Abstract
The objective of this study was to elucidate the normal functional adaptations of the cervix in pregnancy. Utilizing a Long-Evans rodent model, the cervix was divided into distal and proximal portions for virgin, mid-pregnant, and four weeks postpartum animals. The quasi-linear viscoelastic theory describes the elastic and viscous behavior of the cervix. A hydroxyproline assay was used to measure collagen content. The nonlinearity of the elastic response significantly increased throughout the entire cervix during pregnancy when compared to virgin samples (p < 0.05) and was similar to virgin samples postpartum. All viscous behavior, except for the short-term relaxation of the proximal cervix, significantly differed for pregnant specimens (p < 0.05) and remained similar to pregnant samples postpartum. Collagen content was found to increase by mid-pregnancy only in the proximal cervix when compared to virgin. Distal and proximal portions, however, were found to differ in collagen content at all time points (p < 0.05). This study finds that the cervix becomes elastically stiffer with increasing strain and exhibits increased viscous behavior during pregnancy, with incomplete recovery postpartum. These alterations allow for quick dissipation of loads, and are likely related to altered matrix organization and porosity reported by others.
Keywords: Preterm labor, cervix, quasi-linear viscoelasticity, biomechanics
1. Introduction
Preterm labor is a serious event that negatively impacts up to 12% of all pregnancies in the United States and is the leading cause of neonatal mortality in North America.1 In the United States, preterm labor is associated with 70% of all neonatal deaths and costs an estimated 5–6 billion dollars annually.2 The main cause of preterm labor is the premature softening of the cervix,3 a structure within the female reproductive tract that acts as a conduit between the vagina and uterus. In pregnancy, the cervix is maintained in a firm, closed position until term when it allows for passage of the fetus. Throughout pregnancy the cervix undergoes progressive structural and compositional changes, which include disruption of collagen structure, smooth muscle apoptosis, and increased water concentration associated with a rise in glycoaminoglycan (GAG) content.3,4 Ideally, the cervix retains sufficient mechanical stiffness and strength to retain the fetus until term. If the cervix becomes too soft or structurally insufficient prior to term, the fetus is more likely to be prematurely delivered.
Currently, the best clinical method to assess cervical remodeling is a transvaginal ultrasound at approximately 18 weeks of gestation (term pregnancy is 37–41 weeks) to measure cervical length. A short cervix identified at this time point is associated with a significantly elevated risk of preterm delivery. However, this method yields a low percentage of accurate predictions with approximately 10% accuracy for cervical lengths between 5 and 10 mm and rises to 50% for lengths under 5 mm.5 Although preterm labor is often considered idiopathic, other risk factors include intrauterine infection, previous preterm deliveries, and a short duration between pregnancies (<6 months).2 Thus, understanding the functional adaptations requisite of a cervix to maintain a pregnancy and evaluating the recovery of the cervix postpartum is critical to assess previous delivery as a risk factor of preterm labor.
In order to design an effective clinical test for preterm labor prediction, the cervix must also be analyzed, as a mechanical structure.6 Studies that address the mechanical properties of cervix are relatively scarce, yet this perspective is required to understand the mechanics of cervical failure. Due to the limited availability of normal virgin and pregnant human cervices, an animal model must first be appropriately selected. Defining an animal model for pregnancy allows for the mechanics of the cervix to be studied at various time points, which will provide a greater understanding of the adaptations of this tissue throughout pregnancy. Like the human, the cervix of the rodent model is largely comprised of collagen, gylcoaminoglycans, smooth muscle, elastin, and water. In addition, the functional role of the rat cervix mimics that of the human cervix in that both connect the uterus to the vagina and retain the fetus(es) during pregnancy (term pregnancy ~22 days).3
Previous studies have shown compositional variation through the cervix as it bridges the uterus and vagina, including smooth muscle concentration, which is most concentrated at the proximal portion and tapers off distally.3,6 These findings correlate with the changes in the mechanical properties of the human cervix based on anatomical location.6 Though the mechanical properties of the rodent cervix may differ from those of the human, the timing and degree of change in this model will be useful in understanding events that precede preterm labor. Such data would be useful for elastographic analyzes that may link changes in mechanical properties of the cervix to imaging modalities. An analysis of the normal mechanical adaptations of the cervix throughout pregnancy is, therefore, an important first step to future work aimed at defining the abnormal adaptations leading to preterm labor.
Additionally, by examining the time-dependent, or viscoelastic, properties of the cervix we can assess how the functional capabilities of the cervix adapt throughout pregnancy and develop more accurate computational models. The quasi-linear viscoelastic (QLV) theory developed by Professor Fung has been utilized to describe the nonlinear time- and history-dependent behavior, the medial collateral ligament (MCL), anterior cruciate ligament (ACL), aortic valve, stomach, liver, and esophagus.7–11 From the QLV theory, specific forms of the generalized relaxation function and the elastic response are utilized along with an established approach to simultaneously fit the theory to experimental data from both ramping and relaxation portions from a static stress-relaxation test.12,13 Since the cervix is a well-hydrated structure, with an extracellular matrix (ECM) largely comprised of collagen, viscoelastic analysis will provide insight into the mechanical changes of both viscous and elastic components while allowing for correlations to be made to alterations in tissue constituents. Knowledge of such parameters and how they are altered will provide us with a greater understanding of the functional behavior of the cervix during pregnancy and postpartum.9
Since the mid-pregnant and four weeks postpartum (approximately three months in the human) time points are the most clinically relevant in terms of elucidating adaptations to pregnancy and subsequent recovery, the objective of this study was to define the normal functional adaptations of the cervix at mid-pregnancy and the degree of recovery at four weeks postpartum using the QLV theory. The results will be utilized in future studies as baseline data to evaluate differences in cervical tissue that has yielded premature delivery. Based on literature that shows increased water content and collagen disorganization in the cervix throughout pregnancy, we hypothesize that the entire cervix from pregnant animals will display a less stiff elastic response with increased viscous behavior when compared to virgin animals. Additionally, derived from clinical observations that the cervix reconstitutes quickly following delivery, we expect that the viscoelastic properties of the entire cervix to return to virgin levels by four weeks postpartum. Furthermore, the proximal portion of the cervix has been shown to have a higher smooth muscle content6; therefore, we expect it to provide a less stiff elastic response with increased viscous behavior and structurally contain less collagen content when compared to the distal portion regardless of time point.
2. Methods
This study was performed with the approval of IACUC at the University of Pittsburgh (#0702224). For mechanical testing, cervical specimens were collected from virgin (3 months, distal n = 8, proximal n = 6), mid-pregnant (15–16 day, distal n = 6, proximal n = 7), and four weeks postpartum (distal n = 9, proximal n = 8) Long-Evans rats. The cervix was isolated from the surrounding connective tissues and vagina by transecting proximally at the fusion with the uterine horns and distally at the dense fibers which are transition to the vagina. The entire cervix was stored in saline soaked gauze at −20°C until the day of testing (~2–6 weeks). Preliminary data and previous research suggests that freezing has little or no effect on the gross viscoelastic behavior of cervical tissue as assessed in this study.14 On the day of testing the cervix was gradually thawed to room temperature and was further dissected to isolate it from the uterine horns and remove any nonload bearing, loose connective tissue. Then the cervix was then transected into distal and proximal halves at the dense fibers located at the midsection of the cervix (~5 mm from the distal end in the virgin cervix). The disparity in the number of distal and proximal specimen from each group is attributed to removal of the proximal or distal cervix with the uterus or vagina. This was to ensure that only cervical tissue was tested. Cervical mucus was not removed prior to testing to prevent the introduction of artificial damage resulting from its removal. The length, width, and thickness of the each half were then measured using digital calipers (accuracy ±0.025). The average of three measurements was used to obtain a representative cross-sectional area of each cervical half, assuming rectangular geometry. Lagrangian stress was calculated by normalizing load by the cross-sectional area and strain was defined as displacement of the top compression plate divided by sample thickness.
Each sample was centered on a custom steel plate at room temperature and hydrated with a 0.9% saline solution. The plate was fixed to the base of a materials testing machine (EnduraTec Elf 3220, Bose Corporation, Eden Prairie, MN) and an additional plate was attached in series to a load cell (50 lbs, Honeywell, Columbus, Ohio) and the actuator of the material testing machine. While the cervix likely experiences complex, multidirectional loading conditions in vivo, we choose to test specimens in response to a simple unconfined compression loading condition along the ventral–dorsal direction for this experiment due to the relatively planar geometry of the rat cervix (~3 mm thick along the ventral–dorsal axis, ~6–8 mm wide along the medial–lateral axis, and ~7–9 mm long along the proximal–distal axis). Specimens were preloaded to 0.15 N and the position of the top plate was set to zero. After 10 min for the tissue to equilibrate, each specimen underwent stress-relaxation testing during which it was compressed to 20% strain at a rate of 0.167 mm/s and held at 20% strain for a period of 4 min. Preliminary trials were performed at various rates and strain levels between 5% and 30%. At strain levels below 10%, the load response of the cervix was not outside the error of the load cell used in this test setup and deformations at 25% and 30% strain cause excessive tissue damage making the results nonrepeatable. A displacement rate of 0.167 mm/s was chosen as it provided a relatively slow strain rate for all tissues and prevented issues such as overshoot. Each specimen underwent a total of 3 ramp stress-relaxation trials and was given 30 min of recovery between trials. In preliminary trials the peak load experienced by the tissue noticeably decreased during the consecutive stress-relaxation experiments; however the tissue response became consistent after the 3rd trial. Rather than eliminating this effect through cyclic preconditioning, we recorded three separate stress-relaxation tests to further analyze this reduction in peak load.
The QLV theory, developed by Professor Fung, was utilized to characterize the viscoelastic properties of the virgin, mid-pregnant, and postpartum cervical halves. In the experimental setting, we can assume that the strain history begins at t = 0. Based upon a continuous spectrum of relaxation, Fung proposed the following expression for soft tissues which are relatively insensitive to strain rate15,16
| (1) |
where E1 is the exponential integral, C, τ1 (s), and τ2 (s) are material constants. The dimensionless constant C defines the magnitude of viscous effects present and is related to the percentage of relaxation while the time constants τ1 and τ2 govern the initial and late relaxation, respectively. These constants are also related to the slope of the stress-relaxation curve defined by12:
| (2) |
The instantaneous elastic response was approximated using the exponential function.5
| (3) |
where A (kPa) and B are material constants. The constant B and the product A × B (kPa) are the rate of change of the slope of the stress–strain curve and the initial slope of the curve, respectively.
Rather than assuming instantaneous step change in strain, a method developed by our laboratory utilizes simultaneous curve-fitting of the QLV convolution integrals describing the ramping and relaxation portions of the data to determine the material constants.12,13,16 This procedure extracts relaxation data, which occurs during the ramping portion of the experiment. Therefore, the assumption of instantaneous deformation is no longer needed, allowing slow strain rates to be used in order to accurately approximate a ramp and hold strain history. Constants were estimated by entering the data from the third stress-relaxation trial for each specimen and optimization was performed using Mathematica (Wolfram Research, Inc. Champaign, IL).16
A hydroxyproline assay was used to analyze the variation of collagen content of the cervix throughout pregnancy, recovery, and by anatomical location. Additional cervices, which had not undergone mechanical testing, from virgin (n = 4), mid-pregnant (15–16 day, n = 5), and four weeks postpartum (n = 5) were transected into proximal and distal portions as previously mentioned. Tissues were lyophilized for 8 h in a vacuum (0 mbar) and −50°C in a freeze-dry system (FreeZone 2.5, LABCONCO, Kansas City, MO) and the dry weight was measured. The tissue was subsequently digested for 18 h in a papain solution, diluted to 400 ug/ml, and the collagen was denatured with 4 M NaOH at 115.56°C in an autoclave for 60 min. After allowing the tissues to cool to room temperature, 200 μl OF 4 M HCl and 1.2 mL of chloramine solution were added to each vial and the samples were given 20 min to react at room temperature. To create a chromophore complex, 1.2 mL of Ehrlich’s reagent was added to each vial and the vials were placed in a 65°C water bath for 20 min. Samples were then placed in a spectrophotometer (VMax, Molecular Devices, Sunnyvale, CA) and the optical density of each solution was read at 550 nm. Collagen concentration was calculated by inserting the measured optical density into a linear regression equation created in Microsoft Excel (Microsoft, Redmond, WA) using four standards of type I collagen with known optical densities of 0, 200, 400, and 800 ug/ml. The mass of collagen was calculated by multiplying the calculated concentration by 200 ul, which was the initial volume of the standards.
3. Statistics
Animal characteristics, cervical geometries, and collagen content were compared using a one-way ANOVA with a Bonferroni or Dunnet T3 post-hoc, and are presented as mean (standard deviation). Viscoelastic constants were analyzed using a Kruskal–Wallis test with a Mann–Whitney post-hoc and are represented as median (25th percentile–75th percentile). Statistical analysis was performed on all viscoelastic constants between virgin, mid-pregnant, and postpartum tissues. A total of six groups were created, including distal and proximal groups for each of the three time points (virgin, mid-pregnant, and four weeks postpartum). Overall p-values from the ANOVA and Kruskal–Wallis tests were obtained using all six groups. For post-hoc analysis, proximal cervices were compared between the three time points, independent of the distal cervices. Similarly the distal cervices were compared between the three time points independent of the proximal halves. In addition, the proximal and distal cervices were compared within the same time point. All tests had a significance level of 0.05. All statistical analysis was done using a statistical software package (12.0.1 SPSS Inc, Chicago, IL). Experimental observations were quantified as percent changes and are represented as mean ± standard deviations.
4. Results
As expected, both the average weight and length of the Long-Evans rat increased during pregnancy and four weeks postpartum (Table 1). The postpartum increase was likely due to the linear growth of the rat over time. Additionally, the total cervical length decreased during pregnancy by an average of 1.7 mm (p = 0.027) when compared to the virgin cervix and returned to virgin lengths postpartum (p = 0.64). The distal cervix experienced significant reduction in length during pregnancy (p = 0.008), while there was no change in length of the proximal cervix (p = 0.56) compared to virgin tissues. There were no gross differences in the overall shape of the cervix at the different time points, the dense region of collagen fibers located midsection of the cervix, was most pronounced in the virgin cervix, although it was still palpable in the mid-pregnant and postpartum cervices. Additionally, the virgin cervix was firm, while the mid-pregnant cervix appeared more loosely constructed. Four weeks postpartum, the cervix recovered most of its structure to that of virgin tissue; however, the recovery was clearly incomplete as the two tissues were distinct in appearance. Finally, it was noted that the amount of mucus was greater in mid-pregnant and postpartum tissues relative to the virgin structure. While this was not quantified, a repeatable response following the second stress relaxation trial suggests that mucus was expelled during the first trial and therefore the extracellular matrix dominated the mechanical behavior. This was confirmed by the visualization of mucus on the compression plates following testing.
Table 1.
Baseline characteristics (weight and length) of rodents and the geometric dimensions (length, width, and thickness) of the distal and proximal cervix. In addition, the total length of the cervix was recorded. Data represented as mean (S.D.).
| Rat weight (g) | Rat length (cm) | Distal length (mm) | Distal width (mm) | Distal thick (mm) | Proximal length (mm) | Proximal width (mm) | Proximal thick (mm) | Total length (mm) | Number of pups | |
|---|---|---|---|---|---|---|---|---|---|---|
| Virgin | 225.57 (5.42) | 28.66 (0.54) | 5.31 (0.88) | 5.64 (0.74) | 3.13 (0.38) | 4.20 (0.88) | 5.73 (0.57) | 2.64 (0.31) | 9.51 (1.35) | — |
| Mid-pregnant | 253.03 (13.96) | 29.16 (0.52) | 4.14 (0.60) | 5.38 (0.76) | 3.08 (0.40) | 3.68 (0.69) | 5.98 (0.80) | 2.76 (0.34) | 7.81 (0.86) | 12.13 |
| Four weeks postpartum | 282.76 (19.03) | 31.14 (0.54) | 4.46 (0.60) | 6.53 (0.83) | 3.39 (0.40) | 4.29 (0.76) | 6.27 (1.29) | 2.56 (0.42) | 8.75 (1.34) | — |
|
| ||||||||||
| Overall p-value | < 0.001 | < 0.001 | 0.008 | 0.019 | 0.265 | 0.263 | 0.496 | 0.561 | 0.03 | — |
| Virgin versus Mid-pregnant | 0.002 | 0.189 | 0.008 | 1 | 1 | 0.563 | 1 | 1 | 0.027 | — |
| Virgin versus Four weeks postpartum | 0.001 | < 0.001 | 0.07 | 0.89 | 0.557 | 1 | 0.726 | 1 | 0.638 | — |
| Mid-Pregnant versus Four weeks postpartum | 1.01 | < 0.001 | 1 | 0.023 | 0.413 | 0.403 | 1 | 0.871 | 0.412 | — |
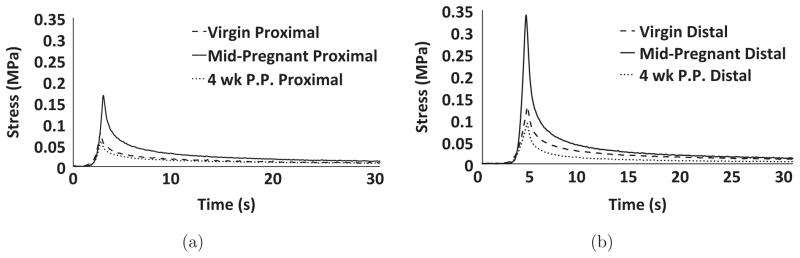
Immediately following the application of the preload to establish our reference length, all specimens displayed a rapid rate of relaxation that plateaued at a load that was undetectable with our load-cell. Therefore, it was assumed that each specimen was experiencing zero stress for the initiation of the stress-relaxation trials (Fig. 2). For all trials, all samples displayed a rapid and significant dissipation of energy, with more than 90% of the peak stress dissipated by the end of the 4 min test. For all samples, a majority of the relaxation occurred in the first 30 s as the change in the percent relaxation during the last 210 s of the test was typically 3%–7%.
Fig. 2.
Representative stress–time curves for (a) proximal and (b) distal; virgin (short dashed), mid-pregnant (solid), and postpartum (dotted). Note that the graphs have been shortened to 30 s in order to more clearly show the slope of the stress-relaxation curve.
Across all experimental groups, the specimen experienced a reduction in maximal load during successive stress-relaxation trials. On average the maximal load decreased by 48% ± 14% between the first and third trials, whereas the load decreased by no more than 10% between the second and third trials. Our preliminary experiments demonstrated that after three trials, the tissue response was repeatable. Thus, it was assumed that the reduction in maximal load, which was substantial (+60%) for some samples, was attributed in part to nonrecoverable phenomena (Mullins effect) possibly related to passive smooth muscle behavior or the observed expulsion of mucus.17 As our objective was to define the remodeling response of connective tissue to pregnancy, we deemed this outside of the scope of the present study. Under the current protocol, there were no obvious trends between groups in terms of the magnitude of this effect.
For the remainder of this paper only the 3rd stress-relaxation trial will be considered. During the ramping phase, which lasted 2 to 5 s depending on samples thickness, the observed load increased following an exponential trend to a peak value (Fig. 2: note all loads were normalized by cross sectional area to calculate Lagrangian stress). On average, the distal cervix exhibited peak stress values 41.5% ± 26.7% greater than the corresponding proximal halves for virgin and mid-pregnant groups; however, no such trend was observed for postpartum samples. In addition, mid-pregnant tissues often reached noticeably greater peak stresses for both the distal and proximal portions. The observed load quickly decreased seconds after reaching the peak load quickly approaching an asymptotic value (Fig. 2). After normalizing to area, it was observed that tissues stress approached values near 0 MPa approximately 30 s from the beginning of each trial. The total percentage of stress relaxation was 98.5% ± 1.37% for the distal groups and 97.0% ± 2.46% for the proximal groups with no observable differences between groups. Peak stress was calculated from data obtained during the third trial for all groups. Representative samples for both distal and proximal halves are shown in Fig. 2.
4.1. Elastic response
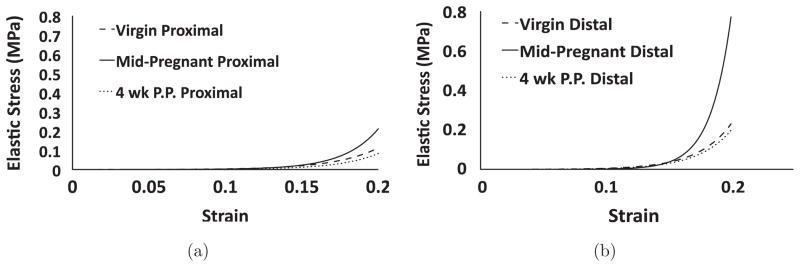
In terms of the constants describing the instantaneous elastic response, constant B, which governs the nonlinearity of the elastic response, was the only parameter found to be significantly different. Proximally, virgin tissues were found to have a median B value of 34.7 (Table 2). At mid-pregnancy, median B values increased by 27.3% when compared to virgin values for the proximal cervix (p = 0.002). By four weeks postpartum, B values were found to return to virgin values (p = 0.189) and 27.4% lower than mid-pregnant values (p = 0.004) for the proximal cervix. Distally, virgin B values were found to have a median value of 38.8 (Table 3). Similar to the proximal cervix, mid-pregnant tissues displayed an increase of 19.3% in median B value (p = 0.008) for distal cervix. At four weeks postpartum, parameter B returned to virgin levels (p = 0.54) and was found to be significantly lower than mid-pregnant samples (p = 0.05) for the distal cervix. When comparing the proximal and distal locations, parameter B was found to be significantly different in both the virgin and postpartum groups. For virgin tissues, median B values were 10.6% greater for distal cervices (p = 0.006) when compared to the proximal cervices. Similarly median B values were 21.8% greater for the distal cervix in the postpartum group (p = 0.002). Inserting parameters A and B into Eq. (3) yields the theoretical instantaneous elastic response curve for a tissue sample. Figure 3 illustrates the effect of parameter B, as the slope of the elastic response increases at a greater rate for mid-pregnant specimen when compared to the elastic response curves for the virgin and postpartum samples.
Table 2.
Median (25th percentile–75th percentile) values of the constants describing the instantaneous elastic response of the proximal portion of virgin (n = 7), mid-pregnant (n = 7), and postpartum (n = 8) Long-Evans rat cervix.
| A × 10−4 (kPa) | B | A × B (kPa) | |
|---|---|---|---|
| Virgin | 1.01 (0.773–1.11) | 34.7 (33.8–36.2) | 0.0034 (0.0022–0042) |
| Mid-pregnant | 0.295 (0.206–2.02) | 43.0 (37.1–47.2) | 0.0013 (0.0006–0.0078) |
| Four weeks postpartum | 1.97 (0.840–8.0) | 31.2 (28.3–33.9) | 0.0217 (0.00248–0.0227) |
Table 3.
Median (25th percentile–75th percentile) values of the constants describing the instantaneous elastic response of the distal portion of virgin (n = 8), mid-pregnant (n = 6), and postpartum (n = 9) Long-Evans rat cervix.
| A × 10−4 (kPa) | B | A × B (kPa) | |
|---|---|---|---|
| Virgin | 1.09 (0.894–1.27) | 38.8 (36.2–45.9) | 0.0042 (0.0030–0.0053) |
| Mid-pregnant | 0.125 (0.0478–1.14) | 53.4 (44.0–59.7) | 0.0007 (0.0002–0093) |
| Four weeks postpartum | 0.5 (0.105–0.669) | 39.9 (37.6–49.8) | 0.00251 (0.000532–0.00231) |
Fig. 3.
Theoretical elastic stress–strain curves for representative (a) proximal and (b) distal; virgin (short dashed), mid-pregnant (solid), and postpartum (dotted) cervical tissues in response to unconfined compression.
4.2. Viscous response
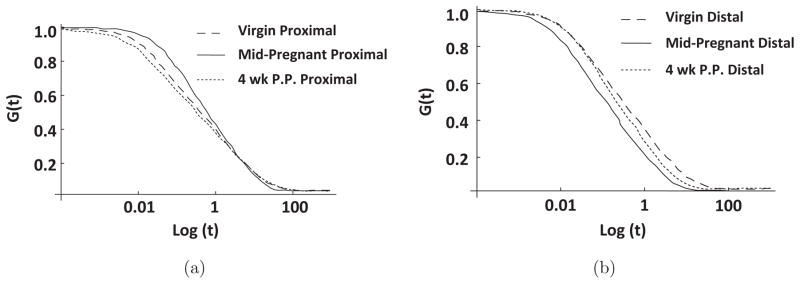
In terms of the constants describing the viscous response, differences were observed in all four parameters. For the proximal cervix, virgin samples were found to have median values of 3.15, 0.0143 s, 45.5 s, and 0.120%/ln (s) for the c, τ1, τ2, and slope of relaxation, respectively (Table 4). By mid-pregnancy, median slope of relaxation increased 17.2% (p = 0.017) while the median C value increased by 60.1% (p = 0.007) when compared to virgin values. Median values for τ2 decreased by 58.7% in the mid-pregnant specimen when compared to virgin tissues (p = 0.011). At four weeks postpartum in the proximal cervix, the viscous parameters fell between those of the virgin and mid-pregnant and were not found to differ from either time point (p > 0.05). Distally, virgin tissues had median values of 6.45, 0.0123 s, 26.3 s, and 0.124%/ln (s) for parameters c, τ1, τ2, and slope of relaxation, respectively (Table 5). When compared to the distal virgin values, the distal mid-pregnant specimen also displayed an increased slope of relaxation (p = 0.029) and a 65.9% increase in median C value (p = 0.008). In the distal cervix, both τ1 and τ2 were significantly decreased by mid-pregnancy (p = 0.020 and 0.005, respectively). After four weeks of recovery postpartum, values for the viscous parameters remained similar to those of the mid-pregnant tissues with an increased slope of relaxation (p = 0.046) and a median C value 66.6% greater (p = 0.046) than those of the virgin samples. For the viscous parameters, the only observed difference between proximal and distal cervices occurred within the mid-pregnant group, as the median value of τ1 was found to be an order of magnitude less for the distal cervix. By inserting parameters C, τ1, and τ2 into Eq. (1), the theoretical stress-relaxation curve, G(t), can be obtained for a specimen (Fig. 4). As illustrated in Fig. 4, mid-pregnant tissues experience a greater rate of relaxation than both virgin and postpartum specimen.
Table 4.
Median (25th percentile–75th percentile) values of the constants describing the reduced relaxation function of the proximal portion virgin (n = 7), mid-pregnant (n = 7), and postpartum (n = 9) Long-Evans rat cervix.
| C | τ1 (s) | τ2 (s) | dG/d(ln(t)) | |
|---|---|---|---|---|
| Virgin | 3.15 (2.51–4.05) | 0.0143 (0.0137–0.0185) | 45.5 (36.5–62.5) | 0.120 (0.117–0.124) |
| Mid-pregnant | 7.90 (6.05–17.1) | 0.0154 (0.0116–0.0164) | 18.8 (6.64–27.3) | 0.145 (0.126–0.156) |
| Four weeks postpartum | 19.5 (2.78–23.7) | 0.0185 (0.0146–0.0205) | 24.5 (6.98–45.1) | 0.155 (0.122–0.165) |
Table 5.
Median (25th percentile–75th percentile) values of the constants describing the reduced relaxation function of the distal portion virgin (n = 8), mid-pregnant (n = 6), and postpartum (n = 9) Long-Evans rat cervix.
| C | τ1 (s) | τ2 (s) | dG/d(ln(t)) | |
|---|---|---|---|---|
| Virgin | 6.45 (3.33–10.8) | 0.0123 (0.0091–0.0147) | 26.3 (15.4–3.56) | 0.124 (0.1201–0.134) |
| Mid-pregnant | 18.9 (14.0–30.7) | 0.0071 (0.0049–0.0097) | 6.42 (4.92–9.90) | 0.1436 (0.136–0.155) |
| Four weeks postpartum | 19.3 (11.4–27.1) | 0.00131 (0.0096–0.0146) | 14.9 (8.70–17.8) | 0.148 (0.131–0.154) |
Fig. 4.
Theoretical reduced stress relaxation curves for representative (a) proximal and (b) distal; virgin (short dashed), mid-pregnant (solid), and postpartum (dotted) cervical tissues in response to unconfined compression.
4.3. Hydroxproline assay
For the proximal cervix, the collagen content increased by 35.6% during pregnancy compared to the virgin cervix (p = 0.05). There was no significant change in collagen content between proximal portions of the virgin to four weeks postpartum samples (p = 1) or mid-pregnant to four weeks postpartum samples (p = 0.403). Distally, no differences were observed between the three experimental time points in terms of collagen content. When comparing the proximal and distal cervices within the same experimental time point it was noted that the proximal portion consistently had lower collagen content. For virgin samples the proximal portion had 56.5% less collagen (p < 0.001) when compared to the distal cervix. Similarly the mid-pregnant and four weeks postpartum cervices had 29.5% and 34.5% less collagen proximally (p = 0.007 and p < 0.001, respectively).
5. Discussion
The objective of this study was to define the change in the viscoelastic properties of the proximal and distal portions of the cervix in pregnancy and recovery from this process postpartum. Utilizing Fung’s QLV theory, parameters describing the viscoelastic behavior of the cervix were determined from ramp relaxation testing of virgin, mid-pregnant, and postpartum specimen. The critical findings of the study were that the elastic response constant B, viscous response constant C, and the slope of the relaxation curve increased during pregnancy. In addition, at four weeks postpartum the constant B returned to virgin levels while the viscous parameters C and slope of relaxation remained similar to mid-pregnant values. Finally, the proximal and distal cervix were shown to differ in collagen content with the distal cervix containing a higher amount of collagen in virgin, mid-pregnant, and four weeks postpartum tissues corresponding to the stiffer elastic response distally.
The results from this study refute our hypothesis that pregnant tissue will exhibit a less stiff elastic response. Interestingly, the parameter B, which governs the nonlinearity of the stress–strain curve, was found to be significantly higher for pregnant tissues in both the proximal and distal sections. Therefore, as strain increases, the slope of the stress–strain curve becomes greater, resulting in a stiffer tissue response. During pregnancy, the growing fetus places an increasing demand on the cervix. This finding suggests that the cervix may be remodeling to meet this increased demand in load. Intuitively, it seems that increased water content and extracellular matrix (ECM) disorganization would construct a less stiff tissue. In order to increase stiffness as observed in the mid-pregnant cervix, collagen disorganization which affords a large influx of water, may increase swelling and lower the porosity of pregnant cervix.5 Reduced porosity would restrict water movement, trapping more water within the tissue at higher levels of strain and elicit a stiffer stress response. This is further supported by the great degree of energy dissipation in the mid-pregnant group (parameter C). Additionally, studies have found considerable changes in the collagen fibril size and arrangement even at early pregnancy using second harmonic generation (SHG) microscopy. Utilizing this imaging technique in the murine model it has been reported that pore size increases during pregnancy, while the number of pores in the matrix decreases.18
The results supported our hypothesis that pregnant tissue will demonstrate increased viscous behavior when compared to virgin and postpartum samples. Pregnant samples were found to have higher C values, which are proportional to the percent relaxation experienced by the tissue. This would increase the ability of the pregnant cervix to dissipate loads, and hence, absorb the weight of the fetus. The data is consistent with previous studies that have shown the water content to increase during pregnancy, as C is related to the water content of soft tissues.3,4 Throughout the entire cervix, pregnancy was also shown to increase the slope of the stress-relaxation curve and reduce the time at which relaxation terminates. The increased slope of G(t) signifies that the tissues more readily releases water when strain is applied. The increased ability for water to move through the tissue may result from an adaptation of proteoglycans within the matrix, allowing for loads to be more quickly dissipated. The increased rate of load reduction leads to shorter time periods of stress-relaxation and remarkably efficient absorption of increasing loads.
Additionally, the distal cervix was found to have higher B values in both virgin and postpartum tissues when compared to proximal samples at the same time point. The location dependent response is consistent with the findings by Myers et al. in the human cervix.6 Since the passive properties are dependent on the ECM, the stiffer response of the distal rat cervix is likely due a higher fraction of collagen, which has also been observed in the human cervix.6 The fact that no such trend was observed for mid-pregnant tissues suggests that during pregnancy the ECM of the cervix becomes more homogenous throughout its entirety as a result of increased collagen content (decrease in collagen concentration) and proteoglycan production, collagen structure disruption, and smooth muscle apoptosis.4–6,20 Additionally, postpartum values for the parameter B returned to virgin levels, suggesting that the tissue successfully reconstructs the ECM following delivery.
In order to assess the predictive capabilities these QLV parameters for the cervix, the several specimen also underwent cyclic testing following the three trials of stress-relaxation. During these trials the tissues were cycled between 0% and 20% strain at a rate of 0.167 mm/s for a total of 10 cycles. These preliminary results have shown that the obtained parameters do not predict the viscous loss exhibited by the cervix, although these parameters did fit the ramping and relaxation data well (minimum overall R2 > 0.92). The inability of the model to predict this response may result from the tissue behaving more nonlinearly than the QLV model is capable of describing. Thus, the use of the QLV model in this study is simply as a tool to help us interpret differences in the stress-relaxation response between groups and not as a generalized description of the viscoelastic behavior of the cervix over a range of strain levels.
In terms of collagen content, the hydroxyproline assay supported our hypothesis, with greater amounts of collagen found in the distal cervix when compared to the proximal cervix. This was observed in all three experimental time points and coincides with the biomechanical results of this study, which found the distal cervix to exhibit a stiffer elastic response and reach greater magnitudes of stress at 20% strain. Furthermore, these results are consistent previous biomechanical and biochemical studies on the human cervix.5,6 While previous literature has found that the total amount of collagen of the cervix increases during pregnancy, these studies have not examined the collagen distribution in terms of the proximal and distal cervix. The present study finds that the overall amount of total collagen insignificantly increased by mid-pregnancy (p > 0.05); however the amount of collagen in the proximal cervix increased by 35.6% (p = 0.05). This increase may arise from apoptosis of smooth muscle, which is most concentrated proximally, allowing collagen to become more prevalent proximally. Additionally, by four weeks postpartum the collagen content remained slightly elevated when compared to the virgin tissue in the proximal cervix while the distal cervix had a noticeable decrease in collagen. Although not statistically different, these postpartum changes, in addition to gross observations and viscous parameters obtained from the QLV theory suggest that postpartum recovery does not return the cervix to its virgin state. Given the location-dependent composition and mechanical response of the cervix, it is important to first test and model anatomical locations of the cervix independently in order to fully understand adaptations during pregnancy as well as the postpartum recovery of this structure.
Overall, the postpartum group displayed increased inter and intra specimen variation in regards to their viscous response. A potential source of this variation arises from the postpartum remodeling of the cervix. While the proximal portion of the cervix appears to have returned more closely to virgin properties, the distal portion was found to be more similar to pregnant tissue in regards to the percentage of relaxation and rate of relaxation. This incomplete recovery may arise from the inability of the cervix to restore proteoglycan and collagen ratios, which were highly altered to prepare for pregnancy and delivery. As a result the cervix retains more water after pregnancy and remains more viscous than virgin tissues, which has been shown in the human cervix.
Although this study has identified differences in the viscoelastic properties resulting from pregnancy, there are several limitations of this data. Previous research has shown that the constituents of rodent cervix are similar to those of the human cervix, yet the cervical adaptations throughout pregnancy have not been thoroughly evaluated in the rodent model. Further analysis of the water, collagen, and GAG content as well as collagen structure and organization of the rat cervix is required to correlate the biochemical properties to the obtained viscoelastic results. In addition, this study may not sufficiently capture the softening, ripening, and postpartum recovery of the cervix due to the limited number of time-points considered. While an infinite number of time points are unrealistic, the current study neglects the conditions at term, delivery, as well as the immediate response of the cervix after delivery. Also, in this study the rodent cervix was compressed along the ventral–dorsal direction, whereas one would expect this tissue to bear a compressive load in the cranial–caudal direction. However, changes in tissue properties are likely detectable through palpation in the ventral–dorsal direction, as clinicians commonly assess the cervix through palpation of the cervical lumen during pregnancy.20
Future studies will evaluate the cervix closer to the time of vaginal delivery, 4 h postpartum, and upon induction of preterm labor. Also, contractile and biochemical assays will be utilized to measure changes in smooth muscle activity along with mass fractions of extracellular matrix constituents. These changes in tissue composition can then be correlated to the observed changes in mechanical behavior. Analyzing the cervix from these diverse perspectives will provide a thorough understanding of the mechanisms that lead cervical failure and preterm labor.
Fig. 1.
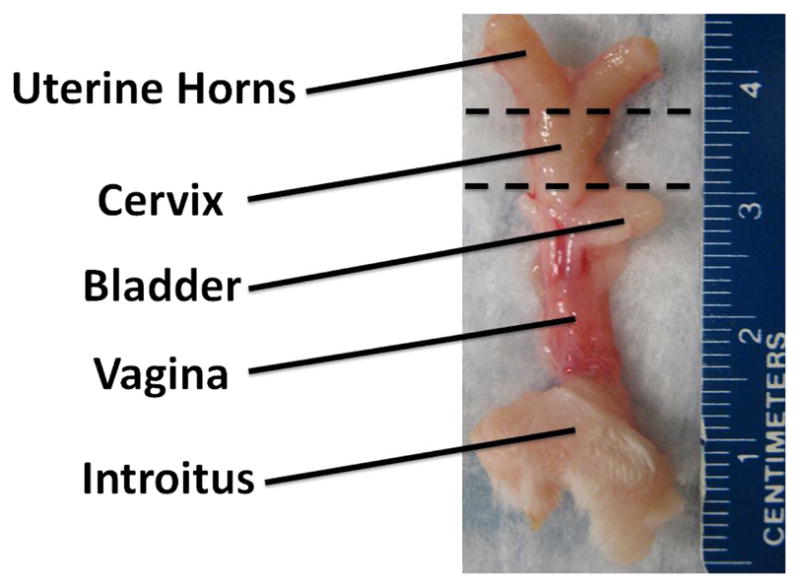
Reproductive tract of the virgin Long-Evans rat with location of uterine transection (superior dashed line) and vaginal transection (inferior dashed line).
Fig. 5.
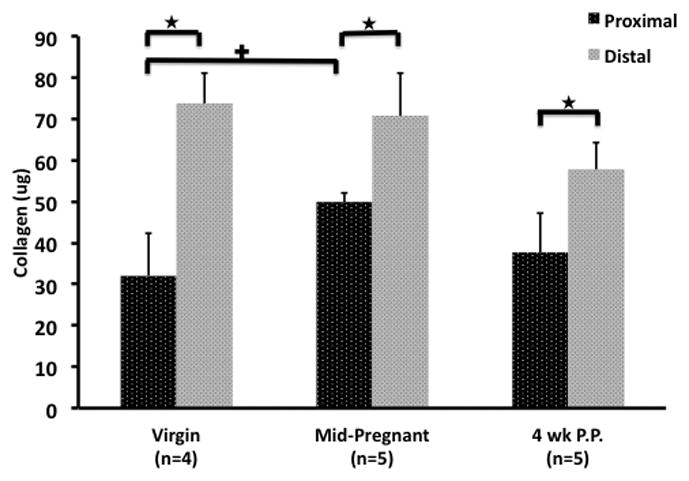
The collagen content of the proximal and distal cervices of the virgin, mid-pregnant, and four weeks postpartum Long-Evans rat. The collagen content was obtained by multiplying the measured collagen concentration by the initial volume of the standard.
★signifies a difference between the proximal and distal cervices within the same experimental time point (p < 0.05).
+ signifies a difference between similar sections of the cervix between two different experimental time points (p < 0.05).
Acknowledgments
The authors acknowledge the financial support provided by NIH Grants-R01HD-045590 and K12HD-043441.
References
- 1.Ross Michael G. Preterm Labor. Emedicine. 2008 http://emedicine.medscape.com/article/260998-overview.
- 2.Challis John RG. Mechanism of parturition and preterm labor. Obstet Gynecol Surv. 2000;55 (10):650–660. doi: 10.1097/00006254-200010000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Carbonne B. Is it possible to improve diagnostic and prognostic criteria of preterm labour? Eur J Obstet Gynecol Reprod Biol. 2004;117S:S6–S9. doi: 10.1016/j.ejogrb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Leppert PC. Anatomy and physiology of cervical ripening. Clin Obstet Gynecol. 1995;38 (2):267–279. doi: 10.1097/00003081-199506000-00009. [DOI] [PubMed] [Google Scholar]
- 5.House M, Kaplan DL, Socrate S. Relationships between mechanical properties and extracellular matrix constituents of the cervical stroma during pregnancy. Semin Pertinatol. 2009;33:300–307. doi: 10.1053/j.semperi.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myers KM, et al. Mechanical and biochemical properties of human cervical tissue. Acta Biomater. 2008;4(1):104–116. doi: 10.1016/j.actbio.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Woo Savio L-Y, Gomez MA, Akeson WH. The time and history-dependent viscoelastic properties of the canine medical collateral ligament. J Biomech Eng. 1981;103(4):293–298. doi: 10.1115/1.3138295. [DOI] [PubMed] [Google Scholar]
- 8.Kwan MK, Lin TH, Woo Savio L-Y. On the viscoelastic properties of the anteromedial bundle of the anterior cruciate ligament. J Biomech. 1993;26(4–5):447–452. doi: 10.1016/0021-9290(93)90008-3. [DOI] [PubMed] [Google Scholar]
- 9.Doehring TC, et al. Fractional order viscoelasticity of the aortic valve cusp: An alternative to quasilinear viscoelasticity. J Biomech Eng. 2005;127:700–708. doi: 10.1115/1.1933900. [DOI] [PubMed] [Google Scholar]
- 10.Lim Y-J, et al. In situ measurement and modeling of biomechanical response of human cadaveric soft tissues for physics-based surgical simulation. Surgical Endosc. 2009;23 (6):1298–1307. doi: 10.1007/s00464-008-0154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang W, et al. Viscoelasticity of esophageal tissue and application of a QLV model. J Biomech Eng. 2006;128:909–916. doi: 10.1115/1.2372473. [DOI] [PubMed] [Google Scholar]
- 12.Fung YC. Biomechanics: Mechanical Properties of Living Tissues. 2. Springer-Science+Business Media LLC; New York: 2004. Quasi-linear viscoelasticity of soft tissue; pp. 277–292. [Google Scholar]
- 13.Lowder JL, et al. Biomechanical adaptations of the rat vagina and supportive tissues in pregnancy to accommodate delivery. Obstet Gynecol. 2007;109(1):136–142. doi: 10.1097/01.AOG.0000250472.96672.6c. [DOI] [PubMed] [Google Scholar]
- 14.Moon DK, Woo Savio L-Y, et al. The effects of refreezing on the viscoelastic and tensile properties of ligaments. J Biomech. 2005;39:1153–1157. doi: 10.1016/j.jbiomech.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Abramowitch SD, et al. An evaluation of the quasi-linear properties of the healing medial collateral ligament in a goat model. Ann Biomed Eng. 2003;32(3):329–446. doi: 10.1023/b:abme.0000017539.85245.6a. [DOI] [PubMed] [Google Scholar]
- 16.Abramowitch SD, et al. An improved method to analyze the stress relaxation of ligaments following a finite ramp time based on the quasi-linear viscoelastic theory. J Biomech Eng. 2004;126:92–97. doi: 10.1115/1.1645528. [DOI] [PubMed] [Google Scholar]
- 17.Speich JE, et al. ROK-induced cross-link formation stiffens passive muscle: Reversible strain-induced stress softening in rabbit detrusor. Am J Physiol Cell Physiol. 2005;289:12–21. doi: 10.1152/ajpcell.00418.2004. [DOI] [PubMed] [Google Scholar]
- 18.Akins ML, Luby-Phelps K. Second harmonic generation imaging as a potential tool for staging pregnancy and predicting preterm birth. J Biomed Opt. 2010;15(2):026020. doi: 10.1117/1.3381184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludmir J, et al. Anatomy and physiology of the uterine cervix. Clin Obstet. 2000;43 (3):433–439. doi: 10.1097/00003081-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Mazza E, et al. Mechanical properties of the human uterine cervix: An in vivo study. Med Image Anal. 2006;10:125–136. doi: 10.1016/j.media.2005.06.001. [DOI] [PubMed] [Google Scholar]