Abstract
Objective
Robot-assisted movement training can help individuals with stroke reduce arm and hand impairment, but robot therapy is typically only about as effective as conventional therapy. Refining the way that robots assist during training may make them more effective than conventional therapy. Here we measured the therapeutic effect of a robot that required individuals with a stroke to achieve virtual tasks in three dimensions against gravity.
Design
The robot continuously estimated how much assistance patients needed to perform the tasks and provided slightly less assistance than needed in order to reduce patient slacking. Individuals with a chronic stroke (n = 26, baseline upper extremity Fugl-Meyer score = 23 ± 8) were randomized into two groups and underwent 24 one hour training sessions over 2 months. One group received the assist-as-needed robot training and the other received conventional table top therapy with the supervision of a physical therapist.
Results
Training helped both groups significantly reduce their motor impairment, as measured by the primary outcome measure, the Fugl-Meyer score, but the improvement was small (3.0 ± 4.9 points for robot therapy, versus 0.9 ± 1.7 for conventional therapy). There was a trend for greater reduction for the robot trained group (p = 0.07). The robot group largely sustained this gain at the three-month follow-up. The robot-trained group also experienced significant improvements in Box and Blocks score and hand grip strength, while the control group did not, but these improvements were not sustained at follow-up. In addition, the robot-trained group showed a trend toward greater improvement in sensory function, as measured by the Nottingham Sensory Test (p = 0.06).
Conclusions
These results suggest that, in patients with chronic stroke and moderate-severe deficits, assisting in three dimensional virtual tasks with an assist-as-needed controller may make robotic training more effective than conventional table top training.
Keywords: Rehabilitation, Robotics, Movement, Stroke, Arm
There has been increasing interest in developing robotics and information technologies for assisting in physical rehabilitation following neurologic injuries and disease 1,2. Such technologies could potentially allow more engaging forms of therapy to be accessed with less supervision, better quantify therapy and its impact, and improve outcomes. While the first two capabilities are being achieved at least to some extent, the third has arguably not been achieved. In its current forms for the upper extremity, robot-assisted therapy has typically been shown to be comparably effective to conventional therapy when the dose of the conventional therapy is matched.3,4 For example, the recent multi-site randomized controlled trial of MIT-MANUS in chronic stroke found comparable reductions in upper extremity impairment following robot or dose-matched therapist-assisted treatment.5
The forms of robot-assisted therapy tested so far are only few among a large set of possibilities. The tasks practiced, the nature of the physical interaction with the robot, and the elements of feedback provided by the robot to the patient can all be varied in multitudinous ways, some yet unimagined. Thus, the field of robot-assisted therapy might be characterized as currently being at a stage in which a search for optimal parameters may lead to achievement of the third capability – that of machine-assisted therapy that is more effective than conventional therapy.
In the randomized controlled study reported here, we tested a form of robot therapy motivated by two primary considerations. First, we desired to have patients practice arm movement in three dimensions against gravity in the context of simulated functional tasks that required use of the hand. Many activities of daily living require three dimensional (3D) movement against gravity and use of the hand, and we hypothesized that training such movements in the context of functional tasks rather than supported arm movements or single-joint arm movements that simply required target tracking would increase the overall benefits of training because of the principle of specificity of motor training. We therefore built a robotic device called Pneu-WREX that allows naturalistic motion of the arm in three dimensions (Figure 1).6–8 Pneu-WREX is a pneumatically actuated version of a non-actuated arm support T-WREX9, which was based on the innovative mobile arm support developed by Rahman et al. for children with arm impairment called WREX.10 We previously tested T-WREX with chronic stroke subjects and found that training with this non-robotic exoskeleton indeed significantly reduced arm motor impairment compared to conventional table top training.11
Figure 1.
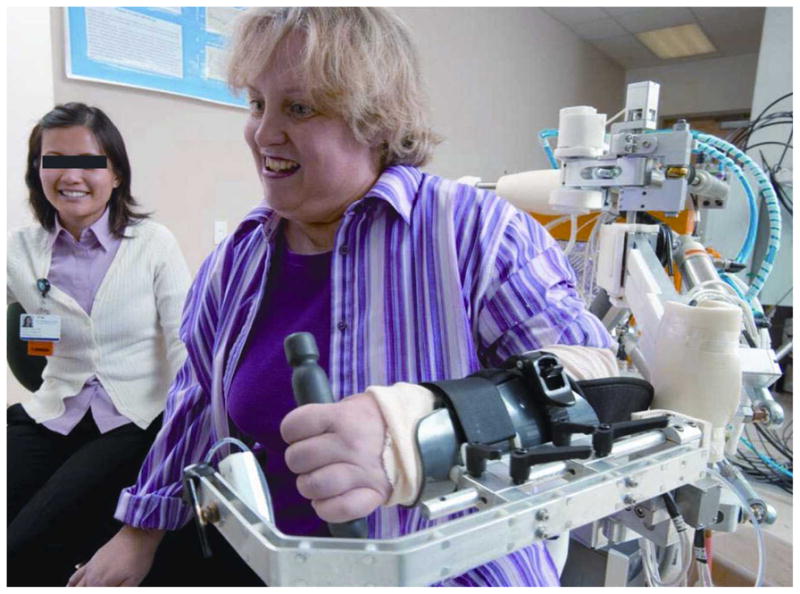
Pneu-WREX is a four degree-of-freedom pneumatically actuated upper extremity orthosis for robot-aided movement training. The user can move her hand in 3D space, and use the grip sensor to grasp and release virtual objects on the computer screen.
However, in our previous study with T-WREX, patients were not assisted robotically in completing movements. Thus, secondly, we desired to test a form of robot therapy that ensured that the patients completed 3D movements, while still requiring the patient to exert substantial effort to complete those movements. Our premise was that helping patients finish target movements would deliver enhanced somatosensory stimulation, and complete and accurate proprioceptive signals, that assist in neural reorganization, perhaps by activating Hebbian-like plasticity processes that depend on afferent input that is correlated with motor output. Yet this premise was conditioned by our and other’s previous findings that assisting in movements with a robot can, on the other hand, induce a slacking response by the patient, measured as a reduction in force output7 or energy consumption12, or a decrease in learning that is apparent in motor learning studies of healthy subjects.13–16 Thus, we considered that the benefits of increased somatosensory stimulation generated by robot-assisted movement may be offset by the negative consequences that a reduction in patient effort17 or learning might bring for recovery, if care is not taken to prevent this reduction. We therefore designed a controller for Pneu-WREX that learns a model of the patient’s ability in order to try to provide a subject-specific, appropriate level of assistance.7 We originally used a standard sliding adaptive controller to learn the position-dependent forces required to assist people in completing tracking games in 3D against gravity, representing those forces using radial-basis functions. However, we found that people with and without stroke subconsciously allowed the adaptive controller to “take-over” the forceful part of the tracking task, relaxing their own force output. To counter this slacking, we added a forgetting term to the adaptive controller that continuously sought to reduce the force output of the robot as well; essentially we made the robot slack as well. Introduction of this term caused individuals to experience small amounts of tracking error, which were enough to cause them to substantially increase their effort for the task.7 Thus, the controller assisted them in achieving the desired movements, while preventing slacking.
In this study, we tested the hypothesis that use of this anti-slacking, assist-as-needed controller for 3D movement training would improve recovery beyond the results possible with conventional table top training for a group of people with chronic stroke. Portions of this work were reported previously in conference paper format.18
METHODS
Subjects
A total of 27 adult stroke survivors were recruited through local hospitals and stroke support groups in Orange County, CA. All participants suffered from a single ischemic or hemorrhagic stroke and were at least three months post-stroke at the time of their enrollment into the program. All participants demonstrated moderate to severe weakness of their affected upper extremities, defined by the upper extremity Fugl-Meyer Motor Scale (score of 10 to 35 out of 66).19 Exclusion criteria included significant pain, instability or subluxation of the affected shoulder, severe elbow or wrist contractures, concurrent severe medical problems, cognitive dysfunction to the extent that would interfere with therapy participation, visual deficits, severe neglect or apraxia, and current enrollment in ongoing upper extremity therapy. All subjects provided written consent, and all procedures were approved by the Institutional Review Board of the University of California in Irvine.
Device: Pneu-Wrex
The robotic device used for this study is called “Pneu-Wrex”. It is a four degrees-of-freedom robot based on a passive arm support called WREX. Pneu-Wrex is a lightweight exoskeleton that allows a wide range of motion of the arm in a 3D space by incorporating pneumatic actuators to generate active forces. The actuated degrees of freedom are elbow flexion/extension, shoulder horizontal abduction/adduction, shoulder flexion/extension, and shoulder forward-backward translation. The device incorporates a passive weight support mechanism similar to the one used in WREX, the design of which constrains the forearm to remain parallel to the ground as it moves through 3D space. As stated above, it relies on an adaptive controller to learn the dynamics of the patient’s arm, ability and effort at the same time, and also contains a forgetting term that reduces patient slacking. Therefore, the device can provide assistance as needed for a patient to actively participate and be able to complete 3D tasks. Hand training through grasp and release is incorporated through a grip sensor that measures the pressure of a water-filled cylinder bladder that the user holds, in order to detect even trace finger movement.
Position sensors located at the joints of the Pneu-Wrex exoskeleton allow it to be used as an input device to measure arm movement of the affected limb. A software package called Vu Therapy designed at the University of California Irvine allowed for the interface of the robotic arm and the simulated environment. The games were designed to be functionally oriented with the patient completing familiar, task-oriented movements. Tasks included grocery shopping (i.e. grasping objects off of a shelf and dropping them into a grocery cart), cleaning a window, playing basketball, and driving a car. Auditory and visual feedback and a game score were provided throughout game play to maintain the patient’s attention and interest.
Assignment and Intervention
This study compared the change in arm movement ability of subjects who participated in Pneu-Wrex training with that of control subjects who exercised for the same duration without the device and received similar amounts of supervision from a therapist. Subjects participated in 24 one-hour treatment sessions, approximately three times per week for eight to nine weeks, except one subject in the Pneu-WREX group experienced 21 sessions due to a temporary medical problem unrelated to the study. All training sessions were supervised by a physical therapist at all times. Each treatment session began with the therapist performing passive range of motion exercise of the arm and obtaining blood pressure and pain ratings. Subjects also recorded blood pressure and pain ratings after each treatment session.
Groups of four to five subjects were enrolled at a time. To ensure a match in age and impairment severity between groups, subjects were first stratified by age (< 60, 61–74, and > 75) and Fugl Meyer Score (9–17, 18–27, 28–66). Subjects in these groups were then randomly assigned to the Pneu-Wrex or control groups by a computerized randomization algorithm. The treating therapists and subjects were blinded to group assignment until each subject was consented and enrolled in the study.
Subjects assigned to the control group participated in conventional exercises, which were developed previously by Sarah Housman, MS OTR/L at the Rehabilitation Institute of Chicago and had been tested in a study of the T-WREX device.11 These exercises were typical of conventional home programs, and consisted of three sections including self range of motion stretches, active range of motion strengthening exercises, and a list of ADL tasks throughout hemiparetic upper extremity. During self range of motion stretches, participants clasped their hands or arms together and used the strength of the less-affected arm to move the affected arm through the available range of motion at each joint. During active range of motion exercises the hemiparetic arm was supported against gravity by a tabletop and a towel was placed under the arm to decrease friction as subjects completed specified movements unilaterally. All sessions were supervised by a physical therapist who provided cueing or assistance as needed.
Individuals in the robot group completed approximately three to five repetitions of five therapy games each session using Pneu-Wrex. Subjects followed the preset movements on the computer screen, which were indicated by a cursor that looked like a hand, in the simulated environment. These movements involved reaching, grasping, grasp release, and horizontal movement. Pneu-Wrex provided assistance as needed for participants to complete the arm movements. Grasp force thresholds required to manipulate virtual objects were adjusted by the supervising therapist according to subject ability; some subjects had no discernible hand function and were unable to use the gripper. All activities on Pneu-WREX were also supervised by the same physical therapist who again provided verbal cueing as needed. The games incorporated auditory feedback to signal successful completion of the task (e.g. placing an item in a shopping cart.), and the screen showed number of successful movements completed or time of completion for each game.
To minimize the possibility of a placebo-like benefit that could arise in the robot trained group because of expectations associated with using a high tech device, subjects in the control group crossed over to the robot treatment group thirty minutes weekly; the intent was to give the control group limited exposure to the robot as well to offset any “high tech” placebo effect. Likewise, to minimize the possibility of a bias that could arise for the control group because they trained unassisted movements close to the ones used in the clinical evaluations, subjects in the robot group crossed over to the conventional training group thirty minutes per week; again, this gave the robot group limited exposure to unassisted, functional training as well. Thus, each group overall experienced a blend of 84% of their primary therapy, and 16% of the other, as the 30 minutes of cross-over were included in the three hours per week of training.
Assessment Procedures
Subjects were assessed twice before the initiation of the treatment sessions, at two weeks prior and one week prior to the study. Subjects were also assessed after the completion of the 24 treatment sessions and at a three month follow-up assessment. A single blinded rater performed all of the clinical assessments during all testing sessions. The primary outcome measure was the arm motor section of the Fugl-Meyer Scale, which assesses arm movement ability outside of synergy patterns.20 Other outcome measures included the Rancho Functional Test for the Hemiplegic/Paretic Upper Extremity 21, which tests the ability of subjects to complete functional activities of graded difficulty, assigning them a level based on the most difficult task they can perform; the Motor Activity Log22, a 5 point self-report of how much and how well the subject is using the arm for 30 daily tasks; the Box and Blocks Test23, which tests how many blocks the subject can grasp and transport in one minute; grip strength, measured with a Jamar Hand Dynamometer; and the Nottingham Sensory Assessment (NSA).24 The NSA assesses tactile sensation (light touch and pinprick) and kinesthesia at the shoulder, elbow, wrist, and hand, and stereognosis using a pen, comb, and sponge. Each item of the NSA was graded from 0 (worst function) to 2 (preserved sensation), except for kinesthesia, which was scored from 0 to 3 points (worst and best function, respectively). The total possible NSA score was 34.
Statistical Analysis
Because we hypothesized that participants would improve their movement ability with robotic or conventional training, pre to post changes were assessed using one sided paired t-tests, comparing the average of the two baseline assessments to the assessments after the training program and at three month follow-up. To test the study hypothesis that robotic training with the assist-as-needed controller improved recovery more than conventional table top training, the motor gains between groups were compared using one-sided t-tests. We did not correct for multiple comparisons 25.
RESULTS
We compared assist-as-needed, 3D arm/hand robotic movement training with conventional table top upper extremity exercises. A total of 52 subjects were screened for the study and 27 subjects were enrolled, 13 in the robot group and 14 in the control group. Follow-up data was not available for one subject who was enrolled in the control group because the subject moved out of the area, so this subject was removed from the analysis. The baseline enrollment characteristics are shown in Table 1. The robot and control groups were well matched in age, time since stroke, and side of hemiparesis, but there were more men in the control group. There were no safety concerns related to use of the robot, and no adverse events attributable to the robot-assisted therapy.
Table 1.
Subject demographics by group
| Robot group (n = 13) | Control group (n = 13) | |
|---|---|---|
| Gender | 5 Male | 12 Male |
| 8 Female | 1 Female | |
|
| ||
| Age (years) | 60 (± 10) | 61 (± 13) |
|
| ||
| Months post-stroke | 65 (± 47) | 67 (± 56) |
|
| ||
| Side of hemiparesis | 6 Left | 5 Left |
| 7 Right | 8 Right | |
|
| ||
| Type of Stroke | 9 Ischemic | 4 Ischemic |
| 2 Hemorrhagic | 6 Hemorrhagic | |
| 3 Unknown | 3 Unknown | |
The clinical motor scores at each assessment are shown in Table 2. The scores did not change significantly from baseline evaluation 1 to baseline evaluation 2, except for the FM score for the robot group. After 24 training sessions, the robot group significantly improved the FM score, the Nottingham Sensory Assessment score, the Motor Activity Log quality of movement score, grip strength and Box and Blocks score. Only the Nottingham Sensory Assessment score remained significantly improved at 3 month follow-up for the robot group, although there was a trend in greater improvement in FM score for the robot group (p = 0.06). The control group improved the FM score and Rancho Level after 24 training sessions; these improvements did not persist at 3 month follow-up, although the control group also exhibited improved sensory scores at 3 month follow-up.
Table 2.
Outcome measures at each evaluation.
| Group | Baseline 1 | Baseline 2 | After 24 sessions of training | At 3 month follow-up | A p betw groups at B1 | B p for B2 – B1 | C p for after 24 sessions | D p for Δ at 3 month follow-up | |
|---|---|---|---|---|---|---|---|---|---|
| Arm Motor Fugl-Meyer (out of 66) | Control | 22.9 ± 7.4 | 23.0 ± 7.5 | 23.8 ± 8.0 | 23.0 ± 8.0 | 0.7 | 0.4 | 0.04* | 0.4 |
| Robot | 24.1 ± 8.8 | 24.6 ± 9.1 | 27.4 ± 11.4 | 26.5 ± 11.2 | 0.02* | 0.02* | 0.06 | ||
| Rancho Level (out of 7) | Control | 3.0 ± 0.7 | 3.1 ± 0.6 | 3.2 ± 0.6 | 3.2 ± 0.7 | 0.1 | 0.2 | 0.05* | 0.08 |
| Robot | 3.4 ± 0.5 | 3.3 ± 0.6 | 3.4 ± 0.5 | 3.4 ± 0.8 | 0.8 | 0.2 | 0.5 | ||
| Nottingham Sensory | Control | 21.1 ± 7.8 | 22.2 ± 8.0 | 22.8 ± 8.6 | 23 ± 7.8 | 0.8 | 0.13 | 0.1 | 0.002* |
| Robot | 21.9 ± 7.8 | 23.4 ± 8.1 | 24.6 ± 7.6 | 25.9 ± 7.1 | 0.07 | 0.02* | 0.01* | ||
| MAL Amount of Use (AOU) (out of 5) | Control | 0.3 ± 0.4 | 0.2 ± 0.3 | 0.3 ± 0.4 | 0.3 ± 0.5 | 0.9 | 0.7 | 0.05 | 0.2 |
| Robot | 0.3 ± 0.4 | 0.3 ± 0.4 | 0.3 ± 0.4 | 0.3 ± 0.5 | 0.1 | 0.4 | 0.2 | ||
| MAL Quality of Movement (QOM) (out of 5) | Control | 0.2 ± 0.3 | 0.1 ± 0.2 | 0.2 ± 0.3 | 0.2 ± 0.4 | 0.8 | 0.79 | 0.05* | 0.4 |
| Robot | 0.2 ± 0.3 | 0.2 ± 0.3 | 0.3 ± 0.3 | 0.3 ± 0.4 | 0.09 | 0.04* | 0.1 | ||
| Grip Strength (kgF) | Control | 3.7 ± 4.8 | 3.8 ± 3.9 | 4.1± 4.4 | 4.7 ± 4.7 | 0.5 | 0.3 | 0.3 | 0.2 |
| Robot | 2.7 ± 2.4 | 2.9 ± 2.6 | 3.9 ± 3.2 | 3.2 ± 3.0 | 0.5 | 0.01* | 0.2 | ||
| Box and Blocks (# blocks in 1 minute) | Control | 0.3 ± 0.6 | 0.5 ± 1.1 | 0.6 ± 1.1 | 0.8 ± 2.0 | 0.6 | 0.3 | 0.2 | 0.2 |
| Robot | 0.5 ± 1.4 | 0.6 ± 1.9 | 2.0 ± 4.1 | 1.9 ± 6.0 | 0.1 | 0.03* | 0.1 |
Means ± Standard Deviations. p values show results of t-test comparing groups at baseline (column A), comparing change from first to second baseline measurements within each group (column B), change from mean of baselines to post-treatment evaluation (column C), and change from mean of baselines to three month follow-up (column D).
denotes significant difference at p < 0.05.
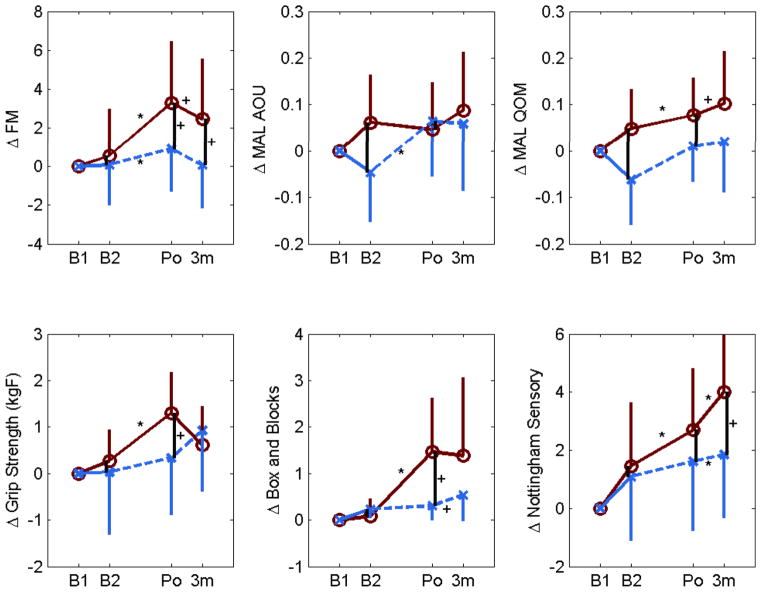
The changes in clinical scores are compared between groups in Table 3 and Figure 2. There was a trend for the robot group to have larger improvements in FM score and Box and Blocks score after 24 training sessions (p = 0.07 and p = 0.06, respectively). There was also a trend for the robot group to have larger improvements in FM score and Nottingham Sensory score at the three month follow-up that (p = 0.06).
Table 3.
Comparison of change in outcome measures for the robot and conventional training group after 24 training sessions and at the 3 month follow-up.
| Group | Δ after 24 sessions | Δ after 3 months relative to baseline | p betw groups after 24 sessions | p betw groups at 6 months | |
|---|---|---|---|---|---|
| Arm Motor Fugl-Meyer (out of 66) | Control | 0.9 ± 1.7 | 0.1 ± 1.4 | 0.07^ | 0.06^ |
| Robot | 3.0 ± 4.9 | 2.4 ± 5.2 | |||
| Rancho Level (out of 7) | Control | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.91 | 0.84 |
| Robot | 0.0 ± 0.1 | 0.0 ± 0.4 | |||
| Nottingham Sensory Test | Control | 1.1 ± 3.3 | 1.8 ± 2.5 | 0.25 | 0.06^ |
| Robot | 2.0 ± 3.2 | 4.0 ± 4.0 | |||
| MAL Amount of Use (AOU) (out of 5) | Control | 0.1 ± 0.2 | 0.1 ± 0.3 | 0.87 | 0.41 |
| Robot | 0.0 ± 0.1 | 0.1 ± 0.3 | |||
| MAL Quality of Movement (QOM) (out of 5) | Control | 0.05 ± 0.1 | 0.02 ± 0.2 | 0.38 | 0.21 |
| Robot | 0.04 ± 0.1 | 0.1 ± 0.3 | |||
| Grip Strength (kgF) | Control | 0.3 ± 2.0 | 0.9± 4.0 | 0.12 | 0.60 |
| Robot | 1.2 ± 1.5 | 0.6 ± 2.3 | |||
| Box and Blocks | Control | 0.2 ± 0.9 | 0.5 ± 1.5 | 0.06^ | 0.27 |
| Robot | 1.4 ± 2.6 | 1.4 ± 4.8 |
Means ± Standard Deviations.
denotes p < 0.1 nearly significant difference
Figure 2.
Changes in outcome measures from first baseline evaluation (B1) to second baseline evaluation (B2), to Post-treatment (Po, i.e. following 24 therapy sessions), to three month follow-up (3M). Bars show one standard deviation. Solid lines = robot group, dashed lines = control group. * denotes a comparisons with p < 0.05, and + denotes a comparison with p < 0.1. FM = Fugl-Meyer Upper Extremity Score, MAL = Motor Activity Log. AOU = Amount of Use. QOM = Quality of Movement.
When asked which training program they preferred, individuals who had experienced the robot program more often picked the robot program (Figure 3). They rated it as less boring and as the program they were more likely to complete at home. Individuals who participated in the control group still preferred the robot therapy based on their limited experience of it, but not as strongly as those who participated in the robot group.
Figure 3.
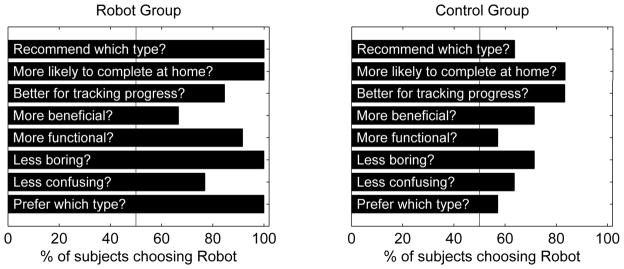
Subjects were asked to choose “robot” or “control” therapy in several categories after completing 24 training sessions that involved some exposure to each training type. Left: responses from subjects enrolled in the robot group. Right: responses from subjects enrolled in the control group.
DISCUSSION
This study suggests that robot-assisted training can safely improve sensory motor recovery for individuals with a chronic, severe to moderate impairment, although it should be noted that the improvements measured were small. There was also a trend toward greater improvements with robot-assisted training compared to conventional table top training. This trend, along with the improved repeatability, quantifiability, and engagingness of the therapy delivered, and the possibility for increased dosage through more semi-autonomous training, are possible benefits of robot-assisted therapy. We first discuss what aspects of the robot-assisted therapy tested here may have contributed to the observed results, then discuss study limitations and key directions for future research.
What Aspects of the Robot-Assisted Therapy Contributed to the Observed Results?
Robotic therapy devices provide an engaging way for people with a stroke to practice meaningful tasks, and they provide somatosensory stimulation beyond what a patient could normally achieve during unassisted practice. These features may assist in brain reorganization of motor networks.26 On the other hand, by virtue of physically assisting in movement, robotic therapy devices may have the unintended consequence of causing patients to diminish their own effort in achieving a desired task. The human motor system can be viewed in part as an “effort optimizer” or “slacker”: studies have found that helping people complete movement tasks with robotic devices causes the motor system to systematically reduce its output7,12,27,28 and can reduce normal motor learning.13–16 If the magnitude of motor output is important for improving motor performance, as it is in strength training29, and as implied by a study of robotic rehabilitation therapy that found little benefit to patient-passive therapy 17, then the implication is that care must be taken in robot-assisted therapy to not “over-assist” the patient. In other words, assistance may have both positive effects (increased somatosensory stimulation, complete and accurate proprioceptive signals, better engagement, better ability to do tasks and thus receive positive feedback about efforts) and negative effects (decreased patient effort and reduced need to “solve” the problem of relearning arm control). A key goal for robotic therapy device research is to increase the positive effects while decreasing the negative ones.
In this study, the robot continuously estimated the assistance the patient needed to achieve the current task, and then provided slightly less assistance than the estimated amount. We had found previously that this approach induces subjects with stroke to exert substantially more effort than when the estimated amount of assistance is provided.7 The benefits of robot-assisted therapy found in this study may thus have been in part due to the way the robot encouraged subjects to exert more effort.
The benefits may also have arisen in part due to the fact that the robot allowed 3D movements that incorporate hand grip and release, rather than just planar or single-joint movements. We found previously that movement training with a non-actuated exoskeleton similar in geometry to the actuated robot used in this study, which allowed similar 3D movements and hand grip and release, also produced better results than conventional table top training11. Although it is difficult to compare between studies because of the heterogeneity of individuals with stroke, the benefits with the non-actuated exoskeleton measured previously were similar to the benefits with the actuated exoskeleton used here. This suggests that the 3D nature of therapy rather than the particular form of assistance provided may have been the determinant of recovery, although it should be noted that both exoskeletons demanded significant effort from their users. Consistent with this interpretation, one of the few studies to find that robot-assisted arm therapy produced better outcomes than a matched dose of conventional therapy used a robot that allowed 3D movement of the hand.30 It may be that training 3D movements with use of the hand is beneficial because the arm is normally used in 3D in conjunction with the hand, including in many clinical evaluations such as the ones used here.
Limitations and Future Directions
This study had several limitations. We discuss them here, and use them to illustrate and discuss more general limitations in the field of robotic therapy.
One limitation of the study is that there were multiple differences between the two treatment arms, and so no single variable was explicitly tested. For example, anti-slacking robotic therapy was not directly compared with non-anti-slacking robotic therapy. Thus, it is impossible to determine definitively if the anti-slacking component of the therapy was in fact the beneficial component. Likewise, the study did not compare 3D to non-3D therapy, and thus it is impossible to determine definitely that 3D therapy was the cause of the observed trend (or that inclusion of the hand grip and release in training was better). Future research should make these isolated comparisons to increase insight into the attributes of robotic therapy that are most important to achieving maximum behavioral gains. However, we point out that there are a huge number of possible isolated comparisons to make when considering the elements of robot assisted therapy, including comparisons of the tasks practiced, the nature of the physical interaction with the robot, and the elements of feedback provided by the robot to the patient, as well as yet unimagined variations. Making isolated comparisons is time consuming and expensive. In our view, one solution would be the development of short-term assays that accurately assess the sensitivity of long-term recovery to individual changes in robot-assisted therapy paradigms. We envision, specifically, a behavioral or brain-imaging probe (or combination of both) that can be made during or shortly after a single session of therapy, and which gives insight into whether the parameters used in that single session of therapy were beneficial in provoking learning (e.g. 31). Determining how to assay short-term pre-cursors of long-term plasticity and learning is an important goal for the field.
Another limitation of this study is that the comparative benefits of the robot therapy did not cross the 0.05 level of significance routinely used in statistical testing, even when using one-sided tests and without correcting for multiple comparisons. The use of multiple t-tests (i.e. unrestricted LSD procedure) has been argued to improve consistency of multiple comparisons 25. The fact that significance levels only bordered on 0.05 requires accepting a 6% chance of a type I error rather than a 5% chance of type I error, which is perhaps reasonable given that that historical selection of the 5% level was somewhat arbitrary 32. The lack of significance may have been due to the relatively small sample size. The field of robot-assisted therapy, and rehabilitation science in general, often suffers from low power, due mainly to difficulties in recruiting subjects and the substantial resources needed to deliver months of focused therapy. In addition, large levels of inter-patient variability reduce the power to detect a difference when one is truly present. Entry criteria or patient stratification might best be achieved by including some biological measures of neural injury or function33, increasing study power.
Another limitation of the study, which again is a limitation of the field of stroke motor rehabilitation as a whole5, is that the observed benefits of the training were small and could be considered as having limited clinical significance. The primary benefits of robot-assisted training measured here were a small reduction in impairment as measured by the primary outcome measure, the FM score, which focuses on motor ability, and the Nottingham Sensory Assessment, which measures tactile and proprioceptive perception. The functional scales that we used, the Rancho Test and the Motor Activity Log, did not detect much benefit of either training type. Conventional table top training showed even less benefit, and this may have been because relatively few movements are typically performed in this type of training, as was quantified previously in a study of conventional upper extremity treatment approaches 34. These results are consistent with the previous study of T-WREX that also used this type of control training, and found only a small improvement due to the control training (2.2 FM points) which again faded at long-term follow-up. In addition, volunteers who participated in this study were on average quite impaired, and so they may not have had the neural resources needed to substantially improve their arm and hand function, due to the anatomy of stroke-induced neural injury. Making a larger impact with robot-assisted therapy may depend in part on better identifying the patients who can most benefit from the therapy, using brain imaging33,35,36 or quantitative records of the amount of therapy people have previously engaged in. Ultimately, the hope for greater impact lies with combining robotic therapy and biological, pharmaceutical, or electrical-stimulation based approaches to enhancing plasticity and regeneration (see for example,37). Such combination therapies are already benefitting from robotic delivery of therapy because the robot helps control the type and amount of training delivered, while also quantifying the behavioral outcomes of that therapy.
Footnotes
Disclosures:
Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article. This report summarizes work supported by NCRR M01RR00827 and under Federal Contract N01-HD-3-3352 by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and co-funded by the National Institute of Biomedical Imaging and Bioengineering (NIBIB). The findings and conclusions in the report are those of the authors and do necessarily represent the views of NICHD, NIBIB, or NCRR. David Reinkensmeyer has a financial interest in Hocoma, A.G., a company that makes robotic therapy devices. The terms of this arrangement have been reviewed and approved by the University of California, Irvine, in accordance with its conflict of interest policies.
References
- 1.Adamovich SV, Fluet GG, Tunik E, Merians AS. Sensorimotor training in virtual reality: a review. NeuroRehabilitation. 2009;25(1):29–44. doi: 10.3233/NRE-2009-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brewer BR, McDowell SK, Worthen-Chaudhari LC. Poststroke upper extremity rehabilitation: a review of robotic systems and clinical results. Topics in stroke rehabilitation. 2007 Nov-Dec;14(6):22–44. doi: 10.1310/tsr1406-22. [DOI] [PubMed] [Google Scholar]
- 3.Kwakkel G, Kollen BJ, Krebs HI. Effects of robot-assisted therapy on upper limb recovery after stroke: a systematic review. Neurorehabilitation and neural repair. 2008 Mar-Apr;22(2):111–121. doi: 10.1177/1545968307305457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prange GB, Jannink MJA, Groothuis CGM, Hermens HJ, Ijzerman MJ. Systematic review of the effect of robot-aided therapy on recovery of the hemiparetic arm after stroke. J Rehabil Res Develop. 2006;43(2):171–184. doi: 10.1682/jrrd.2005.04.0076. [DOI] [PubMed] [Google Scholar]
- 5.Lo AC, Guarino PD, Richards LG, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med. 2010;362(19):1772–1783. doi: 10.1056/NEJMoa0911341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchez RJ, Wolbrecht E, Smith R, et al. A pneumatic robot for re-training arm movement after stroke: Rationale and mechanical design. Rehabilitation Robotics, 2005. ICORR 2005; 9th International Conference on; 2005. pp. 500–504. [Google Scholar]
- 7.Wolbrecht ET, Chan V, Reinkensmeyer DJ, Bobrow JE. Optimizing compliant, model-based robotic assistance to promote neurorehabilitation. IEEE Transactions Neural Systems and Rehabiltation Engineering. 2008;16(3):286–297. doi: 10.1109/TNSRE.2008.918389. [DOI] [PubMed] [Google Scholar]
- 8.Wolbrecht ET, Chan V, Reinkensmeyer DJ, Bobrow JE. Pneumatic control of robots for rehabilitation. International Journal of Robotics Research. 2010;29(1):23–38. [Google Scholar]
- 9.Sanchez RJ, Liu J, Rao S, et al. Automating arm movement training following severe stroke: functional exercises with quantitative feedback in a gravity-reduced environment. IEEE Transactions on Neural and Rehabilitation Engineering. 2006;14(3):378–389. doi: 10.1109/TNSRE.2006.881553. [DOI] [PubMed] [Google Scholar]
- 10.Rahman T, Sample W, Seliktar R, Alexander M, Scavina M. A body-powered functional upper limb orthosis. Journal of Rehabilitation Research and Development. 2000;37(6):675–680. [PubMed] [Google Scholar]
- 11.Housman SJ, Scott KM, Reinkensmeyer DJ. A Randomized Controlled Trial of Gravity- Supported, Computer-Enhanced Arm Exercise for Individuals With Severe Hemiparesis. Neurorehabilitation and Neural Repair. 2009;23(5):505–514. doi: 10.1177/1545968308331148. [DOI] [PubMed] [Google Scholar]
- 12.Israel JF, Campbell DD, Kahn JH, Hornby TG. Metabolic costs and muscle activity patterns during robotic- and therapist-assisted treadmill walking in individuals with incomplete spinal cord injury. Phys Ther. 2006 Nov;86(11):1466–1478. doi: 10.2522/ptj.20050266. [DOI] [PubMed] [Google Scholar]
- 13.Lippman LG, Rees R. Consequences of error production in a perceptual-motor task. J Gen Psychol. 1997;124(2):133–142. doi: 10.1080/00221309709595512. [DOI] [PubMed] [Google Scholar]
- 14.van Asseldonk EH, Wessels M, Stienen AH, van der Helm FC, van der Kooij H. Influence of haptic guidance in learning a novel visuomotor task. J Physiol Paris. 2009;103(3–5):276–285. doi: 10.1016/j.jphysparis.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Milot MH, Marchal-Crespo L, Green CS, Cramer SC, Reinkensmeyer DJ. Comparison of error amplification and haptic guidance training techniques for learning of a timing-based motor task by healthy individuals. Experimental Brain Research. 2009;201(2):119–131. doi: 10.1007/s00221-009-2014-z. [DOI] [PubMed] [Google Scholar]
- 16.Heuer H, Rapp K. Active error corrections enhance adaptation to a visuo-motor rotation. Exp Brain Res. 2011;211(1):97–108. doi: 10.1007/s00221-011-2656-5. [DOI] [PubMed] [Google Scholar]
- 17.Hu XL, Tong KY, Song R, Zheng XJ, Leung WW. A comparison between electromyography-driven robot and passive motion device on wrist rehabilitation for chronic stroke. Neurorehabil Neural Repair. 2009;23(8):837–846. doi: 10.1177/1545968309338191. [DOI] [PubMed] [Google Scholar]
- 18.Reinkensmeyer DJ, Maier MA, Guigon E, et al. Do Robotic and Non-Robotic Arm Movement Training Drive Motor Recovery after Stroke by a Common Neural Mechanism? Experimental Evidence and a Computational Model. Proceedings of the 2009 IEEE Engineering in Medicine and Biology Conference. 2009;2009:2439–2441. doi: 10.1109/IEMBS.2009.5335353. [DOI] [PubMed] [Google Scholar]
- 19.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13–31. [PubMed] [Google Scholar]
- 20.Gladstone DJ, Danells CJ, Black SE. The fugl-meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabilitation and neural repair. 2002 Sep;16(3):232–240. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- 21.Wilson DJ, Baker LL, Craddock JA. Protocol: Functional Test for the Hemiplegic/paretic Upper Extremity. Rancho Los Amigos; 1984. [Google Scholar]
- 22.van der Lee JH, Beckerman H, Knol DL, de Vet HCW, Bouter LM. Clinimetric properties of the Motor Activity Log for the assessment of arm use in hemiparetic patients. Stroke. 2004;35(6):1410–1404. doi: 10.1161/01.STR.0000126900.24964.7e. [DOI] [PubMed] [Google Scholar]
- 23.Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther. 1985;39(6):386–391. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- 24.Lincoln NB, Jackson JM, Adams SA. Reliability and revision of the Nottingham Sensory Assessment for stroke patients. Physiotherapy. 1998;84:358–365. [Google Scholar]
- 25.Saville DJ. Multiple comparison procedures: The practical solution. The American Statistician. 1990;44(2):174–180. [Google Scholar]
- 26.Takahashi CD, Der-Yeghiaian L, Le V, Motiwala RR, Cramer SC. Robot-based hand motor therapy after stroke. Brain : a journal of neurology. 2008 Feb;131(Pt 2):425–437. doi: 10.1093/brain/awm311. [DOI] [PubMed] [Google Scholar]
- 27.Scheidt RA, Reinkensmeyer DJ, Conditt MA, Rymer WZ, Mussa-Ivaldi FA. Persistence of motor adaptation during constrained, multi-joint, arm movements. Journal of Neurophysiology. 2000;84(2):853–862. doi: 10.1152/jn.2000.84.2.853. [DOI] [PubMed] [Google Scholar]
- 28.Emken JL, Benitez R, Sideris A, Bobrow JE, Reinkensmeyer DJ. Motor adaptation as a greedy optimization of error and effort. Journal of Neurophysiology. 2007;97:3997–4006. doi: 10.1152/jn.01095.2006. [DOI] [PubMed] [Google Scholar]
- 29.Taylor NF, Dodd KJ, Damiano DL. Progressive resistance exercise in physical therapy: a summary of systematic reviews. Physical Therapy. 2005 Nov;85(11):1208–1223. [PubMed] [Google Scholar]
- 30.Lum PS, Burgar CG, Shor PC, Majmundar M, Van der Loos M. Robot-assisted movement training compared with conventional therapy techniques for the rehabilitation of upper-limb motor function after stroke. Arch Phys Med Rehabil. 2002;83(7):952–959. doi: 10.1053/apmr.2001.33101. [DOI] [PubMed] [Google Scholar]
- 31.Koski L, Mernar TJ, Dobkin BH. Immediate and long-term changes in corticomotor output in response to rehabilitation: correlation with functional improvements in chronic stroke. Neurorehabil Neural Repair. 2004;18(4):230–249. doi: 10.1177/1545968304269210. [DOI] [PubMed] [Google Scholar]
- 32.Cowles M, Davis C. On the origins of the .05 level of statistical significance. American Psychologist. 1982;37:553–558. [Google Scholar]
- 33.Riley JD, VL, LD-Y, et al. Anatomy of stroke injury predicts gains from therapy. Stroke. 2011;42(2):421–426. doi: 10.1161/STROKEAHA.110.599340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lang CE, JRM, Reisman DS, et al. Observation of amounts of movement practice provided during stroke rehabilitation. Arch Phys Med Rehabil. 2009;90(10):1692–1698. doi: 10.1016/j.apmr.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stinear CM. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130(Pt 1):170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 36.Cramer SC, Parrish TB, Levy RM, et al. Predicting functional gains in a stroke trial. Stroke; a journal of cerebral circulation. 2007 Jul;38(7):2108–2114. doi: 10.1161/STROKEAHA.107.485631. [DOI] [PubMed] [Google Scholar]
- 37.Courtine G, Gerasimenko Y, van den Brand R, et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12(10):333–342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]