Abstract
Background
Although maintaining near normal glycemia delays onset and slows progression of diabetes complications, many diabetes patients and their physicians struggle to achieve glycemic targets. Best methods to support patients as they follow diabetes prescriptions and recommendations are unclear.
Methods
To test the efficacy of a behavioral diabetes intervention in improving glycemia in long-duration, poorly-controlled diabetes, we randomized 222 adults with diabetes (49% type 1, 53±12 years old, 18±12 years duration, hemoglobin A1c=9.0±1.1%) to attend 1) a 5-session manual-based, educator-led structured group intervention with cognitive behavioral strategies (structured behavioral arm), 2) educator-led attention-control group education program (group attention control), or 3) unlimited individual nurse and dietitian education sessions for 6 months (individual control). Outcomes were baseline, and 3, 6, and 12-month post-intervention hemoglobin A1c levels (primary), frequency of diabetes self-care, 3-day pedometer readings, 24-hour diet recalls, average number of glucose checks, physical fitness, depression, coping style, self-efficacy, and quality of life (secondary).
Results
Linear mixed modeling found that all groups improved hemoglobin A1c (p<0.001). However, the structured behavioral arm improved more than group and individual control arms (3-month HbA1c change: −0.8% versus −0.4% and −0.4%; groupXtime interaction p-value=0.04). Further, type 2 participants improved more than type 1 participants (type of diabetesXtime interaction p-value=0.04). Quality of life, glucose monitoring, and frequency of diabetes self-care did not differ by intervention over time.
Conclusions
A structured, cognitive behavioral program is more effective than two control interventions in improving glycemia in adults with long-duration diabetes. Educators can successfully utilize modified psychological and behavioral strategies.
(ClinicalTrail.gov registration number: NCT000142922)
Despite the availability of new medications and treatment devices and the emphasis placed on diabetes treatment adherence over the last decade, National Health and Nutrition Examination Survey (NHANES) data show that 45% of diabetes patients have not achieved glycemic targets of <7%1,2. While some diabetes patients may not receive optimal treatment (e.g., necessity for higher targets, severe comorbidities, inappropriate treatment regimens), an important reason for poor glycemic control is patients’ difficulty in following treatment prescriptions and self-management and lifestyle recommendations3. Although non-specific behavioral/psychological approaches may be effective in addressing these problems4, whether clinicians are able to incorporate these techniques into their clinical practice is not clear5–7.
Many psychosocial factors impact how well diabetes patients are able to follow their treatment prescriptions and self-care recommendations. Depression, which is more common in diabetes patients compared to the general population8,9, is associated with poor glycemic control10, reduced self-care behaviors11,12, and increased morbidity13 and mortality14. Interestingly, treatment of depression alone is not enough to improve hemoglobin A1c levels15,16. High stress and chaotic lifestyles also can lead to other poor self-care and resultant inability to improve glycemia. While several diabetes adherence and lifestyle interventions have been developed by behavioral scientists and psychologists,17–22 few are well-used in clinical practice, possibly because psychologists, physicians, and other medical disciplines treating diabetes all have different skill sets and practice patterns and may have difficulty utilizing behavioral techniques. Further, few well-designed longer-term randomized controlled trials have examined this issue.
Thus, the goal of this randomized controlled trial was to test the efficacy of a highly structured behavioral diabetes education program in helping long-duration diabetes patients in poor glycemic control improve glycemia through comparisons with curriculum-based standard group education and one-to-one education with nurse and dietitian educators. The secondary objective was to assess which factors (e.g., coping processes, affective issues, type of diabetes, and adherence to recommendations) were associated with an improvement in glycemic control.
METHODS
Design Overview
After baseline assessment, this three-arm trial parallel-designed randomized participants to the structured behavioral experimental arm or to one of two control arms: 1) a 5-session (over 6 weeks) manual-based, highly structured group diabetes education that included behavioral support for implementing self-care behaviors and cognitive behavioral strategies (structured behavioral intervention), 2) a 5-session (over 6 weeks) manual-based attention control, group diabetes education, i.e. a control condition that was matched to the structured behavioral arm in terms of exposure to health professionals and diabetes education content (placebo group control), or 3) unlimited individual diabetes education sessions (individual control) for 6 months. Different teams of experienced diabetes nurses and dietitians who were certified diabetes educators (CDE) provided education for each arm. A steering committee comprised of study investigators and coordinators and a data safety monitoring board oversaw the conduct of the study. The Joslin Diabetes Center Committee on Human Subjects approved the protocol and all recruitment procedures and materials. All participants provided informed, written consent prior to participation.
Setting and Participants
Participants were recruited from the clinical practice of the Joslin Clinic, advertisements in its Newsletter, extensive mailings from Joslin’s database, and advertisements in local papers and radio stations.
Adults aged 18 to 70 years diagnosed with type 1 or type 2 diabetes for at least two years who were taking insulin and/or oral medication for at least one year, able to walk briskly, and free of severe complications, and whose hemoglobin A1c level >7.5% were eligible for enrollment. Exclusion criteria included inability to read and speak English, current or planned pregnancy, severe psychopathology, unstable depression, albumin/creatinine ratio>300 mcg/mg, untreated proliferative retinopathy, unstable heart disease, severe hypertension (≥ 160/90), recent alcohol or drug dependence, initiation of insulin treatment within one year, participation in diabetes education six months prior, severe neuropathy or any physical issue such as arthritis that prevented brisk walking. Inclusion/exclusion criteria were assessed via telephone screening, chart review, and a screening visit. Eligible participants were scheduled for a baseline and a randomization visit.
Randomization and Interventions
Randomization consisted of a two-step process to ensure approximately equal groups and minimize waiting time prior to interventions. The first step assigned participants by type of diabetes to either the individual or group program using a computer-generated block assignment scheme (done by the principal investigator) that research assistants unveiled during the randomization visit. Individual arm participants began education immediately. When 7 to 10 participants were assigned to group, the second step randomized them to either the control or structured behavioral arms. Educators and study physicians had no role in randomization.
All group sessions were separated by type of diabetes. Structured behavioral and control group participants received similar core education on nutrition, medication management, exercise, and glucose monitoring; both programs were manual-based and balanced for time and homework. The group control arm sessions and nurse educator sessions for the individual arm were held in the Joslin Clinic. Dietitians from the Clinical Research Center who work on large multisite lifestyle studies but not in the Clinic provided nutrition education for the individual arm. Experienced nurse and dietitian educators currently employed in diabetes outside the Clinic taught the structured behavioral arm within the behavioral research laboratory.
The structured behavioral intervention consisted of 5 two-hour sessions, delivered over 6 weeks, of highly structured behavior-based activities and information including a) group review of glucose logs to identify patterns and dietary, exercise, and medication factors that influence those patterns, b) educator-facilitated self-care goal-setting to help participants achieve and evaluate progress toward self-care goals, and c) instruction, modeling, and practice of problem-solving skills to help participants identify and problem-solve barriers to implementing self-care behaviors. Each session opened with a review of the prior week’s homework including glucose logs, food choices, and physical activity. The educators leading the structured behavioral arm received six hours of group-training in behavioral strategies (cognitive behavioral approaches, use of goal setting techniques that helped participants identify specific steps necessary to reach their goals, and the structured cognitive-behavioral-based curriculum). These strategies were brief, focused, and adapted to the educators’ skills and practice patterns and stressed their role as educators, not therapists.
The attention control arm’s program was designed with the same length of time and amount of contact with health professionals and of homework. The curriculum consisted of prepared slides, a detailed curriculum manual, and specific learning activities including homework and the importance of goal-setting but not training in cognitive behavior strategies or structured goal-setting activities. These educators received three hours of training in the curriculum.
Participants assigned to the individual control arm had access to unlimited one-on-one appointments with diabetes nurse and dietitian educators for 6 months after randomization; however, they were not required to attend any education appointments. The content was determined by the educator based on her assessment and not by study protocol. Participants were sent two reminders about the availability of these education services and research assistants were available to help them schedule appointments. Educators in the two control arms had access to all Clinic teaching materials and assessment guides.
Integrity of the interventions was ensured via written curriculum, pre-approved education materials, separate educator trainings, investigator observation of group education, and separate teams of trained, experienced diabetes educators to prevent carry over of education strategies.
Outcomes and Follow-up
We collected data at baseline and 3, 6, and 12 months post group intervention (5, 8 and 14 months after the baseline visit) and at 5, 8 and 14 months after starting individual education in the one-to-one control arm. The primary outcome was hemoglobin A1c using the HPLC ion capture method (Tosoh Medics, Inc, San Francisco, California; reference range is 4.0 to 6.0%).
In addition to sociodemographic factors (age, sex, race/ethnicity, education level, marital status, occupation) and health factors (duration of diabetes, body mass index (BMI), waist circumference, blood pressure), we also measured frequency of diabetes self-care behaviors on a 5-point Likert scale (Self-Care Inventory-R23), mean 3-day pedometer readings (Accusplit Eagle, Livermore, California), 24-hour diet recalls, and the mean daily blood glucose meter checks. To assess physical fitness, participants not on beta-blockers underwent a YMCA bicycle test24,25. Finally, we measured diabetes-related distress (Problem Areas in Diabetes26,27, a validated scale that rates distress on a 5-point Likert scale), depression and anxiety symptoms (Brief Symptom Inventory-1828, which renders a t-score for each subscale), emotion-based and controlled coping styles (Coping Styles29,30), diabetes-specific self-efficacy (Confidence in Diabetes Self-Care Scale31 rated on a 5-point Likert scale), and diabetes quality of life (Diabetes Quality of Life Questionnaire32,33, scored on a 100-point scale where a high score indicates a high quality of life).
Statistical Analysis
Sample Size
For the primary endpoint of hemoglobin A1c, we estimated that we needed 64 participants per arm to detect a clinically significant 0.5 point difference with 80% power (alpha=0.05, two-tailed). Based on prior experience with poorly controlled diabetes participants3,34, we assumed a 15% attrition rate and targeted recruitment at approximately 74 participants per arm.
Statistical Analysis
We used SAS 9.235 for data analysis. We examined descriptive statistics to ensure that data met statistical test assumptions. We compared baseline characteristics using Chi-square, Wilcoxon Two-Sample or Kruskal-Wallis Test to examine between-group differences and assess the randomization procedure effectiveness.
For primary analyses, we used a linear mixed model for repeated measures over time by type of diabetes (SAS Proc Mixed) to analyze the impact of the three education interventions on hemoglobin A1c at baseline and follow-up with fixed effects of time, group, type of diabetes, the interactions between time and group and between time and type of diabetes. This procedure prevented listwise deletion due to missing data. We also tested whether baseline characteristics including sociodemographic and psychological variables were associated with changes in hemoglobin A1c levels over time. To assess group differences in the proportion achieving a 0.5 point improvement in hemoglobin A1c we used logistic regression with SAS Proc NLMixed.
To assess the impact of missing data, we conducted a sensitivity analysis using SAS Proc MI and MIAnalyze. First, Proc MI generated 15 imputed data sets and then we used multivariate regression models that included baseline characteristics, group assignment, and numbers of hours of education to analyze the imputations. Next, we used Proc MIAnalyze to combine the analysis results to derive valid inference for missing hemoglobin A1c data. We present the most conservative p-value estimates. For continuous secondary outcomes (quality of life, diabetes-related distress and self-care behaviors), we used the same approach as the primary analysis, controlling for demographic and psychosocial variables.
RESULTS
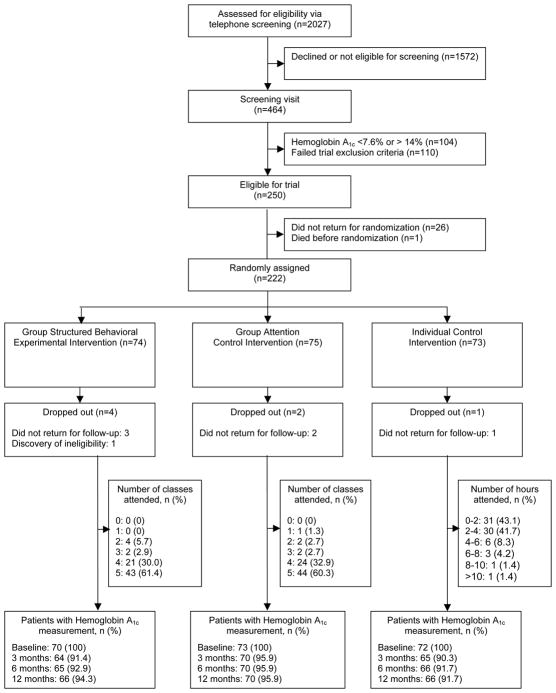
Between 2003 and 2008, we telephone-screened 2027 people, of whom 464 were eligible for a screening visit, and randomized 222 (110 with type 1 diabetes and 112 with type 2 diabetes; Figure 1). The most common reasons for exclusion at screening were not meeting criteria for hemoglobin A1c (49%), presence of complications (8%), age (6%), or inability to walk briskly (3%). Twenty-six eligible people did not return for randomization. Baseline groups did not differ on demographic or psychosocial characteristics. However, those in the structured behavioral arm were more active (steps per day) and a subset of those were more fit on the YMCA bicycle test than those in the other arms (Table 1). The intervention groups also did not differ by type of treatment. For those with type 1 diabetes at baseline: 66.4% were on multiple daily injections, 7.3%-insulin pump insulin, and 28% on NPH insulin plus sliding scale. For those with type 2 diabetes, 21.4% were on insulin only, 33.9% were on insulin plus oral diabetes agents, 18.75% were on only one oral agent (no insulin), 25.9% were on 2 or more oral agents (no insulin). As expected, some baseline characteristics differed by type of diabetes (Table 1).
Figure 1.
Study Flow Diagram
Table 1.
Baseline Characteristics of Type of Diabetes and Participants Randomly Assigned to Structured Behavioral Intervention, Attention Control or Individual Control Groups
| Type of Diabetes | Intervention Group | ||||
|---|---|---|---|---|---|
|
| |||||
| Variable | Type 1 Diabetes (N=110) | Type 2 Diabetes (N=112) | Structured Behavioral Group (N=74) | Attention Control Group (N=75) | Individual Control Group (N=73) |
| Age, (Median)(Minimum, maximum) | 46.6 (21.6, 74.2) | 58.4 (36.6, 75.1) * | 51.8 (23.7, 74.2) | 54.7 (25, 75.1) | 56.2 (21.6, 74.8) |
| Women, n (%) | 62 (56.4) | 50 (44.6) | 34 (46) | 36 (48) | 42 (57.5) |
| Non-Hispanic white, n (%) | 105 (95.5) | 89 (79.5) * | 65 (87.8) | 67 (89.3) | 62 (84.9) |
| Type 1 Diabetes, n (%) | 37 (50) | 37 (49.3) | 36 (49.3) | ||
| Type 2 Diabetes, n (%) | 37 (50) | 38 (49.7) | 37 (49.3) | ||
| Patients of Joslin Diabetes Center, n (%) | 85 (77.3) | 67 (59.8) | 44 (59.5) | 54 (74) | 54 (72) |
| Type 1 Diabetes, n (%) | 28 (75.7) | 29 (78.4) | 28 (77.8) | ||
| Type 2 Diabetes, n (%) | 16 (43.2) | 25 (65.8) | 26 (70.3) | ||
| Education level, (Median)(Minimum, maximum) | 16 (6, 20) | 14.5 (10, 20) * | 16 (9, 20) | 16 (10, 20) | 14 (6, 20) |
| Duration of diabetes, (Median)(Minimum, maximum) | 23.7 (2.2, 66.1) | 10.7 (1.3, 41.1) * | 14.9 (1.3, 66.1) | 15 (2.6, 48.5) | 16.8 (2.2, 45.7) |
| HbA1c, (Median)(Minimum, maximum) | 8.7 (7.6, 12.6) | 9 (7.6, 13.6) | 9 (7.6, 12.6) | 8.8 (7.6, 13.6) | 8.6 (7.6, 13.1) |
| LIPID Panel | |||||
| LDL-Cholesterol, (Median)(Minimum, maximum) | 95.8 (55, 189) | 104 (39.6, 208) * | 98.5 (56, 186) | 95 (39.6, 197) | 100.5 (55, 208) |
| HDL-Cholesterol, (Median)(Minimum, maximum) | 57 (31, 128) | 42 (22, 98) | 50 (22, 101) | 43 (27, 90) | 50 (24, 128) |
| Triglycerides, (Median)(Minimum, maximum) | 66 (21, 273) | 138 (22, 536) * | 100 (29, 299) | 118 (21, 437) | 82.5 (32, 536) |
| Body mass index, (Median)(Minimum, maximum) | 26.1 (17.8, 48.9) | 32.4 (19, 57.8) * | 29.4 (18.6, 51.5) | 29.4 (20.3, 57.8) | 29 (17.8, 50.44) |
| Pedometer steps per day, (Median)(Minimum, maximum) | 7175 (595,21567) | 4681 (156,18938)* | 7273 (156.20121) | 5641 (595,15339) | 5524 (275,21567) * |
| Daily energy expenditure(PAR), (Median)(Minimum, maximum) | 2613 (1504,5134) | 3100 (1893,6170)* | 2882 (1547,5133) | 2924 (1743,6170) | 2880 (1504,4241) |
| Dietary Recall, (Median)(Minimum, maximum) | 213.2 (62.2,738.3) | 183.35 (62.5,536) | 188.4 (62.2,401.9) | 202.2 (75,452.6) | 224.1 (72.8,738.9) |
| Estimated level of Fitness, (Median)(Minimum, maximum) | 27.3 (15.6, 46.9) | 22 (7.9, 46.1) * | 26.8 (9.2, 46.9) | 24.2 (7.9, 43.6) | 23.5 (15.6, 46.1) * |
| Problem Areas in Diabetes, (Median)(Minimum, maximum) | 30.6 (0, 91.3) | 32.5 (1.3, 73.8) | 34.4 (2.5, 91.3) | 30 (3.8, 85) | 32.5 (0, 80) |
| Brief Symptom Inventory: Depression, (Median)(Minimum, maximum) | 48 (40, 73) | 45 (40, 79) | 48 (40, 79) | 45 (40, 79) | 48 (40, 79) |
| Brief Symptom Inventory: Anxiety, (Median)(Minimum, maximum) | 48 (38, 71) | 47 (38, 81) | 47 (38, 69) | 48 (38, 81) | 47 (38, 71) |
| Self-Care Inventory-R, (Mean)(SD) | 56.3 (14.5) | 57.9 (15.7) | 56.3 (14.6) | 57.1 (13.2) | 57.9 (17.5) |
| Diabetes Quality of Life Total Scale (DQOL), (Mean)(SD) | 64.7 (10.8) | 69.6 (10) * | 67.1 (10.4) | 66.6 (10.4) | 67.8 (11.3) |
| DQOL: Diabetes worry subscale, (Median)(Minimum, maximum) | 67.5 (28.8, 90) | 75 (45, 97.5) * | 72.5 (28.8, 97.5) | 72.5 (46.3, 90) | 72.2 (45, 90) |
| DQOL: Global health subscale, (Median)(Minimum, maximum) | 66.7 (0, 100) | 66.7 (0, 100) | 66.7 (0, 100) | 66.7 (0, 100) | 66.7 (0, 100) |
| DQOL: Satisfaction subscale, (Mean)(SD) | 57.9 (15.1) | 60.8 (16.3) | 57.9 (15.1) | 59.1 (15.8) | 61 (16.2) |
| DQOL: Social worry subscale, (Median)(Minimum, maximum) | 75 (18.8, 100) | 81.3 (43.8, 100) * | 81.3 (31.3, 93.8) | 75 (25, 100) | 81.3 (18.8, 100) |
| Rosenberg Self-esteem Scale, (Median)(Minimum, maximum) | 50 (36.7, 70) | 50 (30, 80) | 53.3 (30, 80) | 53.3 (36.7, 80) | 50 (36.7, 70) |
| Social Provisions Total Scale, (Median)(Minimum, maximum) | 79 (46, 96) | 80 (32, 96) | 79 (32, 96) | 79 (48, 96) | 80 (53, 96) |
Values were missing for LDL (n=15), HDL (n=15), Triglycerides (n=15), Pedometer steps per day (n=17), Dietary Recall (n=7), Estimated level of Fitness (n=46), BSI: Depression (n=1), BSI: Anxiety (n=1), and DQOL subscale (n=1)
indicated p<0.05 based on Chi-square, Wilcoxon Two-Sample or Kruskal-Wallis Test
Protocol violations
Unknown to us during screening, one randomized participant did not meet eligibility for being free of severe psychopathology and, after one class, could not continue in the study. Six other participants completed education but did not return for follow-up visits. They did not differ on baseline characteristics from those who completed the study. Finally, for one group of 7 participants in the structured behavioral arm, 6 weeks elapsed between the first and second classes due to severe winter storms. Follow-up visits were scheduled based on the last class.
Hemoglobin A1c levels
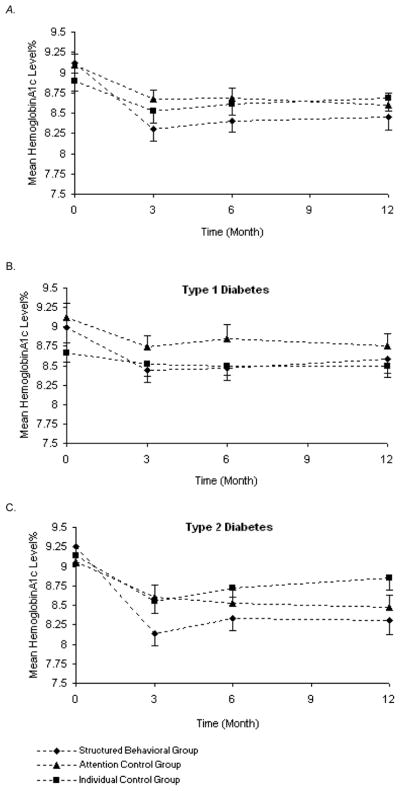
The linear mixed model found that participants improved glycemia (p<0.0001); also both the group/time interaction and the type of diabetes/time interaction being statistically significant at p<0.04 (Table 2 and Figure 2). Thus, although all three groups improved hemoglobin A1c at 3 months, participants in the structured behavioral condition improved more than the control conditions (mean hemoglobin A1c change at 3 months: −0.8% vs −0.4% (attention group control) and −0.4% (individual control)). Those with type 2 diabetes improved more than those with type 1 diabetes (hemoglobin A1c change at 3 months: −0.7% vs −0.3%). Figure 2a shows the mean hemoglobin A1c over time for the three groups for total participants and then by type of diabetes (Figure 2b and 2c). Glycemia deteriorated slightly at 6 months but was basically maintained at 12 months for the two group interventions (Table 2 and Figure 2). When we controlled for age, duration of diabetes, and baseline pedometer steps, the association with the interventions remained statistically significant at the same levels; however, the association with type of diabetes was lost (p=0.09). When we controlled for baseline fitness level, both the intervention effect and the effect of type of diabetes remained intact, however, 27% type 2 and 11% type 1 participants were on beta-blocker medications and therefore did not participate in the YMCA bicycle protocol.
Table 2.
Hemoglobin A1c Levels and Secondary Outcomes of Type of Diabetes and Intervention Group at Baseline, and 3, 6, and 12 Months
| Type of Diabetes | Intervention Group | Mixed Model Analysis With Interactions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Type 1 | Type 2 | Structured Behavioral Group | Attention Control Group | Individual Control Group | ||||||||
|
| ||||||||||||
| N | Mean(SD) | N | Mean(SD) | N | Mean (SD) | N | Mean(SD) | N | Mean(SD) | Effect | P-Value | |
| Hemoglobin A1c % | ||||||||||||
| Baseline | 106 | 8.93(1.0) | 109 | 9.15(1.2) | 70 | 9.12(1.1) | 73 | 9.09(1.2) | 72 | 8.9(1.1) | Time | <.0001 |
| 3 Month | 98 | 8.57(0.9) | 101 | 8.45(1.3) | 64 | 8.3(1.1) | 70 | 8.67(0.9) | 65 | 8.53(1.2) | Typedm | 0.88 |
| 6 Month | 101 | 8.6(0.9) | 100 | 8.53(1.2) | 65 | 8.4(1.1) | 70 | 8.68(1.1) | 66 | 8.61(1) | Group | 0.54 |
| 12 Month | 99 | 8.61(1) | 103 | 8.55(1.5) | 66 | 8.45(1.3) | 70 | 8.6(1.3) | 66 | 8.69(1.3) | Group*Time | 0.04 |
| Typedm*Time | 0.04 | |||||||||||
| Low-density lipoprotein | ||||||||||||
| Baseline | 94 | 101.1(27.4) | 108 | 110.1(34.2) | 65 | 105.8(33.5) | 69 | 108.5(35) | 68 | 103.4(25.2) | Time | 0.15 |
| 6 Month | 77 | 104.2(23.7) | 93 | 106.6(33) | 53 | 108.3(32) | 62 | 100.4(26.5) | 55 | 108.6(28.8) | Typedm | 0.08 |
| 12 Month | 91 | 98.7(25.1) | 99 | 104.4(36.8) | 64 | 103.1(29) | 65 | 98.7(31.9) | 61 | 103.4(34.7) | Group | 0.72 |
| Group*Time | 0.09 | |||||||||||
| Typedm*Time | 0.50 | |||||||||||
| High-density lipoprotein | ||||||||||||
| Baseline | 94 | 59.7(17.8) | 108 | 43.3(11.3) | 65 | 50.9(15.2) | 69 | 48.9(16.2) | 68 | 53(18.7) | Time | 0.01 |
| 6 Month | 78 | 61.6(19) | 93 | 43.1(13.2) | 53 | 52.8(19.3) | 62 | 49.7(18.2) | 56 | 52.4(18.1) | Typedm | <.0001 |
| 12 Month | 91 | 59.3(20.6) | 99 | 42.1(13) | 64 | 52.1(21.4) | 65 | 47.6(17.1) | 61 | 51.5(18.6) | Group | 0.20 |
| Group*Time | 0.81 | |||||||||||
| Typedm*Time | 0.37 | |||||||||||
| Body mass index | ||||||||||||
| Baseline | 106 | 26.7(4.9) | 109 | 33.2(6.9) | 70 | 29.1(6.6) | 73 | 31(7.3) | 72 | 29.9(6.6) | Time | 0.04 |
| 3 Month | 99 | 26.7(5) | 102 | 32.7(7.1) | 64 | 28.6(6.3) | 72 | 31.1(7.5) | 65 | 29.5(6.4) | Typedm | <.0001 |
| 6 Month | 101 | 27(5) | 98 | 32.7(6.7) | 64 | 28.4(5.5) | 71 | 31.5(7.3) | 64 | 29.5(6.3) | Group | 0.16 |
| 12 Month | 98 | 27(4.7) | 101 | 33.1(7.3) | 66 | 28.9(6.7) | 68 | 31.3(7.4) | 65 | 30.1(6.5) | Group*Time | 0.27 |
| Typedm*Time | 0.04 | |||||||||||
| Glycemia checks per day | ||||||||||||
| Baseline | 97 | 3(1.7) | 88 | 1.4(1.1) | 53 | 2.1(1.4) | 67 | 2.2(1.5) | 65 | 2.4(2) | Time | <.0001 |
| 3 Month | 98 | 3.9(1.9) | 90 | 2(2.1) | 60 | 3(2.0) | 66 | 3.2(2.7) | 62 | 2.8(1.9) | Typedm | <.0001 |
| 6 Month | 99 | 3.6(1.8) | 91 | 1.9(1.4) | 62 | 2.7(1.9) | 69 | 3.0(1.9) | 59 | 2.7(1.7) | Group | 0.63 |
| 12 Month | 95 | 3.6(1.9) | 96 | 2.1(1.9) | 66 | 3(2.6) | 67 | 2.9(2) | 58 | 2.6(1.5) | Group*Time | 0.61 |
| Typedm*Time | 0.63 | |||||||||||
| Pedometer readings | ||||||||||||
| Baseline | 101 | 7822(3737) | 98 | 5388(3622) | 65 | 7601(4186) | 68 | 6198(3425) | 66 | 6099(3851) | Time | 0.11 |
| 3 Month | 87 | 8245(3931) | 76 | 5961(4496) | 49 | 8408(4974) | 60 | 6859(3855) | 54 | 6421(4077) | Typedm | <.0001 |
| 6 Month | 90 | 8006(3996) | 79 | 5530(4274) | 54 | 8287(4922) | 62 | 6005(4199) | 53 | 6371(3329) | Group | 0.04 |
| 12 Month | 84 | 7526(3573) | 69 | 5850(4617) | 47 | 7422(4240) | 58 | 6510(4312) | 48 | 6446(3859) | Group*Time | 0.55 |
| Typedm*Time | 0.42 | |||||||||||
| Diabetes related distress | ||||||||||||
| Baseline | 106 | 35.3(22.1) | 109 | 33.1(18.7) | 70 | 34.8(19.3) | 73 | 33.6(20.8) | 72 | 34.0(21.5) | Time | <.0001 |
| 3 Month | 98 | 28.4(17.5) | 91 | 27(20.1) | 62 | 30.5(17.4) | 65 | 25.5(18.3) | 62 | 27.4(20.4) | Typedm | 0.25 |
| 6 Month | 100 | 28.9(18.7) | 94 | 27(19.6) | 63 | 30.4(18.1) | 71 | 26.9(18.8) | 60 | 26.8(20.6) | Group | 0.45 |
| 12 Month | 95 | 26.7(17.8) | 95 | 22.7(15.1) | 65 | 28.5(17.2) | 67 | 22.6(15.9) | 58 | 22.7(16.2) | Group*Time | 0.53 |
| Typedm*Time | 0.67 | |||||||||||
| Self-Care Inventory-R | ||||||||||||
| Baseline | 106 | 56.8(14.2) | 109 | 57.9(15.9) | 70 | 56.9(14.6) | 73 | 56.9(13.4) | 72 | 58.3(17.1) | Time | <.0001 |
| 3 Month | 99 | 63.4(13.9) | 91 | 62.7(14.1) | 62 | 62.6(14.4) | 66 | 64.1(13.7) | 62 | 62.6(14) | Typedm | 0.53 |
| 6 Month | 100 | 63.1(15) | 94 | 60.5(13.6) | 63 | 60.9(14.8) | 71 | 63.3(13.6) | 60 | 61.2(14.9) | Group | 0.53 |
| 12 Month | 95 | 63.4(15.3) | 97 | 62.4(14.1) | 66 | 60.5(14.7) | 67 | 65.9(13.8) | 59 | 62.2(15.3) | Group*Time | 0.09 |
| Typedm*Time | 0.07 | |||||||||||
| Diabetes Quality of Life Scale | ||||||||||||
| Baseline | 106 | 64.7(10.8) | 109 | 69.4(10) | 70 | 67.0(10.2) | 73 | 66.4(10.4) | 72 | 67.8(11.4) | Time | <.0001 |
| 3 Month | 99 | 67.6(10.8) | 91 | 73.1(10.3) | 62 | 69.8(10.7) | 66 | 70.5(11.3) | 62 | 70.5(10.7) | Typedm | 0.001 |
| 6 Month | 100 | 68(12) | 94 | 72(10.6) | 63 | 68.8(10.8) | 71 | 69.4(12.1) | 60 | 71.6(11.6) | Group | 0.76 |
| 12 Month | 95 | 68.6(11.5) | 97 | 73.4(10) | 66 | 69.4(11.3) | 67 | 72.2(10.5) | 59 | 71.6(11.2) | Group*Time | 0.21 |
| Typedm*Time | 0.56 | |||||||||||
P-values associated with Type 3 Tests of Fixed Effects using SAS Proc Mixed
Figure 2.
A–C. Mean Hemoglobin A1c Levels Over Time for the Three Intervention Groups for All Participants and then by Type of Diabetes
Error bars present 1 standard error.
Finally, we used logistic regression to identify characteristics that were associated with a clinically significant improvement in hemoglobin A1c (Table 3). Of baseline characteristics, only a higher hemoglobin A1c predicted a 0.5 percentage point 3-month improvement, and of 3-month characteristics, higher diabetes quality of life, less frustration with treatment, and more emotion-based coping were associated with a hemoglobin A1c improvement.
Table 3.
Logistic Regression Model of Baseline Characteristics Predicting at least a 0.5 Point Improvement in Hemoglobin A1c Levels at Three Months
| Parameter | Odds Ratio Estimates | 95% Wald | Pr > ChiSq | |
|---|---|---|---|---|
| Confidence Limits | ||||
| Intercept | <.0001 | |||
| Hemoglobin A1c at baseline | 2.548 | 1.78 | 3.65 | <.0001 |
| Diabetes Quality of Life Scale - 3 month (unit=10) | 1.521 | 1.01 | 2.28 | 0.0428 |
| Self-care Frustration with Diabetes Treatment - 3 month(unit=10) | 0.833 | 0.70 | 0.99 | 0.0342 |
| Emotion-based Coping - 3 month (unit=10) | 1.582 | 1.18 | 2.11 | 0.0021 |
| Structured Behavioral Group (dummy variable) | 2.537 | 1.14 | 5.62 | 0.0218 |
| Attention Control Group (dummy variable) | 0.754 | 0.35 | 1.62 | 0.4688 |
Secondary outcomes
Diabetes quality of life (total score and subscales), number of daily meter checks, and frequency of self-care behaviors did not differ by type of intervention over time. However, those with type 2 diabetes had higher quality of life scores than those with type 1 diabetes (Table 2).
Participants with type 2 diabetes were heavier at baseline and throughout the study (baseline BMI=33.2 vs 26.7, p<0.001) than those with type 1 diabetes. Those with type 1 diabetes gained 0.45 BMI units while those with type 2 diabetes initially lost ~0.08 units, although they regained this weight at 12 months (main effect of time p<0.04; type of diabetes/time interaction p<0.04). Intervention assignment did not impact BMI (Table 2).
Adverse events
Participants reported no episodes of hypoglycemia that required assistance of others. One participant endorsed ‘sometimes’ on thoughts of suicidal ideation on the Brief Symptom Inventory; this participant was assessed by the study psychologist and found not to be suicidal but was referred for treatment of depression. Three participants reported non-study related adverse events at follow-up: chest pain prior to follow-up visit, breast cancer, and traumatic foot injury resulting in amputation.
Discussion
This single center randomized controlled trial, studying 222 adults with type 1 or type 2 diabetes in poor glycemic control, represented a head-to-head comparison of an intervention with embedded behavioral strategies with two forms of diabetes education: one-to-one nurse and dietitian counseling and standard group education. Although glycemic control improved in all three arms, the group assigned to the highly structured behavioral arm, in which the nurse and dietitian educators were trained to use scaffolding techniques and brief cognitive behavioral strategies, showed more improvement. Further, the structured behavioral group intervention was more effective in terms of improving glycemic control for those with type 2 diabetes while those with type 1 diabetes responded equally well to one-on-one control sessions as to the structured behavioral condition.
The impact of glycemic control on preventing complications in type 2 diabetes has been well-documented in the long-term Diabetes Control and Complications Trial (DCCT)36 and United Kingdom Prospective Diabetes Study (UKPDS)37 clinical trials. Although our participants did not achieve glycemic targets of less than 7%, extrapolating the UKPDS results, a 0.67% reduction in A1c observed at 12 months, if sustained long-term, should by itself result in ~20% reduction in microvascular and ~10% reduction in cardiovascular endpoints37. We also demonstrated that clinical staff can successfully incorporate modified psychological and behavioral strategies designed to support diabetes self-care rather than address psychopathology.
Meta-analyses of small studies of diabetes education interventions found that these interventions were successful in improving glycemia, particularly when a behavioral intervention was incorporated17–19,38. However, little is known about the specific behavioral components and/or education that are necessary to support lifestyle changes and self-care behaviors. The Diabetes Prevention Program39 demonstrated that educator-led lifestyle interventions prevented diabetes for people at risk more than metformin alone. Interestingly, a well-designed cognitive behavioral intervention that was not embedded in an education intervention had a relatively minimal impact on glycemia for people with diabetes22. Few, if any, studies do head-to-head comparisons of interventions to determine if clinical staff can successfully incorporate behavioral techniques into their clinical practices. Thus, our study represents one of the first randomized controlled trials to conduct head-to-head comparisons of three self-care interventions.
Successful diabetes treatment requires participant active involvement in multiple self-care behaviors and treatment prescriptions necessary for achieving glycemic targets40,41. Our findings demonstrate that a diabetes self-management support intervention is an important component of treatment for participants who have not achieved therapeutic targets, evidenced by all three arms achieving an improvement in glycemia at 3 months post-intervention. We also found that nurses and dietitians were able to implement successfully specific behavioral strategies and techniques, including high structure, modified cognitive restructuring, and modeling of behavior, and that when applied, poorly controlled participants were able to improve glycemia. These strategies were not used for therapeutic counseling of psychopathology but rather as support for participants who were attempting to change lifestyle approaches.
Patients often struggle to follow recommended health behaviors. Our study found that participants improved glycemia, although many did not achieve glycemic targets of <7%. One explanation for some patients’ struggles may be their inability to impose their own structure on their life behavior. Our highly structured behavioral intervention provided a scaffold that allowed participants to integrate specific dietary and physical activity behaviors into their busy schedules. Another explanation may be that struggling patients lack contact with others who have diabetes and therefore have little opportunity to discuss or reinforce self-management strategies. The structured behavioral group intervention may have provided social support that led to more engagement in their self-care. However, one of the control conditions was a group education intervention that provided a similar amount of professional and non-health professional support for participants, making the differential improvement solely due to increased social support unlikely.
Further, participants with type 1 diabetes improved equally in the highly structured behavioral group arm and in the individual arm while those with type 2 diabetes improved more in the structured behavioral arm compared to the control group and individual arms. Type 2 diabetes participants were particularly responsive to the education and many maintained that response over time. These findings may result from those with type 1 diabetes receiving more basic educational and behavioral support at diagnosis and throughout the course of their diabetes compared to those with type 2 diabetes. One study examining the long-term value of diabetes education provided at diagnosis found beneficial effects in terms of weight loss and smoking42. Another explanation may be that type 1 diabetes patients who struggle with achieving glycemic targets need more help with emotional and psychological issues than support with diabetes self-management skills.
Our study has several limitations. The interventions did not have follow-up support built into the program. To protect the integrity of each arm of the study, classes and sessions were held in different sections of the Center. By design, only the attention control group was embedded in the clinic as this was the most conservative approach. Further, the structured behavioral arm had more patients receiving their care outside of the Clinic, and may not have received the same intensity of medical/educational follow-up. Thus, the important issue of sustainability will need to be studied in a future trial. Further, the mechanisms underlying the differential response, whether associated with subclinical depression, organization and executive functioning abilities, or some other factor, cannot be addressed.
In summary, our primary objective of this randomized controlled trial was to determine whether a structured, cognitive behavioral group education was more effective in improving glycemic control than an attention control diabetes education or individual education. We also aimed to determine if diabetes clinicians, in this case, educators, could incorporate these psychological/behavioral techniques in their clinical approaches. We found that participants in poor glycemic control in all three education arms improved glycemia, and the highly structured behavioral group arm, which employed cognitive behavioral strategies, was most effective in helping these diabetes participants in poor glycemic control improve glycemia and maintain that improvement over one year.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) grant R01 DK60115 (K.W.), the Diabetes and Endocrinology Research Core NIH P30 DK36836, and the Joslin Diabetes Center Clinical Research Center. The following companies contributed glucose meters and test strips: Abbott Laboratories (Abbott Park, IL), LifeScan (Milpitas, CA), and Roche Diagnostics (Indianapolis, IN). NIDDK and these companies had no role in the conduct of the study or preparation of this manuscript. We thank the patients who participated in the study and the nurses and staff at the Joslin Clinical Research Center.
Footnotes
Presented at the American Diabetes Association Annual Scientific Sessions, June 2008
Conflict of Interest
A. Enrique Caballero, MD serves on the advisory panels of the following companies: Eli Lilly and Company, Amylin Pharmaceuticals, Inc, Takeda Pharmaceuticals America, Inc, sanofi-aventis, Daiichi-Sankyo. No other author has anything to declare.
References
- 1.Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care. 2008;31(1):81–86. doi: 10.2337/dc07-1572. [DOI] [PubMed] [Google Scholar]
- 2.Resnick HE, Foster GL, Bardsley J, Ratner RE. Achievement of American Diabetes Association clinical practice recommendations among U.S. adults with diabetes, 1999–2002: the National Health and Nutrition Examination Survey. Diabetes Care. 2006;29(3):531–537. doi: 10.2337/diacare.29.03.06.dc05-1254. [DOI] [PubMed] [Google Scholar]
- 3.Weinger K, Jacobson AM. Psychosocial and quality of life correlates of glycemic control during intensive treatment of type 1 diabetes. Patient Educ Couns. 2001;42(2):123–131. doi: 10.1016/s0738-3991(00)00098-7. [DOI] [PubMed] [Google Scholar]
- 4.Roter DL, Hall JA, Merisca R, Nordstrom B, Cretin D, Svarstad B. Effectiveness of Interventions to Improve Patient Compliance: A Meta-Analysis. Med Care. 1998;36:1138–1161. doi: 10.1097/00005650-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Doherty Y, Hall D, James PT, Roberts SH, Simpson J. Change counselling in diabetes: the development of a training programme for the diabetes team. Patient Educ Couns. 2000;40(3):263–278. doi: 10.1016/s0738-3991(99)00079-8. [DOI] [PubMed] [Google Scholar]
- 6.Kruijver IP, Kerkstra A, Francke AL, Bensing JM, van de Wiel HB. Evaluation of communication training programs in nursing care: a review of the literature. Patient Educ Couns. 2000;39(1):129–145. doi: 10.1016/s0738-3991(99)00096-8. [DOI] [PubMed] [Google Scholar]
- 7.Naik AD, Palmer N, Petersen NJ, et al. Comparative effectiveness of goal setting in diabetes mellitus group clinics: randomized clinical trial. Arch Intern Med. 2011;171(5):453–459. doi: 10.1001/archinternmed.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63(4):619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24(6):1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 10.Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23(7):934–942. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 11.Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160(21):3278–3285. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]
- 12.Lin EH, Katon W, Von Korff M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27(9):2154–2160. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- 13.Lin EH, Rutter CM, Katon W, et al. Depression and advanced complications of diabetes: a prospective cohort study. Diabetes Care. 2010;33(2):264–269. doi: 10.2337/dc09-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin EH, Heckbert SR, Rutter CM, et al. Depression and increased mortality in diabetes: unexpected causes of death. Ann Fam Med. 2009;7(5):414–421. doi: 10.1370/afm.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Feltz-Cornelis CM, Nuyen J, Stoop C, et al. Effect of interventions for major depressive disorder and significant depressive symptoms in patients with diabetes mellitus: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2010;32(4):380–395. doi: 10.1016/j.genhosppsych.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Georgiades A, Zucker N, Friedman KE, et al. Changes in depressive symptoms and glycemic control in diabetes mellitus. Psychosom Med. 2007;69(3):235–241. doi: 10.1097/PSY.0b013e318042588d. [DOI] [PubMed] [Google Scholar]
- 17.Brown SA. Effects of educational interventions in diabetes care: a meta-analysis of findings. Nurs Res. 1988;37(4):223–230. [PubMed] [Google Scholar]
- 18.Padgett D, Mumford E, Hynes M, Carter R. Meta-analysis of the effects of educational and psychosocial interventions on management of diabetes mellitus. J Clin Epidemiol. 1988;41(10):1007–1030. doi: 10.1016/0895-4356(88)90040-6. [DOI] [PubMed] [Google Scholar]
- 19.Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet. 2004;363(9421):1589–1597. doi: 10.1016/S0140-6736(04)16202-8. [DOI] [PubMed] [Google Scholar]
- 20.Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care. 2001;24(3):561–587. doi: 10.2337/diacare.24.3.561. [DOI] [PubMed] [Google Scholar]
- 21.Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170(17):1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ismail K, Thomas SM, Maissi E, et al. Motivational enhancement therapy with and without cognitive behavior therapy to treat type 1 diabetes: a randomized trial. Ann Intern Med. 2008;149(10):708–719. doi: 10.7326/0003-4819-149-10-200811180-00005. [DOI] [PubMed] [Google Scholar]
- 23.Weinger K, Butler HA, Welch GW, La Greca AM. Measuring diabetes self-care: a psychometric analysis of the Self-Care Inventory-Revised with adults. Diabetes Care. 2005;28(6):1346–1352. doi: 10.2337/diacare.28.6.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McConnel TR. Cardiorespiratory Assessment of apparently healthy populations. In: JLR, editor. ACSM’s Resource Manual for Guidelines for Exercise Testing and Prescription. Baltimore, Maryland: Lippincott Williams & Wilkins; 1998. pp. 347–353. [Google Scholar]
- 25.Vinik AI, Park TS, Stansberry KB, Pittenger GL. Diabetic neuropathies. Diabetologia. 2000;43(8):957–973. doi: 10.1007/s001250051477. [DOI] [PubMed] [Google Scholar]
- 26.Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18(6):754–760. doi: 10.2337/diacare.18.6.754. [DOI] [PubMed] [Google Scholar]
- 27.Welch G, Weinger K, Anderson B, Polonsky WH. Responsiveness of the Problem Areas In Diabetes (PAID) questionnaire. Diabet Med. 2003;20(1):69–72. doi: 10.1046/j.1464-5491.2003.00832.x. [DOI] [PubMed] [Google Scholar]
- 28.Derogatis LR. BSI 18: Brief Symptom Inventory. Administration, Scoring and Procedures Manual. Minneapolis, MN: National Computer Systems, Inc; 2000. [Google Scholar]
- 29.Peyrot M, McMurry JF, Jr, Kruger DF. A biopsychosocial model of glycemic control in diabetes: stress, coping and regimen adherence. J Health Soc Behav. 1999;40(2):141–158. [PubMed] [Google Scholar]
- 30.Peyrot MF, McMurry JF., Jr Stress buffering and glycemic control. The role of coping styles. Diabetes Care. 1992;15(7):842–846. doi: 10.2337/diacare.15.7.842. [DOI] [PubMed] [Google Scholar]
- 31.van der Ven NC, Weinger K, Yi J, et al. The confidence in diabetes self-care scale: psychometric properties of a new measure of diabetes-specific self-efficacy in Dutch and US patients with type 1 diabetes. Diabetes Care. 2003;26(3):713–718. doi: 10.2337/diacare.26.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobson AM, de Groot M, Samson JA. The evaluation of two measures of quality of life in patients with type I and type II diabetes. Diabetes Care. 1994;17(4):267–274. doi: 10.2337/diacare.17.4.267. [DOI] [PubMed] [Google Scholar]
- 33.Jacobson AM. the DCCT Research Group. The Diabetes Quality of Life Measure. In: Bradley C, editor. Handbook of Psychology and Diabetes. London: J. Wiley; 1994. [Google Scholar]
- 34.Kinsley BT, Weinger K, Bajaj M, et al. Blood glucose awareness training and epinephrine responses to hypoglycemia during intensive treatment in type 1 diabetes. Diabetes Care. 1999;22(7):1022–1028. doi: 10.2337/diacare.22.7.1022. [DOI] [PubMed] [Google Scholar]
- 35.SAS 9.2 [computer program] Cary, North Carolina: SAS Institute; 2010. [Google Scholar]
- 36.Diabetes Control and Complications Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 37.UKPDS. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 38.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson RM, Funnell MM, Butler PM, Arnold MS, Fitzgerald JT, Feste CC. Patient empowerment. Results of a randomized controlled trial. Diabetes Care. 1995;18(7):943–949. doi: 10.2337/diacare.18.7.943. [DOI] [PubMed] [Google Scholar]
- 41.Weinger K, McMurrich Greenlaw S. Behavioral strategies for improving self-management. In: Childs B, Cypress M, Spollett G, editors. Complete Nurse’s Guide to Diabetes Care. 2. Alexandria, VA: American Diabetes Association; 2009. [Google Scholar]
- 42.Davies MJ, Heller S, Skinner TC, et al. Effectiveness of the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cluster randomised controlled trial. BMJ. 2008;336(7642):491–495. doi: 10.1136/bmj.39474.922025.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]