- He Q, Li DB, Zhu YS, Tan MP, Zhang DP, Lin XH. Fine mapping of Xa2, a bacterial blight resistance gene in rice. Molecular Breeding. 2006;17:1–6. [Google Scholar]
- Hittalmani S, Parco A, Mew TV, Zeigler RS, Huang N. Fine mapping and DNA marker-assisted pyramiding of the three major genes for blast resistance in rice. Theoretical and Applied Genetics. 2000;100:1121–1128. [Google Scholar]
- Huang N, Angeles ER, Domingo J, Magpantay G, Singh S, Zhang G, Kumaravadivel N, Bennett J, Khush GS. Pyramiding of bacterial blight resistance genes in rice: marker assisted selection using RFLP and PCR. Theoretical and Applied Genetics. 1997;95:313–320. [Google Scholar]
- Inukai T, Nelson RJ, Zeigler RS, Sarkarung S, Mackill DJ, Bonman JM, Takamura I, Kinoshita T. Allelism of blast resistance gene in near-isogenic lines of rice. Phytopathology. 1994;84:1278–1283. [Google Scholar]
- Jia Y, Bryan GT, Farrall L, Valent B. Natural variation at the Pi-ta rice blast resistance locus. Phytopathology. 2003;93:1452–1459. doi: 10.1094/PHYTO.2003.93.11.1452. [DOI] [PubMed] [Google Scholar]
- Joseph M, Gopalakrishnan S, Sharma RK, Singh AK, Singh VP, Singh NK, Mohapatra T. Combining bacterial blight resistance and Basmati quality characteristics by phenotypic and molecular marker assisted selection in rice. Molecular Breeding. 2004;13:377–387. [Google Scholar]
- Kauffman HE, Reddy APK, Hsieh SPY, Merca SD. An improved technique for evaluating resistance of rice varieties to Xanthomonas oryzae. Plant Disease Reporter. 1973;57:537–541. [Google Scholar]
- Khush GS. Progress in irrigated rice research. Manila, The Philippines: International Rice Research Institute; 1989. Multiple disease and insect resistance for increased yield stability in rice; pp. 79–92. http://books.irri.org/9711041847_content.pdf. [Google Scholar]
- Kou Y, Wang S. Toward an understanding of the molecular basis of quantitative disease resistance in rice. Journal of Biotechnology. 2012;159:283–292. doi: 10.1016/j.jbiotec.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Li MM, Xu L, Ren JF, Cao GL, Yu LQ, He HH, Han LZ, Koh HJ. Identification of quantitative trait loci for grain traits in japonica rice. Agricultural Sciences in China. 2010;9:929–936. [Google Scholar]
- Narayanan NN, Baisakh N, Vera Cruz CM, Gnanamanickam SS, Datta K, Datta SK. Molecular breeding for the development of blast and bacterial blight resistance in rice cv. IR50. Crop Science. 2002;42:2072–2079. [Google Scholar]
- Nelson RJ, Baraoidan MR, Cruz CMV, Yap IV, Leach JE, Mew TW, Leung H. Relationship between phylogeny and pathotype for the bacterial blight pathogen of rice. Applied Environmental Microbiology. 1994;60:3275–3283. doi: 10.1128/aem.60.9.3275-3283.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou SH. Rice diseases. 2nd edn. Kew, UK: Commonwealth Mycological Institute; 1985. p. 379. [Google Scholar]
- Pinson SRM, Capdevielle FM, Oard JH. Confirming QTLs and finding additional loci conditioning sheath blight resistance in rice (Oryza sativa L.) using recombinant inbred lines. Crop Science. 2005;45:503–510. [Google Scholar]
- Pinson SRM, Oard JH, Groth D, Miller R, Marchetti MA, Shank AR, Jia MH, Jia Y, Fjellstrom RG, Li Z. Registration of TIL: 455, TIL: 514, and TIL: 642, three rice germplasm lines containing introgressed sheath blight resistance alleles. Journal of Plant Registration. 2008;2:251–254. [Google Scholar]
- Prabhu KV, Somers DJ, Rakow G, Gugel RK. Molecular markers linked to white rust resistance in mustard Brassica juncea. Theoretical and Applied Genetics. 1998;97:865–870. doi: 10.1007/s00122-001-0812-1. [DOI] [PubMed] [Google Scholar]
- Rai AK, Kumar SP, Gupta SK, Gautam N, Singh NK, Sharma TR. Functional complementation of rice blast resistance gene Pi-kh (Pi54) conferring resistance to diverse strains of Magnaporthe oryzae. Journal of Plant Biochemistry and Biotechnology. 2011;20:55–65. [Google Scholar]
- Ronald PC, Albano B, Tabien R, Abenes L, Wu K, McCouch SR, Tanksley SD. Genetic and physical analysis of the rice bacterial blight disease resistance locus, Xa21. Molecular Genetics and Genomics. 1992;236:113–120. doi: 10.1007/BF00279649. [DOI] [PubMed] [Google Scholar]
- Scardaci SC, Webster RK, Greer CA, Hill JE, William JF, Mutters RG, Brandon DM, McKenzie KS, Oster JJ. Rice blast: a new disease in California. Davis: Department of Agronomy and Range Science, University of California; 1997. Agronomy Fact Sheet Series. 1997–2. [Google Scholar]
- SES (Standard Evaluation System for Rice). Manila, The Philippines: International Rice Research Institute; 1996. pp. 1–56. [Google Scholar]
- Sharma TR, Madhav MS, Singh BK, Shanker P, Jana TK, Dalal V, Pandit A, Singh A, Gaikwad K, Upreti HC, Singh NK. High resolution mapping, cloning and molecular characterization of the Pi-kh gene of rice, which confers resistance to M. grisea. Molecular Genetics and Genomics. 2005;274:569–578. doi: 10.1007/s00438-005-0035-2. [DOI] [PubMed] [Google Scholar]
- Singh AK, Gopala Krishnan S, Singh VP, Prabhu KV, Mohapatra T, Singh NK, Sharma T, Nagarajan M, Vinod KK, Singh D, Singh UD, Chander S, Atwal SS, Seth R, Singh VK, Ellur RK, Singh A, Anand D, Khanna A, Yadav S, Goel N, Singh A, Shikari AB, Singh A, Marathi B. Marker assisted selection: a paradigm shift in Basmati breeding. Indian Journal of Genetics and Plant Breeding. 2011;71:1–9. [Google Scholar]
- Singh S, Sidhu JS, Huang N, Vikal Y, Li Z, Brar DS, Dhaliwal HS, Khush GS. Pyramiding three bacterial blight resistance genes (xa5, xa13, Xa21) using marker assisted selection into indica rice cultivar PR106. Theoretical and Applied Genetics. 2001;102:1011–1015. [Google Scholar]
- Singh VK, Singh A, Singh SP, Ellur RK, Choudhary V, Sarkhel S, Singh D, Gopala Krishnan S, Nagarajan M, Vinod KK, Singh UD, Rathore R, Prasanthi SK, Agrawal PK, Bhatt JC, Mohapatra T, Prabhu KV, Singh AK. Incorporation of blast resistance into ‘PRR78’, an elite Basmati rice restorer line, through marker assisted backcross breeding. Field Crop Research. 2012;128:8–16. [Google Scholar]
- Singh VP. The Basmati rice of India. In: Singh RK, Singh US, Khush GS, editors. Aromatic rices. New Delhi, India: Oxford & IBH Publishing Co. Pvt. Ltd.; 2000. pp. 136–153. [Google Scholar]
- Singh VP, Siddiq EA, Zaman FU, Sadananda AR. Improved basmati donors. International Rice Research Newsletter. 1988;13:22–25. [Google Scholar]
- Srinivasachary, Willocquet L, Savary S. Resistance to rice sheath blight (Rhizoctonia solani Kuhn) [(teleomorph: Thanatephorus cucumeris (A.B. Frank) Donk.] disease: current status and perspectives. Euphytica. 2011;178:1–22. [Google Scholar]
- Sundaram RM, Vishnupriya MR, Biradar SK, Laha GS, Reddy GA, ShobhaRani N, Sarma NP, Sonti RV. Marker assisted introgression of bacterial blight resistance in Samba Mahsuri, an elite indica rice variety. Euphytica. 2008;160:411–422. [Google Scholar]
- Sundaram RM, Vishnupriya MR, Laha GS, ShobhaRani N, SrinivasRao P, Balachandaran SM, Reddy GA, Sarma NP, Shonti RV. Introduction of bacterial blight resistance into Triguna, a high yielding, mid-early duration rice variety. Biotechnology Journal. 2009;4:400–407. doi: 10.1002/biot.200800310. [DOI] [PubMed] [Google Scholar]
- Tan CX, Ji XM, Yang Y, Pan XY, Zuo SM, Zhang YF, Zou JH, Chen ZX, Zhu LH, Pan XB. Identification and marker-assisted selection of two major quantitative genes controlling rice sheath blight resistance in backcross generations. Acta Genetica Sinica. 2005;32:399–405. [PubMed] [Google Scholar]
- Tian R, Jiang GH, Shen LH, Wang LQ, He YQ. Mapping quantitative trait loci underlying the cooking and eating quality of rice using a DH population. Molecular Breeding. 2005;15:117–124. [Google Scholar]
- Van Berloo R. GGT: Software for display of graphical genotypes. Journal of Heredity. 1999;90:328–329. [Google Scholar]
- Wang LQ, Liu WJ, Xu Y, He YQ, Luo LJ, Xing YZ, Xu CG, Zhang Q. Genetic basis of 17 traits and viscosity parameters characterizing the eating and cooking quality of rice grain. Theoretical and Applied Genetics. 2007;115:463–476. doi: 10.1007/s00122-007-0580-7. [DOI] [PubMed] [Google Scholar]
- Wang Y., Pinson SRM, Fjellstrom RG, Tabien RE. Phenotypic gain from introgression of two QTL, qSB9-2 and qSB12-1 for rice sheath blight resistance. Molecular Breeding. 2012;30:293–303. [Google Scholar]
- Website 1. APEDA. www.apeda.gov.in (20 November 2011)
- Website 2. Gramene. www.gramene.org (15 December 2011)
- Xu X, Hayashi N, Wang CT, Kato H, Fujimura T, Kawasaki S. Efficient authentic fine mapping of the rice blast resistance gene Pik-h in the Pik cluster, using new Pik-h-differentiating isolates. Molecular Breeding. 2008;22:289–299. [Google Scholar]
- Zhang J, Li X, Jiang G, Xu Y, He Y. Pyramiding of Xa7 and Xa21 for the improvement of disease resistance to bacterial blight in hybrid rice. Plant Breeding. 2006;125:600–605. [Google Scholar]
- Zhou YL, Uzokwe VNE, Zhang CH, Cheng LR, Wang L, Chen K, Gao XQ, Sun Y, Chen JJ, Zhu LH, Zhang Q, Ali J, Xu JL, Li ZK. Improvement of bacterial blight resistance of hybrid rice in China using the Xa23 gene derived from wild rice (Oryza rufipogon) Crop Protection. 2011;30:637–644. [Google Scholar]
- Zuo S, Zhang L, Wang H, Yin Y, Zhang Y, Chen Z, Ma Y, Pan X. Prospect of the QTL-qSB-9Tq utilized in molecular breeding program of japonica rice against sheath blight. Journal of Genetics and Genomics. 2008;35:499–505. doi: 10.1016/S1673-8527(08)60068-5. [DOI] [PubMed] [Google Scholar]