Abstract
Purpose.
The aims were to determine whether exposure to sodium hydroxide results in predictable changes in phosphatidylcholine (PC) in corneal tissue and if PC profile changes correlate to exposure duration. PCs are major components of the cell membrane lipid bilayer and are often involved in biological processes such as signaling.
Methods.
Enucleated porcine (n = 140) and cadaver human eyes (n = 20) were exposed to water (control) and 11 M NaOH. The corneas were excised and lipids were extracted using the Bligh and Dyer method with suitable modifications. Class-specific lipid identification was carried out using a ratiometric lipid standard on a TSQ Quantum Access Max mass spectrometer. Protein amounts were determined using Bradford assays.
Results.
Control and alkali-treated corneas showed reproducible PC spectra for both porcine and human corneas. Over 200 PCs were identified for human and porcine control and each experimental time point. Several PC species (m/z values) consequent upon alkali exposure could not be ascribed to a recorded PC species. Control and treated groups showed 41 and 29 common species among them for porcine and human corneas, respectively. The unique PC species peaked at 12 minutes and at 30 minutes for human and porcine corneas followed by a decline consistent with an interplay of alkali penetration and hydrolyses at various time points.
Conclusions.
Alkali exposure dramatically changes the PC profile of cornea. Our data are consistent with penetration and hydrolysis as stochastic contributors to changes in PCs due to exposure to alkali for a finite duration and amount.
Alkali exposure dramatically changes the phosphatidylcholines (PCs) of the cornea. Findings are consistent with penetration and hydrolysis as stochastic contributors to changes in PCs due exposure to alkali for a finite duration and amount.
Introduction
Damage to the eye may be caused by pathological processes, mechanical trauma, chemical exposures, and a multitude of other events. Chemical exposures resulting in injuries can result from industrial accidents due to equipment failure or operator error, natural disasters, and intentional acts including chemical warfare.1 In the United States, hazardous chemicals are stored at over 15,000 locations and therefore leave a vast population vulnerable to chemical exposures.2 Alkaline agents are basic substances that dissolve in water and produce a solution with a pH greater than 7. Many industrial agents in addition to recorded hazardous chemicals are alkaline in nature. However, sodium hydroxide is an alkali that is commonly used in industrial settings.3 There are many instances in which chemical spills of sodium hydroxide or other alkaline materials have resulted in exposure and blindness.4
The corneal epithelium, the outermost layer of the cornea, is usually the layer of the eye that encounters initial contact with a chemical agent during an exposure.5 Lipids play a vital role in the composition and function of the cornea. Overall, lipids constitute approximately 5% of the cornea by mass.6 Phospholipids are one of the most important categories of lipids that define the cell membrane and help provide structural integrity to cells. Their arrangement and composition in the cell membrane lipid bilayer play an integral role in cell structure and membrane permeability and also in cell signaling.7 Analyses have revealed that phosphatidylcholine (PC), phosphatidylethanolamine, phosphatidylinositol, phosphatidylserine, and sphingomyelin are the major phospholipids in corneal epithelial, fibroblast, and endothelial cell types.8 Alkaline materials have the ability to quickly penetrate corneal layers,9 to saponify the ester linkage of lipids with alcohols such as glycerol, and to hydrolyze the lipids.10 The severity of damage to the cornea often correlates with the strength of the alkali (or its basicity), which relates to its penetrating power and ability to hydrolyze lipids and proteins.10
PCs are usually the major component of the phospholipid bilayer of cellular membranes,9 accounting for approximately half of all membrane lipids.11 Corneal exposure to alkali causes their hydrolyses and therefore has a major effect on the stability of cellular membranes. Thus, it is part of the damage process on exposure to alkali.12 The hydrolysis of phospholipids, proteins, and other biomolecules on exposure to alkali is well known and is generally regarded as part of the damage process. However, some phospholipids, and PCs in particular, have been shown to act as promoters of wound healing in the cornea13 as well as in the repair/healing of other organs.14,15 Indeed, we have observed that some protective and corneal wound-healing lipids appear within a short period of alkali exposure (see Supplementary Material and Supplementary Fig. 1, http://www.iovs.org/content/53/11/7122/suppl/DC1). Subsequently they quickly disappear, most likely due to hydrolysis. Due to their importance in the damage mechanism and in repair-promoting activities, studying the changes in PCs in response to alkali exposure will be the first significant step toward understanding their biological consequences. Precise understanding of the damage process will enable the development of tailored intervention strategies and result in better outcomes. At the same time, it will help to build a better repertoire of new less toxic therapeutic molecules through identification of naturally occurring molecules that appear in response to damage and augment repair. In the present study, we have attempted to identify changes in the PC profile of the cornea in response to exposure to sodium hydroxide using recently developed mass spectrometric methods.16,17
The current study used enucleated porcine corneas as a model system that resembles the human cornea in biomechanical and other properties.18 Our current experiments were guided by some previous experiments performed with porcine corneas exposed to several alkaline agents of varying strengths and concentrations.9 This prior research arrived at a few meaningful experiments with sodium hydroxide. Select experiments with sodium hydroxide were then repeated with a subset of human corneas, ensuring that the results are relevant for human corneas.
Methods
Tissue Procurement
Enucleated porcine eyes (n = 140) were procured from Just Meats, Chillicothe, Ohio, and from the Department of Surgery at the University of Miami Miller School of Medicine, Miami, Florida. Porcine eyes were procured from the Department of Surgery within 1 hour after animals were euthanized and subsequently enucleated under an Institutional Animal Care and Use Committee (IACUC)-approved tissue-sharing protocol. Those from Just Meats were maintained at 4°C during transit and were 48 to 72 hours old at arrival for experiments. All experiments were performed adhering to the ARVO Statement for the use of animals in ophthalmic and vision research and utilized IACUC-approved protocols. Cadaver human eyes (n = 20) were procured from the Florida Lions Eye Bank at Bascom Palmer Eye Institute, Miami, Florida. These eyes ranged from 12 hours to 7 days from death and enucleation to actual experimental utilization. The use of these eyes followed institutional review board–approved protocols and adhered to the tenets of the Declaration of Helsinki. All donors were Caucasians of either sex between 50 and 90 years old at time of death and had no known corneal disease. All enucleated eyes were washed in 1× phosphate-buffered saline (PBS) and stored at 4°C in PBS before experiments were performed. Porcine and human eyes were carefully examined and only those with no epithelial defects were candidates for alkali exposure.
Exposure to Alkali
Porcine and human eyes were placed in an in-house constructed acrylic resin (Plexiglas; Altuglas International, Philadelphia, PA) chamber/apparatus as described in our previous report.9 The acrylic resin (Plexiglas) chamber ensured the safety of the experimenter and contained alkali solutions that might have splashed onto the ocular tissue. A solution of 11 M sodium hydroxide (NaOH) was splashed onto the eye using a syringe. Approximately 5 mL of each alkali solution was splashed onto the fully exposed cornea, ensuring that the entire cornea was covered. After a single exposure, excess alkali was drained off, and each cornea was immediately excised using sterile scissors and forceps. Corneal tissue was divided into two sections, unless stated otherwise, with each assigned to a predesignated time interval (30 seconds, 12 minutes, 30 minutes, 60 minutes) for exposure and was washed immediately after the designated time interval had elapsed. A repeat experiment was conducted if a delay occurred. Control experiments were performed in the same manner, using distilled water.
Lipid Extraction
Corneal lipids were extracted from the exposed corneal tissue using a modified Bligh and Dyer lipid extraction method.19 For membrane disruption and to release lipids, the corneal tissues were minced with scissors and were subsequently flash frozen at −80°C and thawed in a 37°C water bath, alternating for 10 minutes each for a total of five cycles. A solution of chloroform (product no. 650498; Sigma-Aldrich Corp., St. Louis, MO)/methanol (product no. 34966; Sigma-Aldrich Corp.) (1:1) with 10 μM of butylated hydroxytoluene (BHT; W218405; Sigma-Aldrich Corp.) was added to each of the samples and blended with a handheld homogenizer. The BHT was included as a free radical scavenger to prevent lipid oxidation. The specimens were then blended for an additional 30 seconds and subsequently centrifuged at 10,000g for 15 minutes at 4°C for phase separation. The upper alcoholic phase contained all nonlipid molecules and the lower chloroform phase contained the extracted lipids. The chloroform layer was then separated from the alcoholic phase and flushed with argon gas (product no. AR UHP35; Airgas, Inc., Radnor, PA). The argon flush was used in order to remove oxygen from the samples to prevent lipid oxidation. The chloroform was evaporated using a Speedvac (Model 7810014; Labconco, Kansas City, MO), leaving dried lipids. The lipid samples were then flushed with argon again and either analyzed immediately or stored at −80°C prior to analysis. The alcoholic phase was retained and stored at −20°C for later protein quantification.
Thin Layer Chromatography
Thin layer chromatography (TLC) was performed for initial assessment of total corneal phospholipids (see Supplementary Material and Supplementary Fig. 2, http://www.iovs.org/content/53/11/7122/suppl/DC1) after lipid extraction. Intrinsic fluorescence of the TLC plates (catalog number 52011; Analtech, Inc., Newark, DE) was utilized for separation and their detection on a UV transilluminator. This method was found to have much less sensitivity compared to mass spectrometric identification and quantification of PC lipids. With an objective to perform an independent quantification of phospholipids, we used TLC and primuline20 to monitor the progress of the experiment. We attempted to determine whether primuline adduction of PCs20 could be utilized for quantification. Extensive experimentation with TLC-based primuline quantification did not provide reproducible or reliable results.
Evaluation of Storage Conditions
In order to evaluate the extent of lipid degradation with time and storage, control porcine eyes were analyzed in several conditions mirroring those of experimental eyes. First, in order to get the most accurate assessment of the phospholipid profile of porcine corneas with as little time as possible for lipid degradation, porcine eyes were obtained from the Department of Surgery. Lipids from control corneas were extracted and analyzed within 1 to 3 hours of the euthanasia and enucleation. To ensure that extracted lipids did not significantly degrade with storage at −80°C between extraction and analysis, samples extracted immediately after the death of the animal as well as after storage were analyzed. For analyses of effect of storage, lipids were stored at −80°C for 24 and 72 hours and analyzed. Finally, it was important to evaluate samples that had been stored in a manner similar to those received from Just Meats. Furthermore, these analyses were compared to analyses of lipids extracted from porcine eyes, stored whole in 1× PBS at 4°C for 24 and 72 hours before extraction and analysis.
Mass Spectrometry
Extracted lipidswere re-suspended in 1 mL of Sigma Aldrich LC-MS grade propanol–acetonitrile (1:1) solution. They were analyzed using infusion mode on a TSQ Quantum Access Max triple quadrupole mass spectrometer with an electrospray source using published conditions for analyses of PC lipids.16 Positive ion mode precursor ion scan was used for PCs with a daughter mass by charge (m/z) of 184 and collision energy (CE) of 35 eV. Collision gas pressure was set at 1 mTorr. Sheath gas was set to 12 arbitrary units, and auxillary gas was set to 5 arbitrary units. Samples were injected via syringe infusion at a flow rate of 10 μL/min using a Hamilton syringe (part 81265; Hamilton Co., Reno, NV). They were scanned for 1 minute with a 0.5-second scan between 200 m/z and 1000 m/z in profile mode using TSQ Tune software. A fixed half peak width (FWHM) of 0.7 was used. Quantitative lipid standards of PC (1,2-ditridecanoyl-sn-glycero-3-phosphocholine; neutral mass 649.9; catalog no. 850340; Avanti Polar Lipid, Alabaster, AL) were subsequently added with a concentration range of 0.49 pmol to 35 nmol per 500 μL into corneal lipid samples suspended in the propanol–acetonitrile solution and analyzed in the same manner for quantitative analyses. Samples for each time point were scanned three separate times to establish consistency and reproducibility. Independent triplicate sets of experiments were performed for each analysis unless noted otherwise.
Protein Quantification
Soluble proteins were quantified by the Bradford assay21,22 using commercially available reagents (Bio-Rad, Hercules, CA) with amino acid quantified purified BSA as a standard. In order to relate lipid amount to protein amount, a subset of samples were also subjected to electrophoresis on a 4% to 15% gradient PHAST gel system (GE Healthcare Biosciences, Piscataway, NJ), stained with Coomassie Blue (Gel Code Blue; Thermo Scientific Corporation, Rockford, IL) or silver staining (BULLit silver staining kit; Amresco, Solon, OH) with BSA as the standard. Densitometric scans were performed for quantification and the data were used for verification of protein quantified using Bradford's method.
Ratiometric Quantification and Data Analyses
Lipid species identification was performed using MZmine 2.2 software (in the public domain, http://mzmine.sourceforge.net).23 Thermo Finnigan RAW files were converted to netCDF files using Xcalibur for importing into MZmine 2.2. Noise levels were eliminated (usually E2 levels). Remaining peaks were deisotoped using monotonic isotopic shape with ±2.0 m/z. Peaks were then blasted against a custom database created from LipidMaps (Lipidmaps structure database, LMSD; www.lipidmaps.org; Nature Lipidomics Gateway, La Jolla, CA) for identification of PC species. Ratiometric quantification of PCs were performed against the concentration of the lipid standard used (1,2-ditridecanoyl-sn-glycero-3-phosphocholine) and further normalized against amount of protein in the aqueous phase from the same extraction. A comprehensive list of all PC species from each of the three independent replicate experiments was compiled for control and experimental samples for both human and porcine corneas. The averages and standard deviations of lipid amount per protein amount were determined for all identified lipids (see Supplementary Material and Supplementary Tables S1, S2, http://www.iovs.org/content/53/11/7122/suppl/DC1). When the lipid species was not identifiable from the custom LMSD database, the m/z ratio was left as is (see Supplementary Material and Supplementary Tables, http://www.iovs.org/content/53/11/7122/suppl/DC1). Differences in lipid profiles were determined by in-house written Excel macros for differential presence of PC species between control and alkali exposed samples. Symmetrical five-set elliptical Venn diagrams (style following Branko Grünbaum24) were created to display the number of species which were unique or common to control and experimental samples for all time points for human and porcine samples.
Results
PCs of Porcine and Human Corneas
Due to intrinsic and in-deterministic variables as well as deterministic variables, mass spectrometric experiments were repeated to ensure reproducibility of corneal PC spectra for porcine and human corneas for control (as well as alkali-treated eyes).
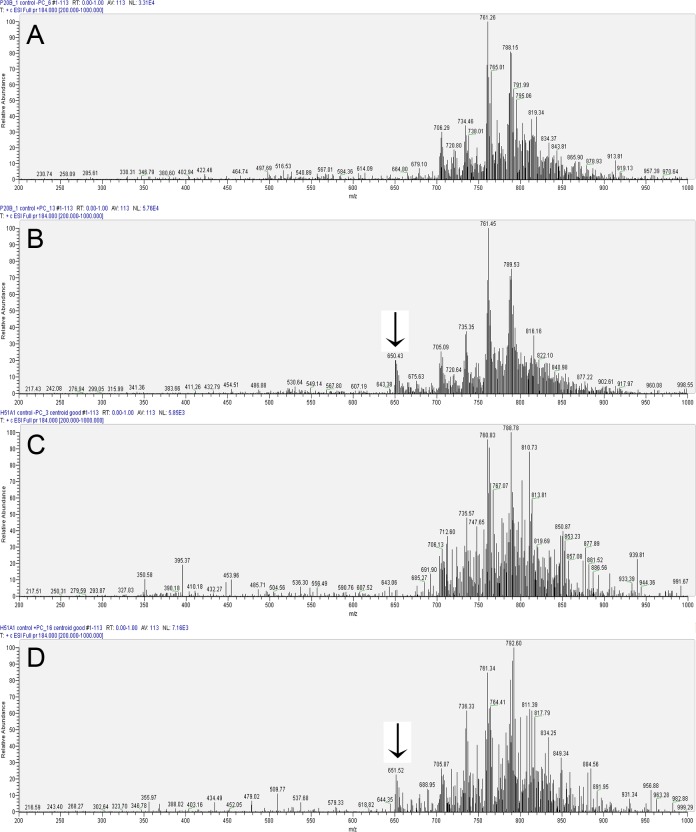
Porcine control (exposed to water) spectra were obtained without the addition of external standard (Fig. 1A) to obtain qualitative results. This was repeated with addition of a class-specific quantitative external standard (Fig. 1B) in order to allow for lipid quantification. Human control corneal PC analyses yielded similar results without and with the external standard, respectively (Figs. 1C, 1D). The two most prominent peaks of PCs in both porcine and human corneas were around m/z 761 and 788. In addition, both porcine and human control corneas also showed a prominent peak around m/z 705, which was the first prominent peak in the m/z range of 200 to 700 (Figs. 1A, 1C). However, there were important differences as well. For example, m/z ∼ 761 peak was the most prominent in the porcine cornea, while m/z ∼ 788 peak was the most prominent in the human cornea. Also, m/z ∼ 816 to 819 were prominent peaks in porcine corneas while ∼ 810 to 812 were the most prominent peaks in the human corneas (Figs. 1A, 1C). The spectra of porcine and human controls therefore resembled one another closely; however, they also had important differences as would be expected for two different mammalian species (Figs. 1A, 1C).
Figure 1. .
Representative LC-MS/MS analysis of control (exposed to water) corneal PCs after immediate extraction. Parent-ion scan (PIS) for daughter ion of 184 was carried out in the positive ion mode with a collision energy of 35 V on a TSQ Quantum Access Max instrument. (A) Representative profile of porcine PCs. (B) Profile of porcine PCs with the addition of an external standard (m/z of 650.43), as indicated with an arrow. (C) Representative profile of human PCs. (D) Profile of human corneal PCs with added external standard indicated by the arrow.
Qualitative Differences in PC Spectra due to Corneal Storage Differences
The possibility of sourcing the animal eyes locally often enables the shortest time gap between the euthanasia (combined with enucleation) and analyses. Except for local experimental tissue sharing, the time gap between euthanasia/enucleation to analyses is considerably increased when eyes are procured from commercial sources such as an abattoir. Eyes derived from either inbred animals or animals from similar genetic stock and raised under identical conditions are expected to present minimal intrinsic variations with respect to their lipid composition. Substantial intrinsic variation in human eyes is expected. In addition, postmortem time, time from death to enucleation, and handling of the eye prior to analyses are important parameters that may affect the PC profiles. For these reasons, we performed a qualitative assessment of PC spectra under different storage conditions for porcine corneas.
The spectra of PC, extracted from the corneas of freshly enucleated (within 1 hour of euthanasia) porcine eyes, showed the overall intensities in the 104 range (see Supplementary Material and Supplementary Fig. 3A, http://www.iovs.org/content/53/11/7122/suppl/DC1). The spectra after storage of whole eyes at 4°C in 1× PBS for 24 hours showed decreased relative overall intensities in 103 range (see Supplementary Material and Supplementary Fig. 3B, http://www.iovs.org/content/53/11/7122/suppl/DC1) for lipid samples scanned for 1 minute with a flow rate of 10 μL/min. The lipids for these analyses were isolated in 1 mL with aqueous phase 10 μg protein derived from approximately 100 mg wet cornea. Prolonged storage up to 72 hours did not further decrease the relative intensities of PC species and spectra showed relative overall intensities in the 103 range (see Supplementary Material and Supplementary Fig. 3C, http://www.iovs.org/content/53/11/7122/suppl/DC1). In general the relative intensities of PC species were uniformly decreased in stored eyes overall across the entire mass/charge range (see Supplementary Material and Supplementary Fig. 3, http://www.iovs.org/content/53/11/7122/suppl/DC1); moreover, we did not find selective omission of any PC species after storage. The most prominent first peak within the m/z range 200 to 700 was ∼ 705; however, the biggest difference at m/z ∼ 761 was somewhat reduced compared to m/z ∼ 788. Other characteristic ions such as m/z ∼ 816 to 819 retained proportional levels in storage up to 72 hours at 4°C in PBS or in Optisol GS medium (not shown).
Storage of PC lipids extracted from the porcine corneas of freshly euthanized and enucleated (within 1 hour) animals at −80°C for up to 24 hours did not decrease the relative intensities of these species for a comparable amount of input lipids as stated above (see Supplementary Material and Supplementary Fig. 4A, http://www.iovs.org/content/53/11/7122/suppl/DC1). In other words, for similar amounts of sample, intensities of PC species remained in the range of 104. Importantly, the distribution and relative proportionality of m/z species around 705, 761, and 788 for −80°C stored lipids (see Supplementary Material and Supplementary Fig. 4A, http://www.iovs.org/content/53/11/7122/suppl/DC1) were similar for PC species extracted from fresh corneas (see Supplementary Material and Supplementary Fig. 3A, http://www.iovs.org/content/53/11/7122/suppl/DC1). The storage of PC lipids at −80°C for 72 hours did decrease the relative intensities of the spectra to 103 range (see Supplementary Material and Supplementary Fig. 4B, http://www.iovs.org/content/53/11/7122/suppl/DC1). However, the distribution and relative proportionality of m/z species around 705, 761, and 788 were better maintained for lipids stored at −80°C than for those derived from cornea stored at 4°C (see Supplementary Material and Supplementary Figs. 4B, 3C, http://www.iovs.org/content/53/11/7122/suppl/DC1). Similar to the effect of storing whole eyes or corneas at 4°C (see Supplementary Material and Supplementary Fig. 3C, http://www.iovs.org/content/53/11/7122/suppl/DC1), the storage of lipid extracts at −80°C for 72 hours decreased PC spectra uniformly (see Supplementary Material and Supplementary Fig. 4B, http://www.iovs.org/content/53/11/7122/suppl/DC1); therefore, the spectra of stored lipids closely resembled that of PC spectra extracted from fresh porcine cornea (see Supplementary Material and Supplementary Fig. 3A, http://www.iovs.org/content/53/11/7122/suppl/DC1). Analyses did not show selective absence of any PC species (usually an average data from five samples were compared). However, the relative ratio of different species was better preserved when lipids, extracted from fresh corneas, were stored for prolonged period in −80°C compared with storage at 4°C and subsequent extraction. These findings are consistent with qualitative demonstrations from representative spectra presented here (see Supplementary Material and Supplementary Figs. 3C, 4B, http://www.iovs.org/content/53/11/7122/suppl/DC1).
Changes in Corneal PC in Response to Alkali Exposure
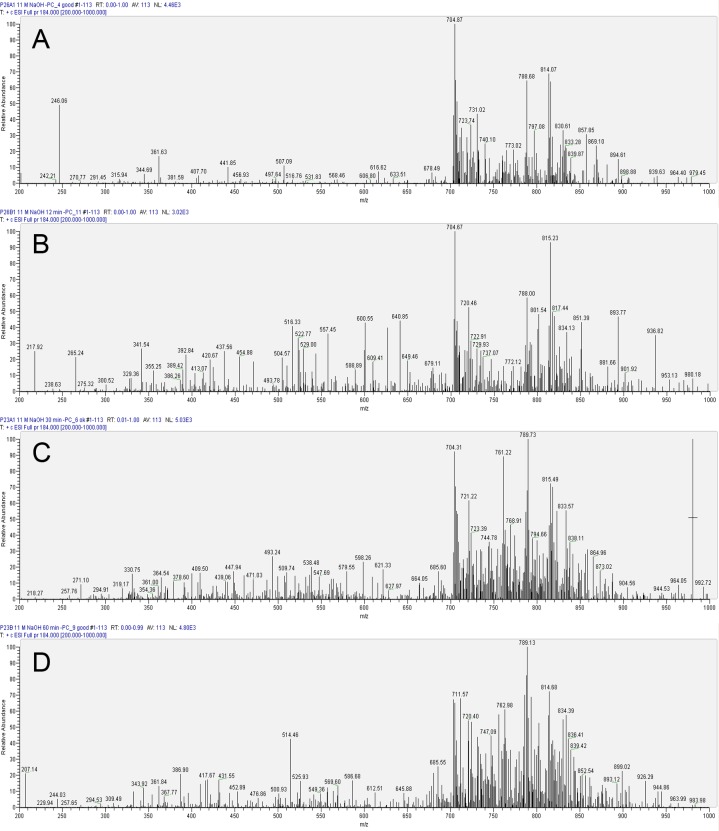
We performed qualitative assessments of PC spectra and subsequently subjected quantitative analyses of the data for control corneas and for corneas exposed to different durations of 11 M NaOH. For even the shortest exposure periods (30 seconds), we found not only a relative decrease in the intensities of PC species, but also dramatic changes in the profile of PC lipids (Fig. 2A). Representative qualitative profiles in the early time points showed profound changes in some selective lipid species; for example, m/z ∼ 761 (Figs. 2A, 2B), which was restored to some extent in later profiles (Figs. 2C, 2D). In subsequent quantitative analyses, we found a reduction in levels but not complete disappearance of m/z ∼ 761 in response to exposure to 11 M NaOH at 30 seconds and 12 minutes. In subsequent time points of 30 and 60 minutes m/z ∼ 761 showed up again in the spectrum. This was likely due to increased permeability and extractability of PCs that are part of organelle membranes and perhaps not derived from cell membranes (which account for most PC species observed in early time points). However, some intrinsic variations in both extractability of lipids and alkali hydrolysis cannot be ruled out. As noted previously, we used quiescent corneas that were devoid of protein and DNA synthesis but not entirely devoid of metabolism, so there remains a possibility of some internal generation of select PC species as well. Some observations (see Supplementary Material and Supplementary Fig. 1, http://www.iovs.org/content/53/11/7122/suppl/DC1) were consistent with metabolic generation of new lipid species at early time points. In alkali-exposed eyes the m/z range of 200 to 700 underwent a dramatic change in which new prominent species appeared (Figs. 2A–D) compared to controls (Fig. 1A). The PC species in this range were likely hydrolytic products that underwent a change with respect to the time length of alkali exposure (Figs. 2A–D).
Figure 2. .
Representative LC-MS/MS analysis of porcine corneal phosphatidylcholines after exposure to 11 M NaOH at various time points. (A) Profile after 30 seconds. (B) Profile after 12 minutes. (C) Profile after 30 minutes. (D) Profile after 60 minutes of exposure.
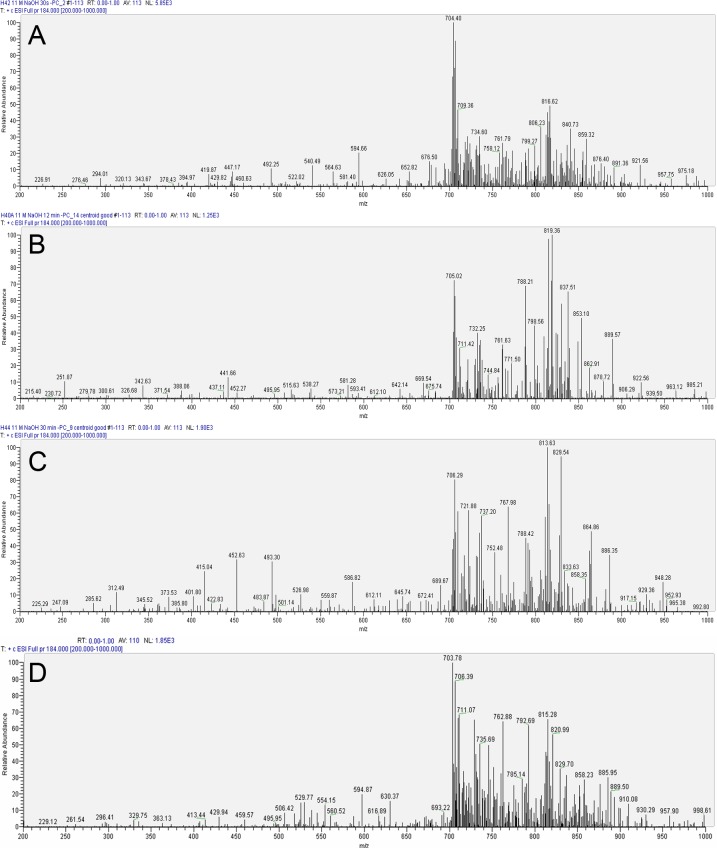
The profile of PC species for human corneas treated with 11 M NaOH (Fig. 3) showed changes similar to that of porcine corneas (Fig. 3). However with 30-second alkali exposure (earliest time point of assessment after alkali exposure) the majority of new PCs were observed around the m/z ratio of 400 to 600 (Fig. 3A) in the human corneas rather than at the m/z 200 to 500 range, which was found in porcine corneas (Fig. 2A). Consistent with our observations in porcine corneas, the peaks that were most prominent at the earliest time point of exposure to alkali (30 seconds), such as m/z ∼ 788, underwent a dramatic decrease (Fig. 3A) only to reappear at later time points (Figs. 3B, 3C). As described before, this could be an intrinsic variation or due to increased extractability from inner cellular membranous structures harboring PC species or simply metabolic production of new PC species. The observations of initial prominent peaks consistent with control corneas (Fig. 1C) in longer alkali-exposed eyes (Figs. 3B, 3C) were most likely due to increased extractability from inner membranous structures. Extractability of PCs, presumably largely membrane associated, is an important issue, which is greatly aided by physicochemical components.
Figure 3. .
Representative LC-MS/MS analysis of human corneal phosphatidylcholines after exposure to 11 M NaOH at various time points. (A) Profile after 30 seconds. (B) Profile after 12 minutes. (C) Profile after 30 minutes. (D) Profile after 60 minutes of exposure.
It is important to note that our initial preliminary experiment, which had compromised lipid extractability, showed a profile in which control corneas had the least lipids. Considerably greater lipid amounts were found with increased time of alkali exposure, presumably due to increase permeability and extractability (see Supplementary Material and Supplementary Fig. 5, http://www.iovs.org/content/53/11/7122/suppl/DC1). If hydrolysis alone was the most prominent factor in this analysis, then we would expect a series of right-angled triangle shapes within the profile consistent with the appearance of hydrolytic products and their disappearance. However, the actual profile in this preliminary analysis was far from a series of right-angled triangles (see Supplementary Material and Supplementary Fig. 5 and Supplementary Table S3, http://www.iovs.org/content/53/11/7122/suppl/DC1). Overall, our qualitative spectra showed a change in PC profile due to alkali exposure in both porcine and human corneas. They had an overall similarity in the pattern of change, though there were differences between both species (Figs. 2, 3).
Similar to that shown for the control samples (Fig. 1), in all actual measurements, data were obtained without and with the addition of the external standard, and the latter was used for quantitative or further analyses. With increasing duration of alkali exposure, the PC species continued to evolve dynamically (Figs. 3, 4). Again, the relative intensities of some species increased from one time point to another, while others decreased; novel species were also captured.
Figure 4. .
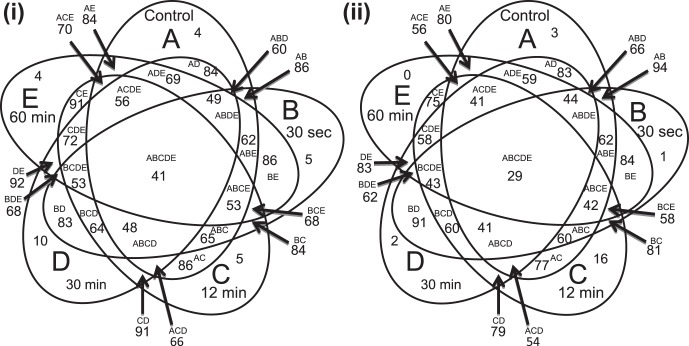
Venn diagram comparing control and experimental cornea. Ellipse A represents phosphatidylcholines (PCs) of the control cornea. Ellipses B to E represent the PCs of the 30-second, 12-minute, 30-minute, and 60-minute NaOH-exposed cornea, respectively. The outer, non-overlapping parts of the ellipses represent the number of PC species unique to each group. Overlapping sections of the diagram represent the number of PC species common to more than one group as indicated by the letters. The sizes of the sections are not proportional to the number of species represented. (i) Porcine cornea; (ii) Human cornea.
Following data analysis and ratiometric quantification we were able to ascribe a number of PC species to known PC entities in porcine (see Supplementary Material and Supplementary Table S1, http://www.iovs.org/content/53/11/7122/suppl/DC1) and human (see Supplementary Material and Supplementary Table S2, http://www.iovs.org/content/53/11/7122/suppl/DC1) control and experimental samples. However, a number of PC species (and presumably the majority of hydrolyses products and intermediates) were not ascribable to any recorded PC species in the available database used in these analyses. These PC species are presented as observed m/z ratios (see Supplementary Material and Supplementary Tables S1, S2, http://www.iovs.org/content/53/11/7122/suppl/DC1). All identified PC species were reported with average lipid amounts normalized to protein amounts (picomoles of lipid per microgram of protein) for three independent analyses of three replicate samples (see Supplementary Material and Supplementary Tables S1, S2, http://www.iovs.org/content/53/11/7122/suppl/DC1).
The porcine control (three replicates combined) scans yielded 244 PC species (see Supplementary Material and Supplementary Table S1, http://www.iovs.org/content/53/11/7122/suppl/DC1; the external standard was excluded from counting). Of these 244 species, only 118 were identified as known PCs in the databases. Porcine cornea exposed to alkali for 30 seconds yielded 215 species (116 were identifiable), 12-minute exposure yielded 233 species (115 were identifiable), 30-minute exposure yielded 244 species (122 species were identifiable), and 60-minute exposure yielded 255 species (116 were identifiable; see Supplementary Material and Supplementary Table S1, http://www.iovs.org/content/53/11/7122/suppl/DC1).
Human control cornea yielded 221 species of which only 119 were identifiable (see Supplementary Material and Supplementary Table S2, http://www.iovs.org/content/53/11/7122/suppl/DC1). Human cornea exposed to 11 M NaOH for 30 seconds, 12 minutes, 30 minutes, and 60 minutes yielded 244 species (125 were identifiable), 199 species (122 were identified), 240 samples (120 were identified), and 206 species, respectively (110 were identified; see Supplementary Material and Supplementary Table S2, http://www.iovs.org/content/53/11/7122/suppl/DC1).
All identified PC species for control and each exposure time point were analyzed to determine which species were unique and which were common to more than one exposure condition. These results have been presented as elliptical Venn diagrams for porcine (Fig. 4i) and human (Fig. 4ii) corneal samples. The number of common PC species in all groups (control and exposed at different time points) was 41 and 26 in human and porcine corneas, respectively (Fig. 4). The PCs common to porcine control and experimental samples (AB, AC, AD, AE) ranged from 84 to 86 species. As would be expected, this is more than double the number of unique species between these samples (Fig. 4; see Supplementary Material and Supplementary Fig. 6, http://www.iovs.org/content/53/11/7122/suppl/DC1). Only four PC species were found to be exclusive to the control group. The following numbers of PC species were found to be entirely unique to each treated group in porcine corneas: five unique species in the 30-second samples, five in the 12-minute samples, 10 in the 30-minute samples, and four in the 60-minute alkali-exposed samples (Fig. 4). It is important to note that we found less than 1% intercorneal variability in porcine or human control corneas (see Supplementary Material and Supplementary Fig. 7, http://www.iovs.org/content/53/11/7122/suppl/DC1) in total PCs, that is, encompassing all m/z ratios for PCs (see Supplementary Material and Supplementary Tables S1, S2, http://www.iovs.org/content/53/11/7122/suppl/DC1).
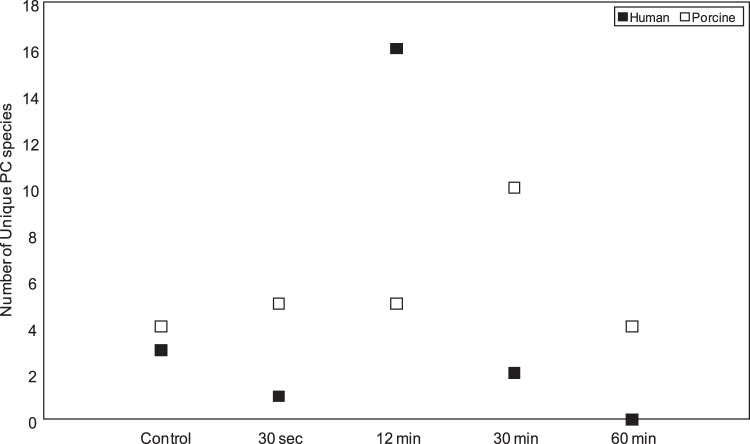
The PCs common to human control and alkali-exposed samples (AB, AC, AD, AE) ranged from 77 to 94 species. Again, the number of common species was more than the number of any unique species among these samples (Fig. 4; see Supplementary Material and Supplementary Fig. 6, http://www.iovs.org/content/53/11/7122/suppl/DC1). The number of common species between human control and alkali-exposed samples showed a greater range (77–94 vs. 84–86) than that of porcine cornea (Figs. 4i, 4ii). Control and alkali-exposed corneas also showed a variation in the number of entirely unique species in each group compared to the rest of the groups combined (Figs. 4, 5). The number of unique species peaked at 30 minutes for porcine and 12 minutes for human samples, respectively, and then declined (Fig. 5). Peaking of unique species and their decline (Fig. 5) was explicable since the unique species was expected to emerge at each time point due to extractability (a function of alkali penetration and other complex factors) and alkali hydrolysis and other chemical modifications (possible oxidation), which eventually reached a plateau and resulted in disappearance of unique species.
Figure 5. .
Unique phosphatidylcholine (PC) species distribution in human and porcine corneal control and alkali treated groups. Human and porcine PC species have been represented by filled and hollow bars, respectively, as indicated. Control corneal samples and corneal samples treated with 11 M NaOH for each time points are as indicated.
Discussion
In several industrial settings,25 11 M NaOH is utilized and is known to cause significant corneal alkali burns.4 Hence, this concentration was selected for experimentation. If the eye is accidentally splashed with NaOH in the laboratory or in industry, it is likely to take approximately 30 seconds to reach a designated wash station. On the other hand, approximately 12 minutes is often the response time for emergency medical personnel to arrive to aid someone who was splashed. In remote areas, warehouse settings, or combat operations, 30 to 60 minutes may elapse prior to arrival of response team. During this time lapse, cumulative damage9 occurs for the duration of the cornea's exposure to active alkaline material. Our study design of alkali exposure was therefore based on these considerations using quiescent corneas that had substantially reduced DNA and protein synthesis as established in our previous study.9 Using quiescent corneas enabled insight into the damage process without (or with limited) added confounding effects of wound healing and repair mechanisms. This not only allows for the development of better-tailored interventions for chemical injuries, but may also allow for the identification of repair- and regeneration-promoting lipid molecules. Identification of such molecules will enable the expansion of our repertoire of therapeutic molecules.
The anti-inflammatory and proresolution properties of lipid mediators in epithelial tissue in general are now well-established.26 This study has characterized the profile of PCs, the most common of the phospholipids of cell membrane and the most prevalent in the mammalian cornea.8 PCs serve a variety of functions including membrane structural support as well as cell signaling.27 PCs and their hydrolysis products potentially play a role in corneal wound healing.28 For example, lysophosphatidic acid and its homologues have been identified as mediators of cellular regeneration following corneal injury.29 Thus, although the hydrolyzed lipids may be regarded as products of the damage process, their role as promoters of wound healing and repair process cannot be ruled out. Indeed, we found that some lipids (likely to be phospholipids) generated within 30 seconds of 11 M NaOH exposure (rapidly lost when the alkali exposure continues for more than 5 minutes) dramatically promote wound healing (see Supplementary Material and Supplementary Fig. 1, http://www.iovs.org/content/53/11/7122/suppl/DC1). Previously PCs have been shown to play a role in the inhibition of alkali-burn induced lipoxygenation. Platelet activating factor (PAF; 1-0-alkyl-2-acetyl-sn-glycero-3-phosphocholine), a PC membrane lipid plays a role in inflammation following corneal alkali exposure.30 A PAF antagonist (BN 52021) has been shown to inhibit lipoxygenase reactions, and the accumulation of PAF has been linked to corneal damage after alkali exposure.30,31 Our data (see Supplementary Material and Supplementary Tables S1, S2, http://www.iovs.org/content/53/11/7122/suppl/DC1) are consistent with up-regulation of PAF in corneal alkali exposures. Furthermore, as the time period of alkali exposure increased, there was an observable up-regulation of PAF in the treated porcine eyes (see Supplementary Material and Supplementary Tables S1, S2, http://www.iovs.org/content/53/11/7122/suppl/DC1), comparable to the trend found in rabbit eyes in prior studies.31 The average amount of PAF present in control porcine corneal tissue (7.56 pmol/μg of protein) increased in treated groups; that is, from 30 seconds to 30 minutes (from 10.56–69.57 pmol/μg; see Supplementary Material and Supplementary Table S1, http://www.iovs.org/content/53/11/7122/suppl/DC1). However, at 60 minutes of alkali exposure, the quantity of PAF present decreased. We conjecture that this was due to the hydrolysis of PAF. In the alkali-exposed human corneal tissue, the PAF species PC(O-18:2(9Z,12Z)/2:0) followed the same trend, ultimately decreasing at 60 minutes (see Supplementary Material and Supplementary Table S2, http://www.iovs.org/content/53/11/7122/suppl/DC1). The detailed knowledge of changes in PC species and subsequent elucidation of their role in biological processes will help guide their use in clinical settings. For example, wound-healing PCs could be used in severely damaged cornea for rapid promotion of repair processes.
The penetrability of the alkali over a time period depends on the strength of the alkali, the amount of the material per unit area, and the intrinsic properties of the tissue, which includes the depth of the easily penetrable structures. The outermost epithelial layer, which serves as a protection barrier for the rest of the eye,32 is approximately 50 μm in thickness.33 Exposure to alkali usually penetrates different cell depths of the epithelial layer, but it also can penetrate other layers. The short exposure time points for alkali showed a dramatic decrease in selective lipid species such as m/z ∼ 761 (Figs. 2A, 2B) in porcine and m/z ∼ 788 (Fig. 3A) in human corneas. These species reappeared at later exposure time points (Figs. 2C, 2D, 3B, 3C). Quantitative analyses corroborated a dramatic reduction in levels but not complete disappearance of m/z ∼ 761 and m/z 788 in early exposure time points as observed in qualitative representative spectrum (Figs. 2A, 2B, 3A, 3B) and their increased amount in 30 and 60 minutes (Figs. 2C, 2D, 3B, 3C; see Supplementary Material and Supplementary Tables S1, S2, http://www.iovs.org/content/53/11/7122/suppl/DC1). The reduction in PC species was likely due to hydrolysis in early time points. Their reappearance in later time points was likely due to increased permeability and extractability of PCs that are part of organelle membranes. At early time points, the PCs were likely derived entirely from cell membranes rather than organelle membranes. It is also possible that different layer penetration by alkali over an extended time period caused the appearance of these PC species (m/z ∼ 761 in porcine and m/z ∼ 788 in humans) upon extended period of alkali exposure (30 and 60 minutes) compared with early time points (30 seconds and 12 minutes).
Some intrinsic variations in both extractability of lipids and alkali hydrolysis cannot be ruled out, but they were not uniformly repeated in all triplicates and across species (porcine and human as well as bovine; not presented). As noted earlier, the internal generation of select PC species remained a possibility despite our use of quiescent corneas. While these corneas were largely devoid of protein and DNA synthesis, they were not completely inactive with respect to metabolism. Upon short exposure to alkali these corneas were able to produce lipids that promote repair or regeneration (see Supplementary Material and Supplementary Fig. 1, http://www.iovs.org/content/53/11/7122/suppl/DC1). These observations were consistent with the ability to generate new lipid species at early time points in response to damage process. Generation of lipids rather than larger proteins in response to damage as a protection mechanism would allow significant conservation of energy for the cornea, which has limited metabolic activity.
The number of common PC species in all groups (control and exposed at different time points) was 41 and 26 in human and porcine corneas, respectively (Fig. 4). The observed variation in PCs in alkali-exposed corneas, subjected to different lengths of alkali exposure, was due to an interplay of penetration and hydrolysis (Fig. 4). The number of common species between human control and alkali-exposed samples showed a greater range (77–94 vs. 84–86) than that of porcine cornea (Figs. 4i, 4ii). This was probably reflective of intrinsic variation between the species, taken together with differences imparted by storage conditions. It is expected that given sufficient time, all unique species would disappear from alkali-exposed corneas compared to control and shorter exposure times. Indeed, this was the trend observed for human cornea and, within close approximation, for porcine corneas as well (Fig. 5).
The ability to use a large number of mammalian eyes with less intrinsic variations will not only enable better understanding of questions pertaining to storage, extraction, and buffer conditions, but will also aid in better identification of less abundant and labile potential therapeutic molecules. Bovine eyes are much larger, but porcine eyes are similar in size to that of humans. However, both these mammalian eyes (porcine and bovine) bear similarity in constituent proteins9 with humans eyes. These eyes (porcine and bovine) can be procured in large numbers from local abattoir and laboratories, from livestock that are similar in genetic make-up and raised under similar conditions, and importantly with some control to the procurement time after death. With human eyes these conditions are rather limited or not possible. Therefore, the use of porcine (or other similar mammalian eyes) greatly minimizes the need for the use of human eyes and at the same time enables the extrapolation of conclusions derived from their utilization. Bovine and porcine eyes are readily available from local abattoirs and their PC profile is similar to that of the human (see Supplementary Material and Supplementary Fig. 8, http://www.iovs.org/content/53/11/7122/suppl/DC1) and amenable to be used for optimization and other controlled studies.
Although the PCs are the most abundant phospholipids in the cornea,8 future work may enable characterization of other classes of phospholipids and their hydrolytic products using recently established specific mass spectrometric and bioinformatics methods.16,17 We used a triple quadrupole instrument that has a resolution of 1 atomic mass unit (at 0.7 FWHM), which was a first pass attempt to capture all PC species. A high-resolution mass spectrometer will help further determine with greater conformity the m/z ratios determined with TSQ Quantum Access Max here. In the future, the unique PC species identified here will need to be carefully characterized using different collision energies and high resolution mass spectrometry to elucidate their structure. This will expand the current PC database and also enable their syntheses and screening to determine the biological roles that they might play in the cornea and in other organs.
Supplementary Material
Acknowledgments
We thank Tom Straub, Florida Lions Eye Bank, and Leonard Real for their assistance with tissue procurement. We thank Bryan Cam and Katyayini Aribindi for their assistance. We thank Rong Wen and Dipankar Ghosh for their comments on the manuscript.
Footnotes
Supported by DOD Grant W81XWH-09-1-0674 (Project 2.2), a career award, and unrestricted funds from Research to Prevent Blindness and NIH core Grant P30-EY14801.
Disclosure: A.M. Crane, None; H.-U. Hua, None; A.D. Coggin, None; B.G. Gugiu, None; B.L. Lam, None; S.K. Bhattacharya, None
References
- 1.Kales SN, Christiani DC. Acute chemical emergencies. N Engl J Med. 2004;350:800–808 [DOI] [PubMed] [Google Scholar]
- 2.Belke JC. Chemical Accident Risks in U.S. Industry: A Preliminary Analysis of Accident Risk Data from U.S. Hazardous Chemical Facilities. Washington, DC: United States Environmental Protection Agency, Chemical Emergency Preparedness and Prevention Office, 2000 [Google Scholar]
- 3.Morgan SJ. Chemical burns of the eye: causes and management. Br J Ophthalmol. 1987;71:854–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Velpandian T, Sobti A, Ravi AK, et al. Rampur chemical spill tragedy: accountability in transporting chemicals. Natl Med J India. 2010;23:375–376 [PubMed] [Google Scholar]
- 5.Pfister RR. Chemical injuries of the eye. Ophthalmology. 1983;90:1246–1253 [PubMed] [Google Scholar]
- 6.Feldman GL. Human ocular lipids: their analysis and distribution. Surv Ophthalmol. 1967;12:207–243 [PubMed] [Google Scholar]
- 7.Dowhan W. Molecular basis for membrane phospholipid diversity: why are there so many lipids? Annu Rev Biochem. 1997;66:199–232 [DOI] [PubMed] [Google Scholar]
- 8.Merchant TE, Lass JH, Roat MI, Skelnik DL, Glonek T. P-31 NMR analysis of phospholipids from cultured human corneal epithelial, fibroblast and endothelial cells. Curr Eye Res. 1990;9:1167–1176 [DOI] [PubMed] [Google Scholar]
- 9.Parikh T, Eisner N, Venugopalan P, et al. Proteomic analyses of corneal tissue subjected to alkali exposure. Invest Ophthalmol Vis Sci. 2011;52:1819–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spoler F, Forst M, Kurz H, Frentz M, Schrage NF. Dynamic analysis of chemical eye burns using high-resolution optical coherence tomography. J Biomed Opt. 2007;12:041203. [DOI] [PubMed] [Google Scholar]
- 11.Aktas M, Wessel M, Hacker S, Klüsener S, Gleichenhagen J, Narberhaus F. Phosphatidylcholine biosynthesis and its significance in bacteria interacting with eukaryotic cells. Eur J Cell Biol. 2010;89:888–894 [DOI] [PubMed] [Google Scholar]
- 12.Brodovsky SC, McCarty CA, Snibson G, et al. Management of alkali burns: an 11-year retrospective review. Ophthalmology. 2000;107:1829–1835 [DOI] [PubMed] [Google Scholar]
- 13.Watsky MA, Griffith M, Wang DA, Tigyi GJ. Phospholipid growth factors and corneal wound healing. Ann N Y Acad Sci. 2000;905:142–158 [DOI] [PubMed] [Google Scholar]
- 14.Buzzelli G, Moscarella S, Giusti A, Duchini A, Marena C, Lampertico M. A pilot study on the liver protective effect of silybin-phosphatidylcholine complex (IdB1016) in chronic active hepatitis. Int J Clin Pharmacol Ther Toxicol. 1993;31:456–460 [PubMed] [Google Scholar]
- 15.Stremmel W, Hanemann A, Braun A. Delayed release phosphatidylcholine as new therapeutic drug for ulcerative colitis—a review of three clinical trials. Expert Opin Investig Drugs. 2010;19:1623–1630 [DOI] [PubMed] [Google Scholar]
- 16.Yang K, Cheng H, Gross RW, Han X. Automated lipid identification and quantification by multidimensional mass spectrometry-based shotgun lipidomics. Anal Chem. 2009;81:4356–4368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herzog R, Schwudke D, Schuhmann K, et al. A novel informatics concept for high-throughput shotgun lipidomics based on the molecular fragmentation query language. Genome Biol. 2011;12:R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng Y, Yang J, Huang K, Lee Z, Lee X. A comparison of biomechanical properties between human and porcine cornea. J Biomech. 2001;34:533–537 [DOI] [PubMed] [Google Scholar]
- 19.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917 [DOI] [PubMed] [Google Scholar]
- 20.Taki T, Kasama T, Handa S, Ishikawa D. A simple and quantitative purification of glycosphingolipids and phospholipids by thin-layer chromatography blotting. Anal Biochem. 1994;223:232–238 [DOI] [PubMed] [Google Scholar]
- 21.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254 [DOI] [PubMed] [Google Scholar]
- 22.Zor T, Selinger Z. Linearization of the Bradford protein assay increases its sensitivity: theoretical and experimental studies. Anal Biochem. 1996;236:302–308 [DOI] [PubMed] [Google Scholar]
- 23.Pluskal T, Castillo S, Villar-Briones A, Oresic M. MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics. 2010;11:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grünbaum B. Venn diagrams and independent families of sets. Mathematics Magazine. 1975;48:12–22 [Google Scholar]
- 25.Hom GG. Chemical, biological, and radiological weapons: implications for optometry and public health. Optometry. 2003;74:81–98 [PubMed] [Google Scholar]
- 26.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Billah MM, Anthes JC. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990;269:281–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenchegowda S, Bazan HE. Significance of lipid mediators in corneal injury and repair. J Lipid Res. 2010;51:879–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liliom K, Guan Z, Tseng JL, Desiderio DM, Tigyi G, Watsky MA. Growth factor-like phospholipids generated after corneal injury. Am J Physiol. 1998;274:C1065–C1074 [DOI] [PubMed] [Google Scholar]
- 30.Bazan HE, Braquet P, Reddy ST, Bazan NG. Inhibition of the alkali burn-induced lipoxygenation of arachidonic acid in the rabbit cornea in vivo by a platelet activating factor antagonist. J Ocul Pharmacol. 1987;3:357–365 [DOI] [PubMed] [Google Scholar]
- 31.Bazan HE, Reddy ST, Lin N. Platelet-activating factor (PAF) accumulation correlates with injury in the cornea. Exp Eye Res. 1991;52:481–491 [DOI] [PubMed] [Google Scholar]
- 32.Turss R, Friend J, Dohlman CH. Effect of a corneal fluid barrier on the nutrition of the epithelium. Exp Eye Res. 1970;9:254–259 [DOI] [PubMed] [Google Scholar]
- 33.Reinstein DZ, Archer TJ, Gobbe M, Silverman RH, Coleman DJ. Epithelial thickness in the normal cornea: three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg. 2008;24:571–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.