Abstract
Using the DUMAS (Dual acquisition Magic Angle Spinning) solid-state NMR approach, we created new pulse schemes that enable the simultaneous acquisition of three dimensional (3D) experiments on uniformly 13C, 15N labeled proteins. These new experiments exploit the simultaneous cross-polarization (SIM-CP) from 1H to 13C and 15N to acquire two 3D experiments simultaneously. This is made possible by bidirectional polarization transfer between 13C and 15N and the long living 15N z-polarization in solid state NMR. To demonstrate the power of this approach, four 3D pulse sequences (NCACX, CANCO, NCOCX, CON(CA)CX,) are combined into two pulse sequences (3D DUMAS-NCACX-CANCO, 3D DUMAS-NCOCX-CON(CA)CX) that allow simultaneous acquisition of these experiments, reducing the experimental time by approximately half. Importantly, the 3D DUMAS-NCACX-CANCO experiment alone makes it possible to obtain the majority of the backbone sequential resonance assignments for microcrystalline U-13C,15N ubiquitin. The DUMAS approach is general and applicable to many 3D experiments, nearly doubling the performance of NMR spectrometers.
Keywords: Dual Acquisition Magic Angle Spinning, SIM-CP, DUMAS, Solid-state NMR, proteins. 3D DUMAS-NCACX-CANCO, 3D DUMAS-NCOCX-CON(CA)CX, microcrystalline ubiquitin
INTRODUCTION
In NMR spectroscopy, the return of nuclear magnetization to thermal equilibrium after absorption of electromagnetic radiation is a relatively slow process. As a result, for both liquid and solid-state NMR spectroscopy, an ensemble of spins can be manipulated using multinuclear and multiple pulse excitation experiments. The longitudinal and transverse spin relaxation rates in liquids and solids occur with substantially different kinetics[1], with solid samples manifesting faster transverse spin relaxation rates and substantially slower longitudinal relaxation rates than the liquid counterparts. We recently exploited these phenomena and used the long living 15N polarization[2] generated by simultaneous cross polarization (SIM-CP) of 1H, 13C, and 15N to create two separate ‘excitation pathways’ in dual acquisition of magic angle spinning (DUMAS) experiments. These experiments take advantage of the 15N-13C dipolar decoupling under magic angle spinning conditions to register two separate multidimensional experiments, and we successfully demonstrated the application of DUMAS on isotopically labeled microcrystalline and integral membrane proteins[3]. In this Communication, we demonstrate that the DUMAS approach enables the acquisition of two 3D experiments simultaneously. The SIM-CP block creates the initial polarization for 13C and 15N that is combined with specific-CP between 15N and 13C to generate two independent pathways for the magnetization, resulting in two separate 3D experiments. The DUMAS method is general and applicable to many 3D pulse sequences. Here, we combine four commonly utilized 3D pulse sequences: NCACX[4], CANCO[5], NCOCX[4], and CON(CA)CX[6] into two 3D pulse sequences namely, 3D DUMAS-NCACX-CANCO and 3D DUMAS-NCOCX-CON(CA)CX. This approach reduces the experimental time and nearly doubling the capacity of the NMR spectrometers. Notably, we show that by using only one experiment (3D DUMAS-NCACX-CANCO), we obtained ~73% of the resonance sequential assignment for microcrystalline U-15N,13C ubiquitin. This new way of concatenating NMR experiments in solids can speed up the characterization of isotopically labeled biomacromolecules and serves as a natural complement to the most recent sensitivity enhancement techniques such as paramagnetic relaxation enhancement (PRE) and dynamic nuclear polarization (DNP). These techniques used in tandem promises to push the boundaries of the spectroscopy of biomacromolecules by MAS ssNMR.
EXPERIMENTAL
All of the NMR experiments were performed using VNMRS spectrometers operating at a 1H Larmor frequency of either 600 or 700 MHz. Approximately 5 mg of U-15N,13C microcrystalline ubiquitin was packed into a 3.2 mm rotor, spinning rate (ωr) of 8.333 kHz was used for all of the experiments. Experiments on ubiquitin were performed at 5°c, while for NAVL 20°C was used. The 15N RF carrier was centered at 120 ppm, while the 13C RF was centered at either 60 ppm or 170 ppm, Cα or CO regions, respectively. The 90° pulse length for 1H, 13C and 15N were 2.5, 6, and 6 μs, respectively. During SIM-CP, 13C and 15N RF amplitudes were set to 35 kHz, whereas 1H RF amplitude was linearly ramped from 80 to 100 %, with the center of the slope set to 51.66 kHz. The optimal Hartmann-Hahn (HH) contact time for the SIM-CP was found to be 300 μs for ubiquitin and 1 ms for NAVL, based on the 1D calibration spectra. For the specific-CP transfer from 15N to 13Cα (or 13CO), the 13C offset was shifted to 60 (or 177) ppm. During specific-CP, the 15N RF amplitude was set to (5/2)·ωr (=20 kHz), whereas 13C RF amplitude was set to (3/2)·ωr (= 12 kHz) and (7/2)·ωr (= 28 kHz) for 13Cα and 13CO specific-CP, respectively. The specific-CP was implemented with an adiabatic ramp (Δ~1.6 kHz and β=0.5 kHz), and the contact times for NCA and NCO transfers were set to 3.035 and 5.029 ms, respectively. For heteronuclear decoupling (CW or TPPM), the 1H RF amplitude was set to 100 kHz. During the DARR mixing period, 1H RF amplitude was set to 8.333 kHz (ωr). The two dimensional NCA and DUMAS-NCA experiments were acquired with 32 scans, whereas CAN and DUMAS-CAN were acquired using 64 scans. The dwell time of t1′ and t1″ were set to 150 and 300 μs, respectively, with 32 increments. 3D DUMAS-NCACX-CANCO was acquired on the ubiquitin sample using 96 scans with 20 ms DARR mixing time. 3D DUMAS-NCOCX-CON(CA)CX was acquired on a dipeptide NAVL using 16 scans, where two DARR mixing times were used for NCOCX element with 8 scans each. The dwell time for the t1″ and t2′ evolution periods was set to 150 μs, while the dwell for the t1′ and t2″ evolution periods was set to 300 μs. The number of increments was 21 in both indirect dimensions. In the indirect dimensions, the total evolution time for 13C and 15N were 3 and 6 ms respectively. All of the spectra were recorded using a dwell time of 10 μs in the direct dimension, and a 13C acquisition time of 20 ms during t3′ and t3″. For the 3D DUMAS-NCACX-CANCO and 3D DUMAS-NCOCX-CON(CA)CX experiments, the 13C RF carrier frequency was centered at 60 and 170 ppm, respectively, while the 15N RF carrier frequency was centered at 120 ppm. The 13C spectra were referenced with respect to the CH2 resonance of adamantane at 40.48 ppm and indirectly to 15N using a relative gyromagnetic ratio of 15N and 13C. A recycle delay of 2s was used in all experiments. All of the spectra were processed using NMRpipe[7] and analyzed with Sparky[8].
RESULTS
Optimization of SIM-CP
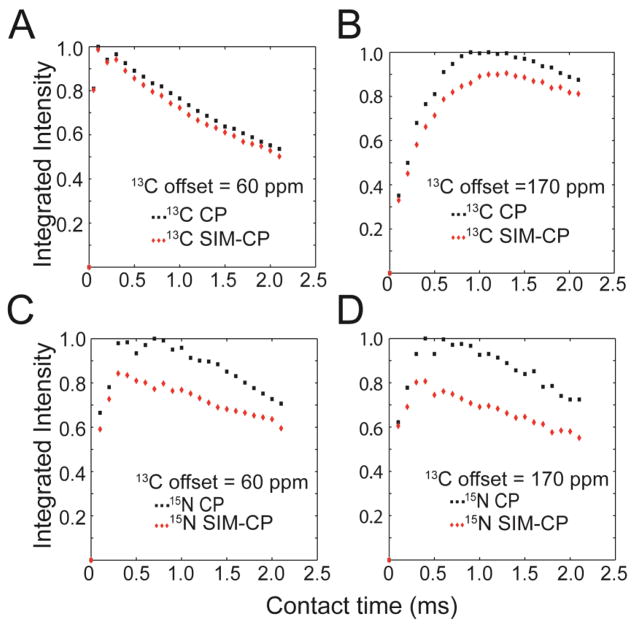
Typically, sequential assignment of proteins in MAS ssNMR is accomplished by using 3D pulse sequences such as NCACX[4], CANCO[5], NCOCX[4], CAN(CO)CX and CON(CA)CX, that enable one to ‘walk’ along the polypeptide backbone resonances. In these experiments, after the initial HH CP from 1H to either 13C or 15N, the polarization of 13Cα, 13CO, or 15N is transferred via different pathways to the backbone and side chain nuclei, and chemical shifts are then encoded in evolution periods and separated into three dimensions. For these experiments the 13C offset is centered to either CA or CO regions during the initial HH CP for optimum polarization transfer and subsequent t1 evolution with relatively smaller spectral width. For instance, during the initial 1H-13C CP for the CAN(CO)CX and CON(CA)CX experiments, the 13C offset is set to the center of CA and CO regions, respectively. In contrast, the DUMAS approach is made possible by simultaneous CP from the 1H bath to the heteronuclear spins (13C and 15N), i.e. SIM-CP. To systematically test the efficiency of polarization transferred during SIM-CP (Figure 1A), we carried out a series of experiments on a 600 MHz spectrometer using microcrystalline ubiquitin. The RF amplitude for 13C and 15N were set to 35 kHz, and the 1H RF amplitude was ramped from 80 to 100 % with the center of the ramp (51.66 kHz) corresponding to n=2 side band matching conditions. Figures 2A and 2B show the comparison between CP and SIM-CP of the integrated intensities of aliphatic and carbonyl carbon resonances at various contact times. In Figure 2A, the 13C offset was centered in the Cα region (60 ppm), while in Figure 2B the offset is centered in the CO region (170 ppm). While we observed a loss of ~10 % in the integrated intensity of the carbonyl region for the spectra with offset centered at 170 ppm, there is virtually no sensitivity loss for aliphatic carbon spectra with the 13C offset centered in the Cα region. We then repeated the above experiments, but detecting the 15N signals. Figures 2C and 2D compare the integrated intensity of the 15N resonances for both the CP and SIM-CP schemes, with 13C offset for SIM-CP centered in the Cα and CO regions. In both cases, we observed a sensitivity loss of ~15–20 %. These results are in agreement with our previous data[3]. Therefore, we conclude that the build-up rates of polarization transfer for CP and SIM-CP are very similar, with SIM-CP showing only marginal loss of sensitivity for 15N and 13CO spectra with respect to conventional CP.
Figure 1.
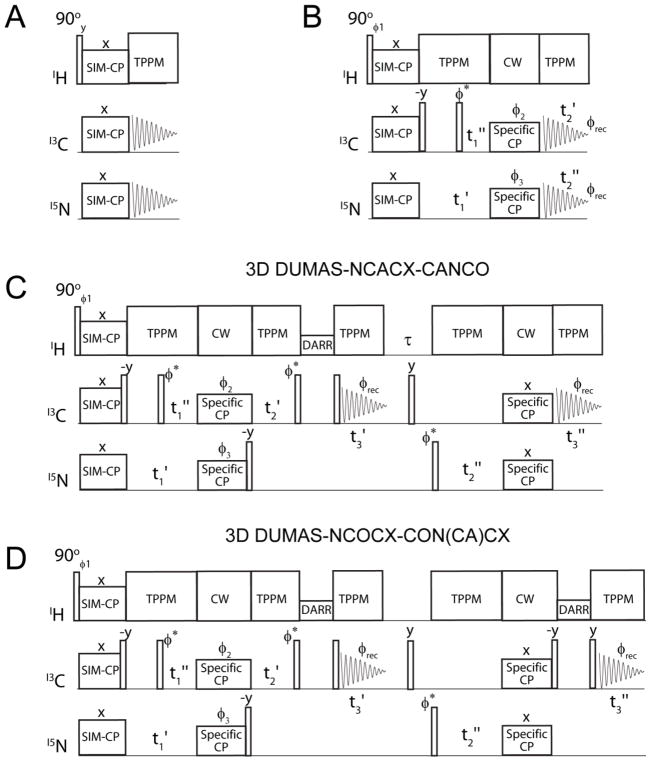
(A) Pulse sequence used for optimizing SIM-CP (simultaneous cross polarization) parameters (B) 2D DUMAS-NCA-CAN pulse sequence using a bidirectional specific-CP transfer preceded by 13C and 15N parallel evolutions. 13C and 15N are detected in separate experiments. The φ2, φ3 phase cycle allows the magnetization from 15N to 13Cα and vice versa. (C) DUMAS pulse scheme for simultaneous acquisition of NCACX and CANCO 3D experiments. The evolution of t1′ and t1″ are incremented in parallel, similarly t2′ is incremented in parallel with t2″. The dwell time of t1′ and t2″ are twice to t1″ and t2′. The phase of φ* is shifted between 0° and 90° for states mode acquisition of the indirect dimensions. (D) DUMAS scheme for simultaneous acquisition of NCOCX and CON(CA)CX 3D experiments. φ1 = (y, −y)4; φ2 = (y, y, y, y, −y, −y, −y, −y); φ3=(x, x, −x, −x)2; φrec=(x, −x, −x, x, −x, x, x, −x).
Figure 2.
Comparison of the relative sensitivity of CP (black) and SIM-CP (red) pulse sequences performed on U-15N,13C microcrystalline ubiquitin at various contact times. For both CP and SIM-CP, the 1H RF amplitude was ramped from 80 to 100 %. The 13C and 15N RF amplitudes satisfy the n=2 side band matching condition. The 13C offset was centered at 60 ppm for (A) and (C), and 170 ppm for (B) and (D). The integrated intensities for the aliphatic and carbonyl regions are shown in (A) and (B) respectively, while the integrated intensities for the 15N spectra are shown in (C) and (D). In each plot, the maximum intensity obtained with CP was normalized to 1. These plots demonstrate that SIM-CP is as efficient as conventional CP for aliphatic 13C region, with a marginal loss (5–15 %) for 13CO and 15N spectra.
Bidirectional versus unidirectional specific-CP
In conventional 3D pulse sequences, polarization transfer between 15N and 13C after the initial double resonance CP is obtained via specific-CP[9]. For the NCACX and CANCO experiments, the specific-CP transfers the polarization from N to CA and CA to N, respectively, in a unidirectional manner. Here, we show that SIM-CP followed by specific-CP enables a bidirectional polarization transfer between 13C (either CA or CO) and 15N. For example, after SIM-CP, the polarization transfer from N to CA and CA to N occurs simultaneously using a specific-CP optimized for the CA region. The SIM-CP and specific-CP are combined in Figure 1B in the 2D DUMAS-NCA-CAN experiment. After SIM-CP, 13C magnetization is aligned along the z-direction for a period of t1′-t1″, during which 15N evolution takes place. 13C magnetization is then brought onto the transverse plane and evolves simultaneously with 15N during t1″. Note that the t1′ and t1″ evolution periods are nested, enabling the evolution of both 13C and 15N, simultaneously. In our experiments, the dwell of t1′ is half of the t1″, which ensures that the total evolution time (t1′) and the dwell time of 15N is twice that of 13Cα (t1″). After the t1 evolution, specific-CP transfers the magnetization from N to CA and vice versa, which is then followed by either 13C or 15N detection. The 13C and 15N coherences are acquired in separate experiments to give NCA and CAN 2D spectra, respectively. The pulse sequence for this experiment is named as 2D DUMAS-NCA-CAN (Figure 1B). The phase cycle (φ2, φ3) of the specific-CP suppresses the direct polarization (if any) from 13C and 15N that is not transferred to 15N and 13C respectively. Figure 1S shows the comparison of NCA and CAN spectra acquired on 600 MHz spectrometer using conventional and DUMAS approach (Figure 1B). For conventional NCA and CAN experiments, SIM-CP of DUMAS-NCA-CAN (Figure 1B) is replaced by double resonance 1H-15N and 1H-13C CP respectively. The DUMAS spectra are similar to conventional spectra, with a marginal loss of sensitivity (~10%) for the DUMAS-NCA spectrum, due to the lower efficiency of the 15N SIM-CP with respect to CP. These results demonstrate that SIM-CP in combination with specific-CP can be used for bidirectional polarization transfer. In the following sections these elements are utilized for the design of the 3D DUMAS experiments.
3D DUMAS experiments
Figure 1C shows the DUMAS pulse sequence for simultaneous acquisition of NCACX and CANCO (3D DUMAS-NCACX-CANCO). After the NCA/CAN block shown in Figure 1B, a 90° pulse on 15N aligns the magnetization along z-direction, while 13Cα evolves during t2′ under 1H decoupling. This is followed by a DARR mixing sequence and the 13Cα signal is detected during the first acquisition t3′ under 1H decoupling. This first acquisition gives the 3D NCACX spectrum. After the first acquisition, a 90° pulse in the middle of τ period (~6 ms) dephases any residual carbon magnetization. A 90° pulse applied on 15N then brings the magnetization to transverse plane, leading to 15N evolution during t2″ under 1H decoupling. Subsequently, the 15N magnetization is transferred to CO using specific-CP, which is then detected during second acquisition under 1H decoupling. This second acquisition gives a 3D CANCO spectrum. The t2′ and t2″ evolutions are incremented simultaneously, setting the dwell time for t2″ to twice that of t2′. Additionally, the pulse sequence shown in Figure 1C can also be used to concatenate the NCOCX and CONCA experiments. In this case, the first and second specific-CP elements are optimized for NCO (CON) and NCA polarization transfers, and the 13C offset is set in the center of the CO region. In Figure 1D, we report the 3D DUMAS-NCOCX-CON(CA)CX experiment, where the first and second specific transfers were optimized for NCO (CON) and NCA transfers, respectively.
Sequential assignment of U-13C,15N ubiquitin using 3D DUMAS-NCACX-CANCO
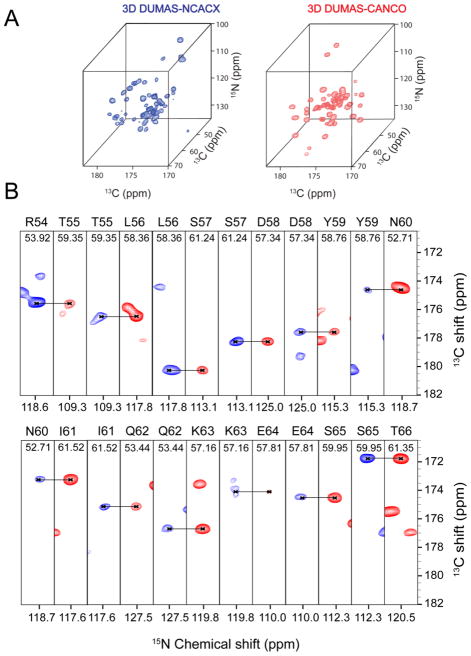
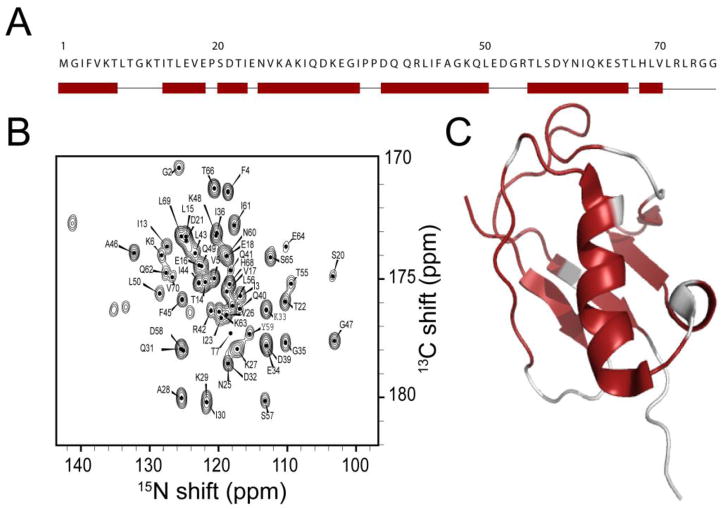
To demonstrate the potential of the DUMAS approach, we tested the 3D DUMAS-NCACX-CANCO on a 700 MHz spectrometer using ~ 5 mg of microcrystalline sample of U-13C,15N ubiquitin. Figure 3 shows the strip plots of NCO planes at various 13Cα chemical shifts obtained from two 3D NCACX and CANCO spectra acquired simultaneously using the pulse sequence 3D DUMAS-NCACX-CANCO (Figure 1C). At short DARR mixing time of 20 ms, for each pair of 15N and 13Cα, the 13CO resonance of NCACX and CANCO correspond to intra (blue) and (i - 1) inter (red) residue correlations. Hence, a sequential walk can be obtained using only these two spectra. Indeed, we are able to detect 56 out of 75 sequential backbone connectivities. Additionally, the NCACX spectrum detects the side chain resonances, enabling the assignment of 45 Cα-Cβ correlations. Most of the unassigned residues are located in the dynamic regions of the protein, where the dipolar based polarization transfer is inefficient[10]. In spite of two specific-CP periods, the sensitivity of CO peaks from CANCO spectrum is about 2 times higher than the corresponding NCACX spectrum. This is due to partial polarization transfer from CA to CO during the DARR mixing period of the NCACX experiment. The sequential walk from residues 54 to 65 is shown in Figure 3. Side chain assignments are then obtained from the NCACX spectrum. Figure 2S shows the resonance assignments for the side chains of residues 55– 66, while Figure 4 summarizes the sequential assignments obtained for ubiquitin using the 3D DUMAS approach. The resonance assignments obtained using 3D DUMAS-NCACX-CANCO are in agreement with those determined by Meier and co-workers[11] BMRB entry: 7111).
Figure 3.
(A) Three dimensional spectra of NCACX (blue) and CANCO (red) acquired using 3D DUMAS-NCACX-CANCO, with 96 scans and 21 increments in both indirect dimensions. Total experimental time was 4.16 days. (B) 15N-13CO strip plots of the above 3D spectra NCACX (blue) and CANCO (red) at corresponding Cα chemical shifts. The sequential backbone resonance assignments for residues spanning the region 54–66 are shown. DARR mixing time of 20 ms was used.
Figure 4.
(A). Sequential assignment of ubiquitin backbone resonances obtained from the 3D DUMAS-NCACX-CANCO is indicated by rectangles along the primary sequence, whereas narrow lines indicate the unassigned residues. (B) NCO spectrum with the resonance assignments obtained by summing the F1 planes of the 3D CANCO spectrum. The assignments are obtained from the sequential walk of 3D spectra as shown in Figure 3. (C) Mapping (red color) of the assigned resonances on the backbone of ubiquitin three-dimensional structure (PDB ID: 2JZZ).
The current selection of solid-state NMR pulse sequences available for backbone and side chain assignments does not possess the same degree of sensitivity. For example, due to an additional specific-CP, the sensitivity of the CAN(CO)CX is about half of the NCOCX experiment for the same DARR mixing time. For an efficient concatenation of multidimensional experiments, one should combine n sensitive 3D experiments each with x number of scans with an insensitive 3D experiment with n times x scans. For instance, we acquired the 3D DUMAS-NCOCX-CON(CA)CX experiment on U-13C,15N NAVL dipeptide, combining two NCOCX experiments (8 scans, 20, and 50 ms DARR mixing times) with a single CON(CA)CX (16 scans, 20 ms DARR mixing time) (Figure 1D). Figure 3S shows the strip plots of NCOCX and CON(CA)CX spectra from the 3D DUMAS-NCOCX-CON(CA)CX experiment. All the strip plots are drawn at 13C chemical shift of 177 ppm, corresponding to the valine signal. While the spectrum acquired with 20 ms DARR mixing time shows intra-residue carbon correlations, the longer mixing time (50 ms) displays intra- as well as inter-residue correlations. Analogously, the pulse sequence shown in Figure 1D can also be used to concatenate the NCACX and CAN(CO)CX experiments. In this case, the first and second specific-CP elements are respectively optimized for NCA (CAN) and NCO polarization transfers with the 13C offset set at the CA region.
DISCUSSION
To achieve the simultaneous acquisition of multidimensional spectra using 3D DUMAS, we polarize two nuclei simultaneously using SIM-CP from the 1H bath to 13C and 15N[3]. The 15N z-magnetization generated under MAS conditions relaxes at a relatively slower rate [2], enabling the acquisition of the second multi dimensional experiment. Based on our data, the SIM-CP is as efficient as the conventional CP for aliphatic carbon region, with a marginal loss for 13CO and 15N spectra. Also, our data show that qualitatively the buildup of polarization for both CP and SIM-CP occur with very similar kinetics. As for the CP, the quantitative theoretical treatment for SIM-CP spin dynamics is complicated by the network of homo- and hetero-nuclear dipolar interactions and is beyond the scope of this paper. Therefore, for the optimization of such experiments, we relied on the integrated intensities of the 1D NMR spectra and the optimization of several experimental parameters such as contact time, RF amplitudes, RF ramping constant to maximize the signal to noise ratio. As pointed out previously[3], the 13C and 15N spin lock periods during SIM-CP do not have to equal. A longer 13C (or 15N) spin lock period would improve the signals arising from the most dynamic regions of the biopolymers or for the mobile side chains.
Moreover, to combine 3D experiments using the DUMAS approach, the relative sensitivity of the 3D experiments must be taken into account. For instance, the sensitivity of the 3D NCOCX is roughly twice that of the 3D CON(CA)CX under identical DARR mixing periods. This is because the CON(CA)CX experiment uses two specific-CP elements, whereas the NCOCX uses only one specific-CP. Nonetheless, the CON(CA)CX experiment can be combined using the DUMAS approach with two or more sensitive NCOCX experiments with different mixing times and mixing blocks (DARR, CHHC, SPC5, DREAM, etc.) and be used to obtain constraints for sequential, medium, and long-range distances as well as side chain restraints. An example is shown in Figure 3S. In other words, the DUMAS approach enables one to combine an insensitive 3D experiment with two or more relatively more sensitive experiments.
Recently, other groups have utilized simultaneous cross polarization schemes to increase the sensitivity of NMR spectra or to acquire specific 2D spectra in parallel[12–14]. In contrast to these experiments, the DUMAS approach is not limited to selected experiments, and can be considered a general scheme for combining several 2D, 3D, and possibly 4D experiments for protein sequential assignment and for inter-nuclear distance measurements. Therefore, DUMAS is a general approach to concatenate multidimensional solid-state NMR experiments, speeding up the current techniques for resonance assignment and distance measurements of biopolymers such microcrystalline proteins[15–17], membrane embedded proteins[18–20], and fibrils[21, 22].
Finally, we would like to stress that the DUMAS approach is not an alternative to other sensitivity enhanced methods such as PRE[23, 24], DNP[25], and 1H detection of perdeuterated samples[26]. Rather, it can be used in combination with these methods to ‘make the best of the polarization’. The future application of the DUMAS experiments will include the use of quadruple CP (1H-2H-13C-15N) on perdeuterated samples in combination with multiple receivers for further sensitivity optimization of NMR experiments in solids.
CONCLUSIONS
In conclusion, we have generalized the DUMAS approach for simultaneous acquisition of three dimensional experiments. The DUMAS scheme reduces the acquisition time of multidimensional datasets by almost 50% and can be implemented on modern spectrometers using a single receiver. The DUMAS approach opens up new avenues for reducing the acquisition time for multi-dimensional experiments of biomolecular solid-state NMR by combining the sensitivity and resolution advancements achieved in the past few years. We anticipate that the combination of DUMAS with DNP, proton detection, PRE, perdeuteration, as well as multiple receivers, will further increase the sensitivity, resolution, and number of experiments that can be acquired simultaneously for structure determination of biomacromolecules.
Supplementary Material
HIGHLIGHTS.
DUMAS enables the simultaneous acquisition of two 3D spectra of proteins.
DUMAS nearly doubles the performance of conventional NMR spectrometers.
3D DUMAS-NCACX-CANCO enables 75% of backbone assignment of ubiquitin.
Acknowledgments
This work is supported by the National Institute of Health (GM 64742 and GM 72701). The authors would like to thank Dave Rice (Agilent), Dr. Frank Delaglio (nmrPipe) for helpful discussions, and to Fa-An Chao for preparation of the microcrystalline ubiquitin. 2D and 3D DUMAS pulse sequences are available for download at www.chem.umn.edu/groups/veglia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abragam A. Principles of Nuclear Magnetism. 1961. [Google Scholar]
- 2.Giraud N, Bockmann A, Lesage A, Penin F, Blackledge M, Emsley L. Site-specific backbone dynamics from a crystalline protein by solid-state NMR spectroscopy. J Am Chem Soc. 2004;126:11422–11423. doi: 10.1021/ja046578g. [DOI] [PubMed] [Google Scholar]
- 3.Gopinath T, Veglia G. Dual Acquisition Magic-Angle Spinning Solid-State NMR-Spectroscopy: Simultaneous Acquisition of Multidimensional Spectra of Biomacromolecules. Angew Chem Int Ed Engl. 2012 doi: 10.1002/anie.201108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pauli J, Baldus M, van Rossum B, de Groot H, Oschkinat H. Backbone and side-chain 13C and 15N signal assignments of the alpha-spectrin SH3 domain by magic angle spinning solid-state NMR at 17.6 Tesla. Chembiochem. 2001;2:272–281. doi: 10.1002/1439-7633(20010401)2:4<272::AID-CBIC272>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Berthold DA, Frericks HL, Gennis RB, Rienstra CM. Partial (13)C and (15)N chemical-shift assignments of the disulfide-bond-forming enzyme DsbB by 3D magic-angle spinning NMR spectroscopy. Chembiochem. 2007;8:434–442. doi: 10.1002/cbic.200600484. [DOI] [PubMed] [Google Scholar]
- 6.Franks WT, Kloepper KD, Wylie BJ, Rienstra CM. Four-dimensional heteronuclear correlation experiments for chemical shift assignment of solid proteins. J Biomol NMR. 2007;39:107–131. doi: 10.1007/s10858-007-9179-1. [DOI] [PubMed] [Google Scholar]
- 7.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 8.Goddard TD, Kneller DG. SPARKY. 1999;3 [Google Scholar]
- 9.Baldus M, Petkova AT, Herzfeld JH, Griffin RG. Cross polarization in the tilted frame: assignment and spectral simplification in heteronuclear spin systems. Mol Phys. 1998;95:1197–1207. [Google Scholar]
- 10.Schanda P, Meier BH, Ernst M. Quantitative analysis of protein backbone dynamics in microcrystalline ubiquitin by solid-state NMR spectroscopy. J Am Chem Soc. 2010;132:15957–15967. doi: 10.1021/ja100726a. [DOI] [PubMed] [Google Scholar]
- 11.Schubert M, Manolikas T, Rogowski M, Meier BH. Solid-state NMR spectroscopy of 10% 13C labeled ubiquitin: spectral simplification and stereospecific assignment of isopropyl groups. J Biomol NMR. 2006;35:167–173. doi: 10.1007/s10858-006-9025-x. [DOI] [PubMed] [Google Scholar]
- 12.Bjerring M, Paaske B, Oschkinat H, Akbey U, Nielsen NC. Rapid solid-state NMR of deuterated proteins by interleaved cross-polarization from (1)H and (2)H nuclei. J Magn Reson. 2012;214:324–328. doi: 10.1016/j.jmr.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen AB, Szekely K, Gath J, Ernst M, Nielsen NC, Meier BH. Simultaneous acquisition of PAR and PAIN spectra. J Biomol NMR. 2012 doi: 10.1007/s10858-012-9616-7. [DOI] [PubMed] [Google Scholar]
- 14.Linser R, Bardiaux B, Higman V, Fink U, Reif B. Structure calculation from unambiguous long-range amide and methyl 1H-1H distance restraints for a microcrystalline protein with MAS solid-state NMR spectroscopy. J Am Chem Soc. 2011;133:5905–5912. doi: 10.1021/ja110222h. [DOI] [PubMed] [Google Scholar]
- 15.Wylie BJ, Sperling LJ, Nieuwkoop AJ, Franks WT, Oldfield E, Rienstra CM. Ultrahigh resolution protein structures using NMR chemical shift tensors. Proc Natl Acad Sci U S A. 2011;108:16974–16979. doi: 10.1073/pnas.1103728108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDermott A, Polenova T. Solid state NMR: new tools for insight into enzyme function. Curr Opin Struct Biol. 2007;17:617–622. doi: 10.1016/j.sbi.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Sun S, Han Y, Paramasivam S, Yan S, Siglin AE, Williams JC, Byeon IJ, Ahn J, Gronenborn AM, Polenova T. Solid-state NMR spectroscopy of protein complexes. Methods Mol Biol. 2012;831:303–331. doi: 10.1007/978-1-61779-480-3_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cady SD, Schmidt-Rohr K, Wang J, Soto CS, Degrado WF, Hong M. Structure of the amantadine binding site of influenza M2 proton channels in lipid bilayers. Nature. 2010;463:689–692. doi: 10.1038/nature08722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi L, Kawamura I, Jung KH, Brown LS, Ladizhansky V. Conformation of a seven-helical transmembrane photosensor in the lipid environment. Angew Chem Int Ed Engl. 2011;50:1302–1305. doi: 10.1002/anie.201004422. [DOI] [PubMed] [Google Scholar]
- 20.Tang M, Sperling LJ, Berthold DA, Schwieters CD, Nesbitt AE, Nieuwkoop AJ, Gennis RB, Rienstra CM. High-resolution membrane protein structure by joint calculations with solid-state NMR and X-ray experimental data. J Biomol NMR. 2011;51:227–233. doi: 10.1007/s10858-011-9565-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helmus JJ, Surewicz K, Apostol MI, Surewicz WK, Jaroniec CP. Intermolecular alignment in Y145Stop human prion protein amyloid fibrils probed by solid-state NMR spectroscopy. J Am Chem Soc. 2011;133:13934–13937. doi: 10.1021/ja206469q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tycko R. Solid-state NMR studies of amyloid fibril structure. Annu Rev Phys Chem. 2011;62:279–299. doi: 10.1146/annurev-physchem-032210-103539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wickramasinghe NP, Parthasarathy S, Jones CR, Bhardwaj C, Long F, Kotecha M, Mehboob S, Fung LW, Past J, Samoson A, Ishii Y. Nanomole-scale protein solid-state NMR by breaking intrinsic 1HT1 boundaries. Nat Methods. 2009;6:215–218. doi: 10.1038/nmeth.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nadaud PS, Helmus JJ, Sengupta I, Jaroniec CP. Rapid acquisition of multidimensional solid-state NMR spectra of proteins facilitated by covalently bound paramagnetic tags. J Am Chem Soc. 2010;132:9561–9563. doi: 10.1021/ja103545e. [DOI] [PubMed] [Google Scholar]
- 25.Maly T, Debelouchina GT, Bajaj VS, Hu KN, Joo CG, Mak-Jurkauskas ML, Sirigiri JR, van der Wel PC, Herzfeld J, Temkin RJ, Griffin RG. Dynamic nuclear polarization at high magnetic fields. J Chem Phys. 2008;128:052211. doi: 10.1063/1.2833582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reif B. Ultra-high resolution in MAS solid-state NMR of perdeuterated proteins: Implications for structure and dynamics. J Magn Reson. 2012 doi: 10.1016/j.jmr.2011.12.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.