Abstract
Previous studies from our laboratory showed that coronary arterioles from type 2 diabetic mice undergo inward hypertrophic remodeling and reduced stiffness. The aim of the current study was to determine if coronary resistance microvessels (CRMs) in Ossabaw swine with metabolic syndrome (MetS) undergo remodeling distinct from coronary conduit arteries. Male Ossabaw swine were fed normal (n = 7, Lean) or hypercaloric high-fat (n = 7, MetS) diets for 6 mo, and then CRMs were isolated and mounted on a pressure myograph. CRMs isolated from MetS swine exhibited decreased luminal diameters (126 ± 5 and 105 ± 9 μm in Lean and MetS, respectively, P < 0.05) with thicker walls (18 ± 3 and 31 ± 3 μm in Lean and MetS, respectively, P < 0.05), which doubled the wall-to-lumen ratio (14 ± 2 and 30 ± 2 in Lean and MetS, respectively, P < 0.01). Incremental modulus of elasticity (IME) and beta stiffness index (BSI) were reduced in CRMs isolated from MetS pigs (IME: 3.6 × 106 ± 0.7 × 106 and 1.1 × 106 ± 0.2 × 106 dyn/cm2 in Lean and MetS, respectively, P < 0.001; BSI: 10.3 ± 0.4 and 7.3 ± 1.8 in Lean and MetS, respectively, P < 0.001). BSI in the left anterior descending coronary artery was augmented in pigs with MetS. Structural changes were associated with capillary rarefaction, decreased hyperemic-to-basal coronary flow velocity ratio, and augmented myogenic tone. MetS CRMs showed a reduced collagen-to-elastin ratio, while immunostaining for the receptor for advanced glycation end products was selectively increased in the left anterior descending coronary artery. These data suggest that MetS causes hypertrophic inward remodeling of CRMs and capillary rarefaction, which contribute to decreased coronary flow and myocardial ischemia. Moreover, our data demonstrate novel differential remodeling between coronary micro- and macrovessels in a clinically relevant model of MetS.
Keywords: type 2 diabetes, vascular mechanics, coronary flow, extracellular matrix, receptor for advanced glycation end products
metabolic syndrome (MetS), also called syndrome X, is a cluster of risk factors that include abdominal obesity, dyslipidemia, hypercholesterolemia, hypertension, and hyperglycemia (19, 38). The prevalence of MetS is increasing in adolescent (13, 44) and adult (18) populations, regardless of sex, and it currently afflicts one-third of the US adult population and one-fourth of the world population (18). Individually, all these components are associated with increased risk of type 2 diabetes [diabetes mellitus (DM)] and cardiovascular disease, and, collectively, MetS patients are at a higher risk of cardiovascular disease, including myocardial infarction (38). The consequences of these cardiovascular sequelae clustered as MetS are poorly understood, although recent studies suggest cardiac and vascular dysfunction (47), including coronary endothelial dysfunction (10, 36). Moreover, MetS patients are at a two- to fourfold increased risk of myocardial infarction, implicating coronary artery disease as a consequence of MetS pathophysiology (38).
The coronary vascular complications of each component of MetS, including hypertension, diabetes/insulin resistance, and obesity, are associated with endothelial and smooth muscle dysfunction, leading to marked alterations in coronary vascular reactivity (7) and coronary flow (24). These observations have been elucidated in humans (32, 37) and in several animal models, including diabetic db/db mice (3, 35), Ossabaw swine with MetS (6, 9, 10, 36), and spontaneously hypertensive rats (1). Concomitantly with endothelial dysfunction, vascular remodeling remains a synergistic complication in DM, as evidenced by recent studies from our laboratory demonstrating inward hypertrophic remodeling of coronary resistance arterioles or outward hypertrophic remodeling of mesenteric resistance arterioles isolated from diabetic db/db mice (22, 43). These findings were associated with impaired coronary flow reserve or augmented mesenteric flow, respectively, providing convincing evidence that resistance microvascular structure can impart changes in regional blood flow. Furthermore, in a departure from reports of increased aortic stiffness in DM, our study further showed that diabetic coronary resistance microvessels (CRMs) were less stiff, a finding associated with a collagen-elastin imbalance (22). Although adverse vascular remodeling has also been reported in hypertension (2, 39), little is known about the impact of MetS on vascular remodeling. Therefore, the aim of the current study was to determine whether 1) CRMs undergo remodeling in MetS and 2) there was any discordant remodeling between CRM and coronary conduit arteries, such as the left anterior descending coronary artery (LAD). To accomplish this aim, we tested the hypothesis that the coronary microcirculation undergoes inward hypertrophic remodeling associated with reduced stiffness, differing from that of upstream conduit arteries in MetS.
MATERIALS AND METHODS
Animals.
Male Ossabaw swine were bred and maintained by the Comparative Medicine Program, jointly maintained by Indiana University and Purdue University. All procedures were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine. Lean Ossabaw swine (n = 7) were fed a normal diet consisting of standard chow with 22% of kilocalories from protein, 70% from carbohydrates, and 8% from fat. Obese Ossabaw swine (n = 7) were fed an excess-calorie high-fat diet (∼4,500 kcal/day, Purina TestDiet, Richmond, IN) consisting of 13% of kilocalories from protein, 40% from carbohydrates, and 47% from fat, with 2% cholesterol provided for 6 mo. Previous studies indicate that this diet induces MetS that closely matches the human phenotype because of the presence of a thrifty genotype in these pigs (30, 33). All experiments, except coronary flow velocity and coronary pressure-flow autoregulation experiments, were performed in this cohort of animals.
Blood pressure, heart rate, fasting plasma glucose, intravenous glucose tolerance test, and cholesterol.
Swine were acclimatized to restraint in a specialized sling for 5–7 days before the intravenous glucose tolerance test (IVGTT) was conducted. The IVGTT was conducted within 1 wk before the animals were euthanized. Swine were fasted overnight and anesthetized with isoflurane (maintained at 4–5% by mask with supplemental O2). The right jugular vein was catheterized percutaneously (12, 17, 45). After catheterization, the swine were allowed to recover for 3 h before the IVGTT to avoid any effect of isoflurane on insulin signaling (34). After restraint of conscious swine in the sling, tail cuff blood pressure and heart rate measurements and baseline (fasting) blood samples were obtained as previously described (34, 50). Pigs were acclimated to the sling and to tail cuff blood pressure measurements for 7–10 days before starting the study. All blood pressure measurements were taken at the same time (9–11 AM). Glucose (1 g/kg body wt iv) was administered, and timed blood samples were collected (34). Blood glucose was measured using a glucose analyzer (model YSI 2300 STAT Plus, YSI Life Sciences, Yellow Springs, OH). Plasma insulin assays were performed by Linco Research Laboratories (St. Charles, MO). Venous blood samples were obtained in the week before the animals were euthanized following overnight fasting and were analyzed for triglyceride and total cholesterol, as previously described by us (15–17).
Preparation of CRMs.
Ossabaw swine were anesthetized using 2–3% isoflurane vaporized with 100% O2. The heart was excised and dissected in 4°C physiological salt solution (PSS; in mM: 130 NaCl, 4 KCl, 1.2 MgSO4, 4 NaHCO3, 10 HEPES, 1.2 KH2PO4, 5 glucose, and 2.5 CaCl2, pH 7.4). CRMs (<150 μm ID, n = 7 per group) were isolated from the left ventricular side of the distal LAD, excised, and mounted onto two glass microcannulas within a pressure myograph chamber (Living Systems, Burlington, VT). Prior to any measurements, vessels were equilibrated for 30 min under constant intraluminal pressure (50 mmHg) at 37°C in PSS. Internal diameter and left and right wall thickness were measured using a video dimension analyzer (Living Systems) and continuously recorded using LabChart 6 data acquisition software connected to a data acquisition module (PowerLab 16/30, ADInstruments, Colorado Springs, CO).
Measurements of coronary resistance microvascular passive structures and mechanical properties.
All experiments were performed in Ca2+-free PSS in the presence of 2 mM EGTA and 100 μM sodium nitroprusside. To generate a passive pressure-diameter curve, intraluminal pressure was increased from a minimum of 0 mmHg to a maximum of 125 mmHg, and left and right ventricular wall thickness (WT) and internal diameter (Di) were recorded at 0, 10, 25, 50, 75, 100, and 125 mmHg. The following structural and mechanical parameters were calculated as previously described by us (22)
where (Di)L and (Di)M are luminal diameters of Lean and MetS vessels, respectively, and (Di)remodel represents the remodeled lumen [(Di)remodel]
where (De)M is external diameter of MetS vessels and CSAL is CSA of control Lean vessels.
where CSAM is CSA of MetS vessels.
where P is pressure (in dyn/cm2).
where Di is the internal diameter for a given intraluminal pressure and D0 is the original diameter measured at 0 mmHg intraluminal pressure.
Elastic modulus [stress (σ)/strain (ε)] is used to determine arterial stiffness. However, since the stress-strain relationship was nonlinear, we obtained the tangential or incremental elastic modulus, or simply the tangential slope of the stress-strain relationship at each incremental pressure (Δσ/Δε).
where 125 and 75 represent ex vivo systolic and diastolic blood pressure (SBP and DPB, in mmHg), respectively, and D125 and D75 are luminal diameters measured during ex vivo SBP and DBP.
where D125 and D75 are luminal diameters measured during ex vivo SBP and DBP (in mmHg), respectively.
Remodeling index and growth index were calculated from group data; therefore, statistical analysis could not be performed.
Morphological assessment of coronary conduit remodeling.
Distal LADs from Ossabaw swine with (n = 6) and without (n = 4) MetS were carefully isolated and fixed in 10% formalin, embedded in paraffin, sectioned at 5-μm thickness, and stained with hematoxylin and eosin. Images (×4 magnification) were captured using DP2-BSW software connected to a microscope (model IX51, Olympus America, Center Valley, PA). To minimize bias in morphological measurements of imperfectly rounded LADs, images were analyzed for luminal and external CSAs by a blinded individual, and morphological parameters, such as diameter and wall thickness, were calculated using the following equations
Coronary conduit mechanical measurements using intravascular ultrasound.
The intravascular ultrasound (IVUS) procedure was performed as previously described by our group (33). Briefly, after an overnight fast, swine received xylazine and telazol (2.2 and 5.5 mg/kg im, respectively). Swine were intubated, and anesthesia was maintained with 2–3% isoflurane vaporized with 100% O2. The isoflurane level was adjusted to maintain anesthesia with stable hemodynamics. Heart rate, aortic blood pressure, respiratory rate, and electrocardiographic data were continuously monitored throughout the procedure. Under sterile conditions, a 7F vascular introducer sheath was inserted into the right femoral artery, and heparin (200 U/kg) was administered. A 7F guiding catheter (sizes 0.75–2.0; Amplatz L, Cordis, Bridgewater, NJ) was advanced to engage the left main coronary ostium under fluoroscopic guidance. Coronary angiograms were obtained in right and left anterior oblique 30° views for clear documentation of coronary conduit anatomy. A 3.2F, 30-MHz IVUS catheter (Boston Scientific, Natick, MA) was advanced over a guide wire and positioned in the coronary artery. Automated IVUS pullbacks were performed at 0.5 mm/s. IVUS video images were acquired with a Sonos Intravascular Imaging System (Hewlett Packard), recorded to VHS tape, and digitized off-line for analysis. IVUS pullbacks were used to assess native atheroma.
BSI is a relevant measure of vascular stiffness that can be used as an aid in the clinical diagnosis of atherosclerosis. However, we employed BSI as a direct index of macrovascular stiffness in plaque-free regions of the coronary conduit arteries. LAD or circumflex coronary artery (CFX) CSAs were measured by a blinded individual during peak diastole and peak systole in atherosclerotic plaque-free coronary regions during three consecutive cardiac cycles, and coronary diameters and BSI were calculated using the following formulas, as previously described (46)
where SBP and DBP are systolic and diastolic blood pressures, respectively, and Ds and Dd are luminal diameters during peak systole and peak diastole, respectively.
Coronary flow velocity.
Coronary flow velocities were measured in a separate set of Lean (n = 5) and MetS (n = 3) Ossabaw pigs, as previously described (33). Briefly, the ostium of the left main artery was engaged with the guiding catheter, and a Doppler flow wire (0.014 in. diameter; JoMed, Rancho Cordova, CA) was advanced down the CFX. After angiography-aided placement of the flow wire in a nonbranching section of the CFX, flow velocity signals were allowed to equilibrate for several minutes. Each average peak velocity value was calculated online as an average of instantaneous peak velocity over two consecutive cardiac cycles. Adenosine (0, 10, 25, and 70 μg average) was introduced into the coronary circulation by a bolus infusion through the guiding catheter method validated by Kern et al. (23). Coronary blood flow velocity was calculated in response to the absolute intracoronary adenosine doses indicated. The peak increase in coronary flow velocity typically occurred within 30 s of the infusion. After each adenosine bolus, coronary flow velocity was allowed to return to baseline. Pilot studies revealed that there were no alterations in coronary artery diameter during adenosine infusions (Alloosh and Sturek, unpublished observations), thereby validating the use of coronary flow velocity as a surrogate of quantitative flow in this model. All flow data were stored on videotape and personal computer for further off-line analysis. Data are reported as hyperemic-to-basal coronary flow velocity ratio, which is the adenosine-induced hyperemic flow velocity divided by baseline flow velocity.
Coronary blood flow autoregulation.
In vivo coronary blood flow responses to alterations in coronary perfusion pressures (CPPs) were measured in a separate set of swine [n = 6 (Lean) and 4 (MetS)], as previously described (8). Briefly, swine were initially sedated with Telazol (tiletamine-zolazepam, 5 mg/kg sc), xylazine (2.2 mg/kg sc), and ketamine (3.0 mg/kg sc). After endotracheal intubation and venous access, anesthesia was maintained with morphine (3.0 mg/kg sc) and α-chloralose (100 mg/kg iv). The animals were mechanically ventilated (Harvard respirator) with room air supplemented with O2. Catheters were placed into the right femoral artery and vein for systemic hemodynamic measurements and administration of supplemental anesthesia, heparin, and sodium bicarbonate, respectively. The left femoral artery was catheterized to supply blood to an extracorporeal perfusion system used to perfuse the LAD at controlled pressures. Arterial blood gases were analyzed periodically throughout the experimental protocol, and adjustments were made as needed to maintain blood gas parameters within normal physiological limits (159 ± 12 mmHg arterial Po2, 43 ± 1 mmHg arterial Pco2, pH 7.4 ± 0.02). A left lateral thoracotomy was performed to expose the heart, and the LAD was isolated and cannulated distal to its first major diagonal branch following heparin administration (500 U/kg iv). CPP was regulated by a servo-controlled roller pump, and coronary blood flow was continuously measured by an in-line flow transducer (Transonic Systems, Ithaca, NY). After a stabilization period (∼20 min postcannulation), in vivo coronary pressure-flow autoregulation was assessed by 10-mmHg increment changes in CPP from 140 to 40 mmHg. Our previous studies indicate that myocardial O2 consumption and venous Po2 in this preparation are stable over a range of perfusion pressures (8). Data were continuously recorded and analyzed using IOX data acquisition software (EMKA Technologies, Falls Church, VA). Autoregulatory gain (Gc) was calculated using the following formula as previously described (8)
where F is flow and P is pressure.
RNA isolation and quantitative PCR analysis.
CRMs (<150 μm, n = 6 pools) and LADs (n = 6) were isolated from the original cohort of Lean or MetS pigs, snap-frozen in liquid nitrogen, and stored at −80°C. Seven to 10 CRMs were isolated per animal, and CRMs from 2–3 animals were pooled for each “n” value and used to prepare total RNA using the Qiagen Micro Array kit according to the manufacturer's instructions. Tissue was allowed to thaw in QIAzol reagent and then disrupted at 4°C by repeated sonication. After chloroform extraction, aqueous phases were removed and RNA was isolated on mini spin columns. An on-column DNase digestion was performed according to the manufacturer's instructions. All samples were eluted with nuclease-free water, and RNA was quantitated using a spectrophotometer (NanoDrop, Thermo Fisher Scientific, Waltham, MA). Total RNA for each sample (400 ng per CRM pool and 600 ng per LAD) was reverse-transcribed using the RevertAid kit (Fermentas, Glen Burnie, MD) according to the manufacturer's protocol. Equivalent control reactions lacking reverse transcriptase were also performed.
Where possible, target mRNA sequences were derived from annotated or predicted Sus scrofa sequences (http://www.ncbi.nlm.nih.gov; Table 1) and verified for homology with annotated Bos taurus orthologs. Where no sequence data were available (elastin), primers with homology to human and bovine sequences were used to amplify cDNA prepared from pig heart tissue for sequence analysis. The elastin primers [5′-ATGGCGGGTCTGACGGCGG-3′ (forward) and 5′-CCCCTGTCCCTGTTGGGTAACCAGC-3′ (reverse)] amplified a 426-bp open reading frame (accession no. JN049646) with 90% nucleotide homology to bovine elastin (accession no. NM_175772.1).
Table 1.
PCR primers
| Primer |
||||
|---|---|---|---|---|
| Target | NCBI Accession No. | Forward | Reverse | Roche Universal Probe No. |
| Rpl7 | NM_001123192 | ttaaacgactggaatggttcct | ggcgcactctgtcacgtag | 72 |
| Col 1a1 | XM_003362054 | caagagcggcgatcgtggtg | gcctgtctcacccttgtca | 18 |
| Col 3a1 | XM_003133535 | agctggaaagaatggtgacag | ccttggggaccaggagcac | 18 |
| Elastin | JN049646 | cgaagccagggaaagtacc | cacccctacacctgggaac | 22 |
| AT1R | XM_003132469 | ctggccctttggcaatta | cacactggcatagaggttgaaa | 41 |
| RAGE | NM_001123218 | ggaagccggacattgttg | acacaagtccccaccttgtt | 34 |
| TGF-β R1 | NM_001038639.1 | cacagctttgcggattaaga | ggagagttcaggcaaagcag | 18 |
| TGF-β R2 | EF396957.1 | gtccttcaagcagacggatg | cgagacgtcatctcccaga | 21 |
NCBI, National Center for Biotechnology Information; Col, collagen; AT1R, angiotensin type 1 receptor; RAGE, receptor for advanced glycation end products; TGF-β R1 and R2, TGF-β receptor types 1 and 2.
Quantitative PCRs were performed in duplicate (or in singlet for control reactions lacking reverse transcriptase). PCRs were performed using primer/universal probe sets designed via the Roche Applied Science website (https://www.roche-applied-science.com/sis/rtpcr/upl/index.jsp?id=uplct_000000; Table 1). All PCRs (25 μl) were performed on an Eppendorf Mastercycler ep realplex in opaque 96-well plates (Abgene, Waltham, MA) using 200 nM forward and reverse primer, 250 nM probe, and 1× Maxima Probe/ROX qPCR master mix (Fermentas). For each target, equivalent amounts of reverse-transcribed CRM and LAD RNA (5–50 ng) were used. The amount of reverse-transcribed RNA was determined from preliminary experiments to obtain cycle threshold (Ct) values in the 24–28 range. Amplification parameters were as follows: 1 cycle at 95°C for 10 min, 95°C for 15 s, and 40 cycles at 60°C for 1 min. Ct values were determined after normalization of thresholds and correction for drift. Relative expression was determined using the 2−ΔΔCT methodology (40) with normalization to the ribosomal subunit Rpl7.
Coronary capillary density/immunohistochemistry.
Sections of cardiac tissue from the original cohort of Lean (n = 7) and MetS (n = 5) pigs harvested from the left ventricular side of the distal LAD were fixed in 10% buffered formalin, embedded in paraffin, and sectioned at 5-μm thickness. Sections were deparaffanized in a graded series from xylenes to alcohol-water, and antigen retrieval was performed in citrate buffer at pH 6.0 (catalog no. 00-5000, Invitrogen, Carlsbad, CA) at 120°C, 15 psi for 30 min. Endogenous nonspecific binding was blocked [hydrogen peroxide, avidin-biotin (catalog no. 00-4303, Invitrogen), and serum] according to the manufacturer's protocol (catalog no. PK-6101, Vectastain Elite ABC Kit, Vector Laboratories, Burlingame, CA). Cardiac sections were incubated overnight at 4°C in 1% BSA, 0.1% Triton X-100, and 0.05% Tween 20 in PBS with a CD31 [platelet/endothelial cell adhesion molecule (PECAM-1)] antibody (1:100 dilution; catalog no. sc-1506, Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibody and ABC Elite reagent incubations were performed according to the manufacturer's protocol. 3,3′-Diaminobenzidine (catalog no. D4293, Sigma, St. Louis, MO) was used as the chromogen, and hematoxylin (catalog no. 6765015, Thermo Fisher Scientific) was used as the counterstain. Three images (×40 magnification) per section were captured using DP2-BSW software connected to a microscope (model IX51, Olympus America, Center Valley, PA). Images were analyzed by a blinded individual using ImageJ software (National Institutes of Health) to count the number of capillaries per image, and data are represented as the number of capillaries per image area (in mm2).
Immunohistochemistry was performed using two antibodies directed against the angiotensin type 1 receptor (AT1R) and the receptor for advanced glycation end products (RAGE) using the avidin-biotin horseradish peroxidase technique. Sections of cardiac and LAD tissue from Lean (n = 4) and MetS (n = 4) pigs were fixed in 10% buffered formalin, embedded in paraffin, and sectioned at 5-μm thickness. Sections were deparaffanized in a graded series from xylenes to alcohol-water, and antigen retrieval was performed in citrate buffer at pH 6.0 (catalog no. 00-5000, Invitrogen) at 120°C, 15 psi for 30 min. Endogenous nonspecific binding was blocked [hydrogen peroxide, avidin-biotin (catalog no. 00-4303, Invitrogen), and serum] according to the manufacturer's protocol (catalog no. PK-6101, Vectastain Elite ABC Kit). Sections were incubated overnight at 4°C in 1% BSA, 0.1% Triton X-100, and 0.05% Tween 20 in PBS with an AT1R antibody (1:100 dilution; catalog no. AAR011, Alamone Labs, Jerusalem, Israel) or a RAGE antibody (1:50 dilution; catalog no. AB9714, Millipore, Billerica, MA). Secondary antibody and ABC Elite reagent incubations were performed according to the manufacturer's protocol. 3,3′-Diaminobenzidine staining and counterstaining were performed as described above.
Statistics.
Values are means ± SE. P < 0.05 denotes significance using GraphPad Prism 5.0 (GraphPad, LaJolla, CA). CRM structural, mechanical, and coronary flow velocity measurements were analyzed using two-way ANOVA followed by Bonferroni's post hoc test. All other measurements were analyzed using an unpaired Student's t-test.
RESULTS
Baseline Ossabaw swine characteristics.
Body weights, total cholesterol, triglycerides, blood pressure, fasting blood glucose, and glucose tolerance tests were augmented in Ossabaw pigs fed a high-fat diet compared with Lean Ossabaw pigs, confirming the presence of MetS in this cohort of animals (Table 2).
Table 2.
Metabolic parameters of Ossabaw swine
| Lean | MetS | |
|---|---|---|
| Body weight, kg | 66 ± 4 | 94 ± 2* |
| Total cholesterol, mg/dl | 56 ± 8 | 440 ± 54* |
| Triglycerides, mg/dl | 23 ± 7 | 63 ± 18* |
| SBP, mmHg | 110 ± 2 | 139 ± 4* |
| DBP, mmHg | 68 ± 2 | 91 ± 2* |
| Heart rate, beats/min | 79 ± 3 | 102 ± 2* |
| Fasting blood glucose, mg/dl | 69 ± 3 | 91 ± 10* |
| Peak glucose, mg/dl | 603 ± 29 | 711 ± 44* |
| Peak insulin, μU/ml | 71.9 ± 6.9 | 122.6 ± 28.6 |
Values are means ± SE; n = 7 per group. MetS, metabolic syndrome; SBP and DBP, systolic and diastolic blood pressure.
P < 0.05 vs. Lean.
Coronary microvascular structure and mechanics.
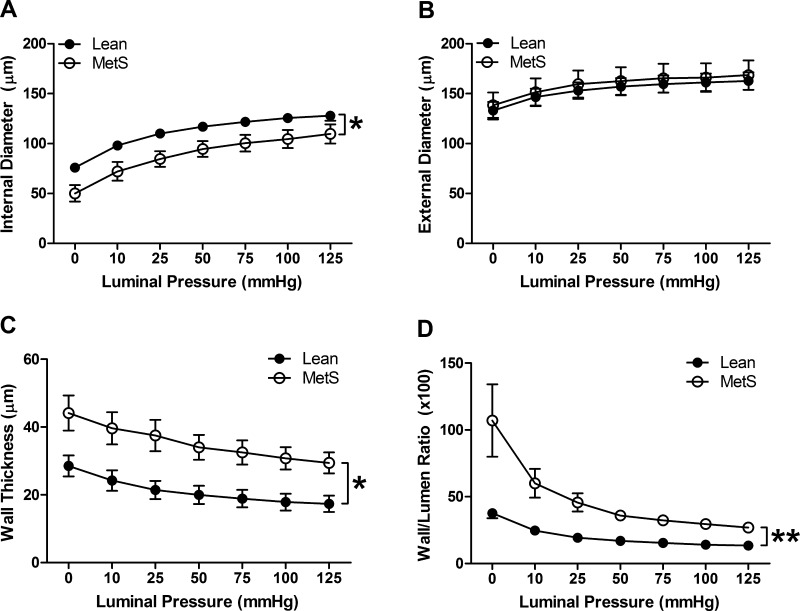
To determine the impact of MetS on CRMs, coronary microvascular structure was measured passively in Ossabaw pigs with and without MetS. CRM from MetS Ossabaw swine exhibited reduced luminal diameter over a range of pressures (Fig. 1A; 126 ± 5 and 105 ± 9 μm at 100 mmHg in Lean and MetS, respectively, P < 0.05), while external diameters were unchanged (Fig. 1B). MetS CRMs displayed a near doubling in wall thickness (Fig. 1C; 18 ± 3 and 31 ± 3 μm at 100 mmHg in Lean and MetS, respectively, P < 0.05), which significantly increased the wall-to-lumen ratio (Fig. 1D; 14 ± 2 and 30 ± 2 at 100 mmHg in Lean and MetS, respectively, P < 0.01). Growth index was augmented by 65% in the CRMs isolated from Ossabaw pigs with MetS compared with Lean pigs, while the remodeling index was reduced by 24%. Collectively, these parameters indicate that CRMs from MetS pigs undergo significant inward hypertrophic remodeling.
Fig. 1.
Coronary resistance microvessel passive structural measurements obtained by pressure myography: internal (luminal) diameter (A), external diameter (B), medial wall thickness (C), and wall-to-lumen ratio (D). MetS, metabolic syndrome. Values are means ± SE; n = 7 per group. *P < 0.05; **P < 0.01.
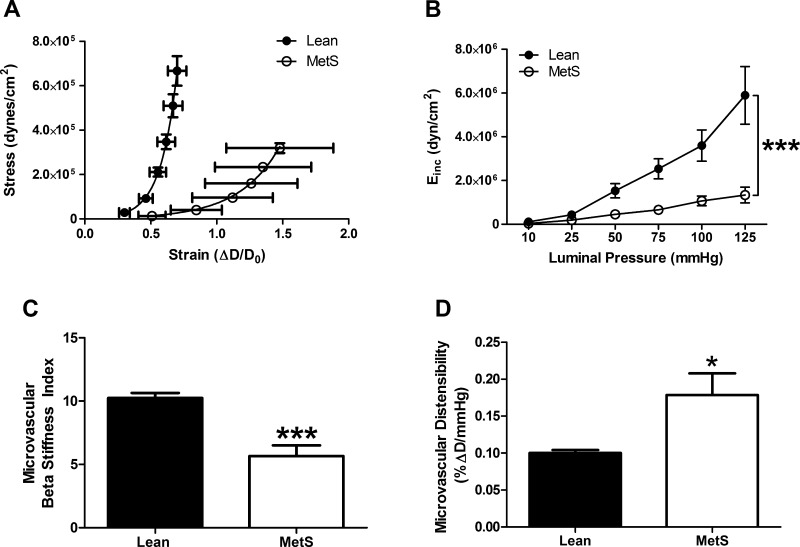
We next calculated mechanical properties of CRMs. Vascular wall stress was significantly reduced by 50% over a range of pressures in coronary microvessels isolated from MetS pigs (data not shown), a finding that contributed to a rightward shift and reduced slope of the CRM stress-strain curve (Fig. 2A). Direct calculations of incremental modulus (3.6 × 106 ± 0.7 × 106 and 1.1 × 106 ± 0.2 × 106 dyn/cm2 at 100 mmHg in Lean and MetS, respectively, P < 0.001) and BSI (10.3 ± 0.4 and 7.3 ± 1.8 at 100 mmHg in Lean and MetS, respectively, P < 0.001) confirmed the reduction in MetS CRM stiffness (Fig. 2, B and C), which was also associated with increased CRM distensibility (Fig. 2D; 0.10 ± 0.004 and 0.18 ± 0.03 at 100 mmHg in Lean and MetS, respectively, P < 0.05).
Fig. 2.
Coronary resistance microvessel passive mechanical properties. Stress-strain curve (A), representing coronary resistance microvessels (CRMs) from MetS pigs, was shifted to the right, corresponding to a reduced incremental modulus of elasticity (Einc; B). D, luminal diameter; D0, original diameter measured at 0 mmHg intraluminal pressure. CRM beta stiffness index was reduced (C), whereas CRM distensibility was increased (D). Values are means ± SE; n = 7 per group. *P < 0.05; ***P < 0.001 vs. Lean.
Coronary macrovascular structure and mechanics.
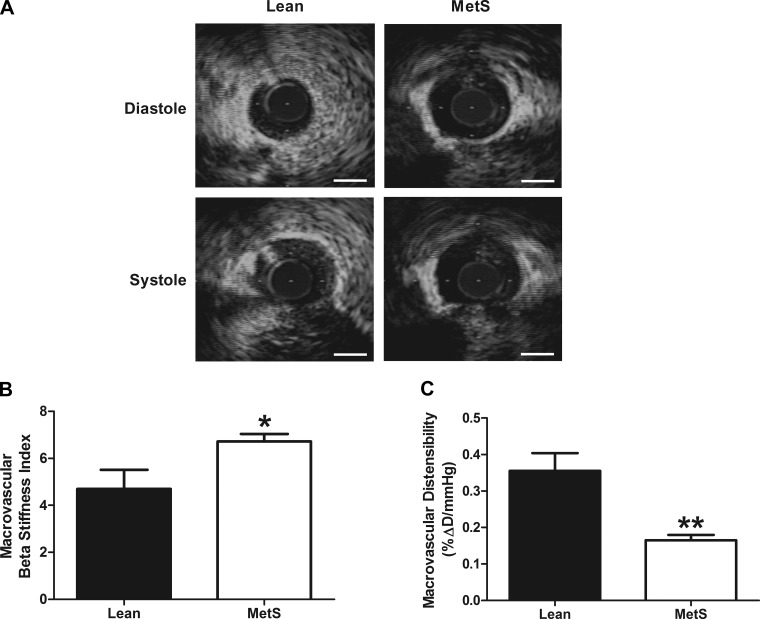
To determine whether MetS alters the structure or mechanics of larger conduit coronary arteries, we measured vascular remodeling of the LAD. Because of technical issues, our morphological measurements were limited to LAD sections that were not fixed at physiological pressures, although macrovascular mechanics were measured in vivo using IVUS. LAD structure was largely unchanged between MetS and Lean pigs, although a slight increase in wall thickness was observed in MetS animals (Table 3). Conversely, an augmented BSI (4.70 ± 0.81 and 6.71 ± 0.32 in Lean and MetS, respectively, P < 0.05) was associated with a 53% reduction in distensibility (0.36 ± 0.05 and 0.17 ± 0.01 in Lean and MetS, respectively, P < 0.01) in LADs from MetS pigs (Fig. 3, A–C), suggesting that MetS imparts a stiffening of the coronary conduit arteries.
Table 3.
Macrovascular remodeling measurements from Lean and MetS LADs
| Lean | MetS | |
|---|---|---|
| Luminal diameter, μm | 647 ± 66 | 631 ± 84 |
| External diameter, μm | 865 ± 81 | 915 ± 86 |
| Wall thickness, μm | 109 ± 9 | 142 ± 9* |
| Wall-to-lumen ratio, ×100 | 17.2 ± 1.1 | 23.7 ± 3.6 |
| Medial CSA, mm2 | 0.27 ± 0.05 | 0.35 ± 0.05 |
Values are means ± SE; n = 6 and 4 for Lean and MetS, respectively. LADs, left anterior descending coronary arteries; CSA, cross-sectional area.
P < 0.05 vs. Lean.
Fig. 3.
Macrovascular stiffness and distensibility. A: representative intravascular ultrasound images from Lean and MetS left anterior descending coronary arteries (LADs) in diastole and systole. Scale bars, 1 mm. B: LAD beta stiffness index. C: LAD distensibility. Values are means ± SE; n = 6–7 per group. *P < 0.05; **P < 0.01 vs. Lean.
Coronary capillary density, coronary flow, and coronary autoregulation.
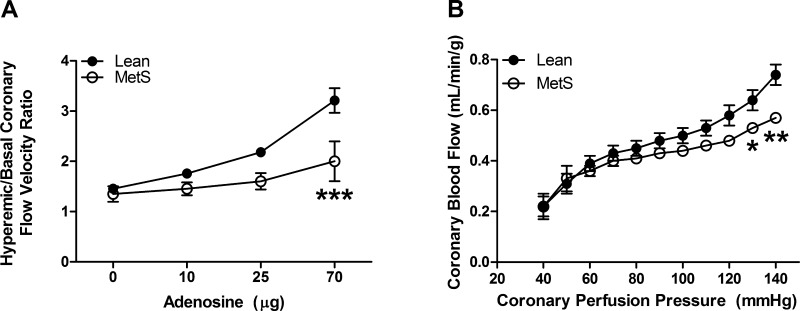
To assess whether MetS is associated with coronary capillary rarefaction, we measured coronary capillary density in hearts from Lean and MetS pigs. Coronary capillary density was significantly reduced by ∼26% in MetS pigs (1,389 ± 101 and 1,024 ± 111 capillaries/mm2 in Lean and MetS, respectively, P < 0.05), indicating capillary rarefaction (Fig. 4). Because we observed a significant increase in cardiomyocyte CSA (mCSA; 674 ± 54 and 848 ± 42 μm2 in Lean and MetS, respectively, P < 0.05), we confirmed the presence of capillary rarefaction by normalizing it to mCSA. The number of coronary capillaries per mCSA remained reduced in MetS (0.068 ± 0.016 and 0.044 ± 0.005 in Lean and MetS, respectively, P < 0.05). Moreover, we also observed a 38% decrease in the ratio of adenosine-induced hyperemic-to-basal coronary blood flow velocity (Fig. 5A; 3.2 ± 0.25 and 2.0 ± 0.4 in Lean and MetS, respectively, P < 0.001). There were no overt alterations in baseline coronary blood flow velocity (14.4 ± 0.8 and 14.0 ± 2.5 cm/s in Lean and MetS, respectively, P > 0.05). The maintenance of coronary blood flow over a range of pressures (autoregulation) was enhanced in MetS pigs by an average of 23%, consistent with augmented myogenic tone (Fig. 5B). The augmented Gc in the MetS coronary circulation from 60 to 120 mmHg failed to reach statistical significance (0.35 ± 0.14 and 0.51 ± 0.08 in Lean and MetS, respectively, P > 0.05). Importantly, the nonsignificant increase in Gc from 120 to 140 mmHg (−0.67 ± 0.42 and 0.07 ± 0.15 in Lean and MetS, respectively, P > 0.05) was associated with a significant increase in coronary vascular resistance in MetS pigs at 140 mmHg (192.2 ± 13.0 and 246.4 ± 7.9 mmHg·ml−1·min·g−1 in Lean and MetS, respectively, P < 0.001).
Fig. 4.
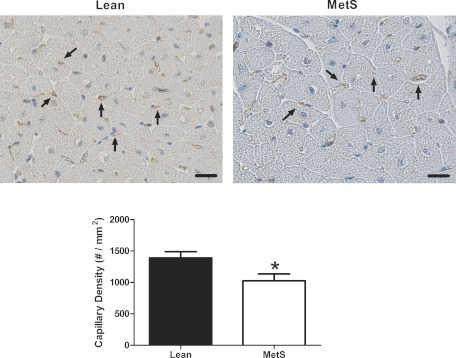
Coronary capillary density. Top: representative sections stained with platelet/endothelial cell adhesion molecule (PECAM-1), an endothelial marker. Scale bars, 20 μm. Bottom: cumulative data. Values are means ± SE; n = 5–7 per group. *P < 0.05 vs. Lean.
Fig. 5.
Hyperemic-to-basal coronary flow velocity ratio and coronary autoregulation in intact Lean and MetS pigs. A: hyperemic-to-basal coronary flow velocity ratio was significantly decreased during infusion of 70 μg of adenosine. B: autoregulatory coronary blood flow maintenance over a range of pressures was augmented in vivo in MetS vs. Lean pigs. Values are means ± SE; n = 3–6 per group. *P < 0.05; **P < 0.01; ***P < 0.001 vs. Lean.
Molecular changes associated with coronary remodeling.
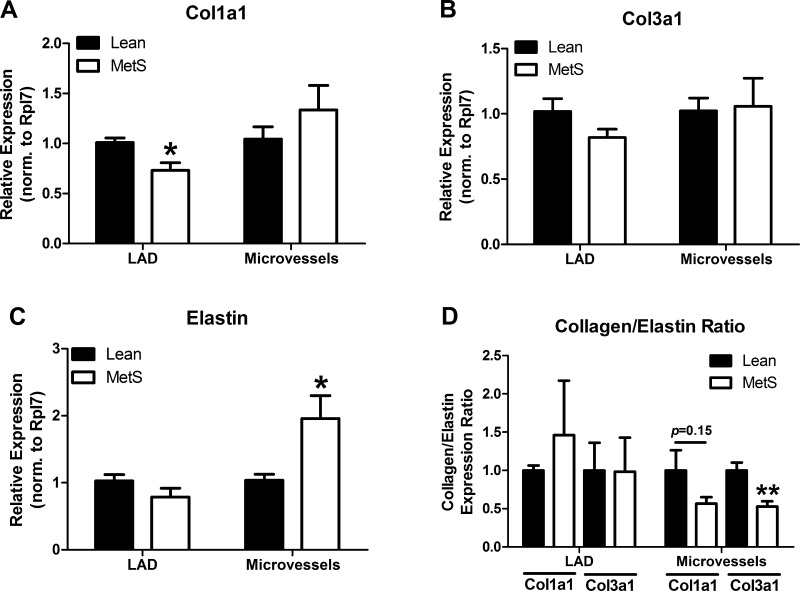
We next investigated molecular mechanisms associated with the structural remodeling of CRMs and LADs. The relative amount of steady-state expression of collagen (Col1a1 and Col3a1) and elastin mRNA was lower in CRMs than LADs (data not shown). Collagen gene expression between Lean and MetS pigs remained largely unaltered in both vessel types (Fig. 6, A and B), although a slight decrease in Col1a1 was observed in LADs from MetS pigs. Conversely, elastin mRNA expression was significantly upregulated nearly twofold only in CRMs from MetS pigs (Fig. 6C). The Col3a1-to-elastin ratio was reduced by 47% (P < 0.01) in MetS CRMs, while the 44% reduction in Col1a1 did not achieve statistical significance (P = 0.15). These ratios remained unchanged in MetS LADs (Fig. 6D).
Fig. 6.
Extracellular matrix mRNA expression in CRMs and LADs from Lean and MetS pigs. A and B: collagen (Col1a1 and Col3a1) mRNA expression. C: elastin mRNA expression increased 2-fold in CRMs but remained constant in LADs. D: Col1a1-to-elastin and Col3a1-to-elastin ratios. Values are means ± SE; n = 6 per group for pooled microvessels and n = 6 per group for LADs. *P < 0.05 and **P < 0.01 vs. Lean.
We found no significant changes in TGF-β receptor types 1 and 2 (data not shown) and AT1R mRNA (Fig. 7A) or apparent differences in protein expression detected by immunohistochemistry (Fig. 7B) between Lean and MetS CRMs or LADs. Interestingly, mRNA for RAGE was not different (Fig. 8A), while RAGE protein appeared increased in LADs from MetS pigs compared with Lean controls (Fig. 8B, top). Conversely, the more than twofold increase in RAGE mRNA in CRMs from MetS pigs (Fig. 8A) did not correlate with a change in protein expression detected by immunohistochemistry (Fig. 8B, bottom).
Fig. 7.
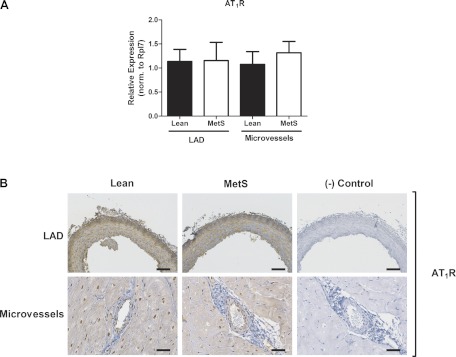
Angiotensin type 1 receptor (AT1R) expression in CRMs and LADs from Lean and MetS pigs. A: AT1R mRNA expression. B: AT1R immunostaining in LADs and CRMs. Scale bars: 100 μm (LADs) and 50 μm (CRMs). Representative images are from 4 animals per group.
Fig. 8.
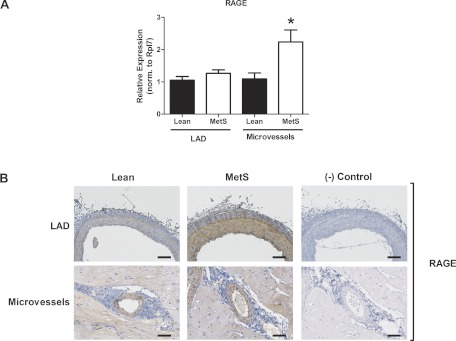
Receptor for advanced glycation end products (RAGE) expression in CRMs and LADs from Lean and MetS pigs. A: RAGE mRNA expression. Values are means ± SE. *P < 0.05 vs. Lean. B: RAGE immunostaining in LADs and CRMs. Scale bars: 100 μm (LADs) and 50 μm (CRMs). Representative images are from 4 animals per group.
DISCUSSION
Traditionally, studies of the impact of MetS on the coronary circulation focused on atherosclerosis and endothelial dysfunction of macrovascular arteries such as the LAD. Indeed, our group and others have characterized coronary artery disease in the Ossabaw MetS model (6, 9, 10, 17, 21, 33, 36). In this study, we compared coronary micro- and macrovascular structure and mechanical properties and related them to coronary blood flow and perfusion in this clinically relevant porcine model of MetS. Our results are the first to show that CRMs isolated from Ossabaw pigs with MetS undergo significant inward hypertrophic remodeling that is associated with reduced microvascular stiffness. We further show that MetS is associated with coronary capillary rarefaction, decreased hyperemic-to-basal coronary flow velocity ratio, and augmented coronary pressure-flow autoregulation. Interestingly, the upstream LAD supplying these arterioles was not largely remodeled and was stiffer in Ossabaw MetS pigs. The selective reduction in the CRM collagen-to-elastin ratio and differential expression of RAGE may partially explain the novel and discordant alterations in vascular remodeling and stiffness between small resistance microvessels and larger conduit vessels in MetS.
Since MetS is a conglomeration of several different risk factors, it is important to find a suitable model that recapitulates most, if not all, of the individual components of MetS simulating the human condition. The Ossabaw swine model of MetS closely mimics the clinical components of the clinical syndrome (19, 38). Data from previous studies (17, 45) and our current study show that Ossabaw swine fed a high-fat diet develop obesity, insulin resistance, hyperglycemia, hypertension, and hypercholesterolemia. Thus this animal model allowed us to determine micro- and macrovascular coronary remodeling in a clinically relevant setting.
Remodeling of the vascular system occurs in response to pathological insults, such as hypertension and diabetes (2, 22, 39, 43), which can be inward (narrowing of the vascular lumen), outward (widening of the vascular lumen), hypotrophic, eutrophic, and/or hypertrophic in nature (31, 39). The mechanisms that direct the different types of remodeling are largely unknown but may reflect a delicate balance between neurohormonal and hemodynamic stimuli. For example, studies from our laboratory showed that increased mesenteric blood flow was associated with outward remodeling of mesenteric arterioles of type 2 diabetic db/db mice (43) in the presence of elevated ANG II reported in this model (41). Our current observations of significant inward hypertrophic remodeling of coronary arterioles in MetS are in keeping with studies from our laboratory showing a similar remodeling profile in coronary arterioles from DM mice (22), as well as in cerebral arterioles in hypertensive rats (2).
Vascular hypertrophic inward remodeling involves the encroachment of vascular smooth muscle cells (VSMCs) and extracellular matrix (ECM) on the vascular lumen. Microvascular remodeling in MetS is multifactorial, involving alterations in transmural and luminal pressure, the renin-angiotensin system, advanced glycation end products (AGE)-RAGE, inflammation, and TFG-β signaling. The AT1R and RAGE regulate pathophysiological VSMC growth and ECM production. Surprisingly, we did not observe significant differences in AT1R mRNA or immunohistochemical expression in CMRs or LADs between Lean and MetS pigs, although AT1R immunostaining was abundantly present in both vessels. Conversely, RAGE gene expression was selectively increased by more than twofold in CRMs isolated from MetS vs. Lean pigs, while RAGE immunostaining revealed robust expression in LADs and CRMs that was visually augmented only in the LAD. The differences between RAGE mRNA and protein levels suggest that posttranscriptional regulation of RAGE may occur in CRMs. Collectively, these data suggest that MetS elicits a distinct pattern of receptor expression in the coronary microvasculature relative to the macrovasculature and that RAGE may be at least partly responsible for the inward hypertrophic remodeling of the CRMs and discordant expression of ECM components. Indeed, RAGE can increase proliferation and growth of VSMCs (48). Furthermore, RAGE-dependent VSMC proliferation may involve the MAP kinases ERK and p38 (53), Rho-associated protein kinase 1 (11), and STAT/Pim1 (29). It is important to note that the majority of these studies used VSMCs from macrovessels such as the aorta or carotid arteries; therefore, it is unclear whether the mechanisms learned from these cells are applicable to coronary microvascular VSMCs. Further studies are required to determine the exact role of the AT1R and RAGE in the pathogenesis of CRM remodeling in metabolic disorders such as DM and MetS.
Concomitant with structural remodeling, alterations in the mechanical properties of blood vessels also regulate flow. The structural stability and integrity of a blood vessel are dictated by the VSMCs and ECM components, such as collagen and elastin; therefore, alterations in the composition of these factors can ultimately determine vessel stiffness. For example, increased macrovascular stiffness reported in diabetes has been traditionally linked to enhanced fibrosis (28) associated with collagen accumulation or impaired ECM turnover due to AGE-related collagen cross-linking (26, 28, 42, 51). Moreover, in addition to their contribution to the production of ECM components (4), the viscoelastic properties of the medial VSMCs make them less stiff than collagen (20). Therefore, in the face of equal collagen expression between Lean and MetS CRMs, as shown in our current study, augmented growth of VSMCs may in part contribute to the reduction in CRM stiffness. Together with medial hypertrophy, we found that elastin expression was elevated only in CRMs isolated from MetS pigs, which reduced the collagen-to-elastin expression ratio. This imbalance in collagen and elastin in CRMs likely contributed to the reduced stiffness and increased distensibility of the coronary microvessels measured under passive conditions. Since elastin is more distensible than other ECM components, it is a prime determinant of the overall elasticity of the vessel wall. However, we cannot rule out the possibility of decreased collagen cross-linking in MetS CRMs in our study as an additional mechanism of reduced microvascular stiffness. Indeed, ECM components are regulated by a complex interplay of production, degradation by matrix metalloproteases/tissue inhibitors of metalloproteases, and cross-linking via AGEs. The exact mechanisms underlying reduced CRM stiffness are unclear but may also involve the renin-angiotensin system, RAGE, and TGF-β. AT1R and RAGE activation augment TGF-β signaling, and TGF-β receptor type 1 signaling activates SMADs to alter elastin (14, 25, 55). Given that AT1R and TGF-β receptors were unchanged in our study, RAGE signaling may predominate in the coronary microvessels to elicit changes in the ECM.
The net physiological effect of these remodeling processes hinges on whether they alter coronary perfusion. The uniqueness of coronary hemodynamics that ultimately dictate myocardial perfusion is regulated by an intimate interaction among the myocardial tissue, the coronary conduit arteries, and the coronary microcirculation (49). Moreover, the impact of these interactions on coronary vascular remodeling is unknown, despite intensive investigation. Reductions in blood flow and myocardial perfusion are typically attributed to blockade of the coronary lumen by atherosclerotic plaques, as found in MetS patients, but significant reductions in blood flow only occur when the blockage has exceeded ∼70% (23), which was not observed in our current study (not shown). Our data provide intriguing evidence that microvascular remodeling accounts for at least some of the deleterious ischemic events that occur in these patient populations. Furthermore, coronary capillary density is an index of myocardial perfusion (27), and our data showing reduced coronary capillary density is in close agreement with our finding of impaired hyperemic-to-basal coronary flow velocity ratio in this MetS model. Since blood flow is a powerful regulatory mechanism in vascular remodeling (43), impaired flow may be caused by or the effect of inward hypertrophic remodeling of the coronary microvasculature shown in this study. Further studies are necessary to delineate these mechanisms. Finally, coronary autoregulation, the ability to maintain blood flow over a range of pressures, was significantly increased in MetS due to enhanced coronary vascular resistance, which may be secondary to alterations in K+ and Ca2+ channel regulation (8). However, since hyperemic coronary flow is minimally influenced by coronary autoregulatory mechanisms, the decrease in hyperemic-to-basal coronary flow velocity ratio likely reflects adverse coronary microvascular remodeling. We cannot rule out alterations in adenosine receptor expression in the coronary vasculature of MetS as an additional mechanism (5); however, we reported impaired coronary flow reserve in type 2 diabetic db/db mice measured using isoflurane or adenosine (22), suggesting that alterations in coronary flow reserve were independent of adenosine responses in a similar rodent animal model.
Increased ischemic events in type 2 DM patients or patients with MetS are historically attributed to increased dyslipidemia-associated atherosclerosis that also typically accompanies metabolic diseases (52). The adverse impact of atherosclerosis on coronary circulation encompasses endothelial dysfunction (54) as well as occlusive hemodynamic blood flow; however, functional impairment in coronary flow only occurs in the presence of very significant atherosclerotic occlusion (∼70%) (23). Our data provide intriguing evidence that microvascular remodeling accounts for at least some of the deleterious ischemic events in these patient populations. The utilization of the Ossabaw porcine model of MetS, which very closely mimics human MetS (19), allowed us to determine whether coronary microvascular remodeling occurred in an animal model with atherosclerosis. Previous studies from our laboratory showed that coronary arterioles from DM mice undergo a similar remodeling profile in the absence of atherosclerosis (22), so inward remodeling with an associated reduction in microvascular stiffness was independent of atherosclerosis. We postulate that enhanced atherosclerosis, as well as adverse microvascular remodeling in MetS in the presence of dyslipidemia, works synergistically to account for increased myocardial ischemic events.
Clinical Perspective
Diabetic and MetS patients have a significantly higher risk for myocardial infarction than patients without. The current consensus, based on data to date, shows that coronary artery narrowing by atherosclerotic plaque formation impairs coronary blood flow and, thus, myocardial O2/nutrient supply as a partial explanation for the higher incidence of heart attack in this population. Our current data showing inward hypertrophic remodeling of resistance microvessels, capillary rarefaction, and enhanced autoregulation in the Ossabaw model of MetS offer an additional mechanism by which DM and MetS patients are more at risk for myocardial infarction. Our study suggests that the functional capacity for coronary dilation and, therefore, coronary perfusion may be impaired by structural limitations of the coronary microvasculature. This may chronically lead to impaired coronary flow reserve and diminished myocardial O2 supply-demand balance (7). Additionally, the distinct stiffness profiles between coronary resistance microvessels and coronary conduit arteries suggest that current therapies targeting reduction of vascular stiffness may be ineffective. The decreased coronary microvascular stiffness in Ossabaw swine with MetS may represent a mechanism to compensate for the reduction in luminal area, enhanced myogenic tone, and/or increased myocardial stiffness. Future studies will determine the clinical impact of coronary remodeling and differential stiffness profiles between coronary microvessels and coronary conduit arteries in the setting of MetS and whether current therapeutic strategies are effective in mitigating adverse coronary remodeling.
GRANTS
This work was supported by National Institutes of Health Grants T32 HL-098039 (to A. J. Trask), HL-092245 (to J. D. Tune), RR-013223 and HL-062552 (to M. Sturek), and HL-056046 and HL-63318 (to P. A. Lucchesi). We gratefully acknowledge funding by The Heart Center at Nationwide Children's Hospital and the support of the Comparative Medicine Center of Indiana University School of Medicine and Purdue University for Ossabaw tissue.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.J.T., P.S.K., M.L.G., M.J.C., Z.C.B., A.G.G., M.A., J.D.T., M.S., and P.A.L. are responsible for conception and design of the research; A.J.T., P.S.K., A.P.K., M.L.G., M.J.C., T.A.W., Z.P.N., Z.C.B., A.G.G., M.A., and M.S. performed the experiments; A.J.T., M.L.G., M.J.C., Z.C.B., A.G.G., J.D.T., M.S., and P.A.L. analyzed the data; A.J.T., P.S.K., M.L.G., M.J.C., Z.P.N., J.D.T., M.S., and P.A.L. interpreted the results of the experiments; A.J.T. prepared the figures; A.J.T. and P.A.L. drafted the manuscript; A.J.T., P.S.K., A.P.K., M.L.G., M.J.C., T.A.W., Z.P.N., Z.C.B., A.G.G., M.A., J.D.T., M.S., and P.A.L. edited and revised the manuscript; A.J.T., P.S.K., A.P.K., M.L.G., M.J.C., T.A.W., Z.P.N., Z.C.B., A.G.G., M.A., J.D.T., M.S., and P.A.L. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the technical assistance of Xiaojin Zhang, Jim Byrd, and Jim Wenzel.
REFERENCES
- 1. Bauersachs J, Bouloumie A, Mulsch A, Wiemer G, Fleming I, Busse R. Vasodilator dysfunction in aged spontaneously hypertensive rats: changes in NO synthase III and soluble guanylyl cyclase expression and in superoxide anion production. Cardiovasc Res 37: 772–779, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Baumbach GL, Heistad DD. Remodeling of cerebral arterioles in chronic hypertension. Hypertension 13: 968–972, 1989 [DOI] [PubMed] [Google Scholar]
- 3. Belmadani S, Palen DI, Gonzalez-Villalobos RA, Boulares HA, Matrougui K. Elevated epidermal growth factor receptor phosphorylation induces resistance artery dysfunction in diabetic db/db mice. Diabetes 57: 1629–1637, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belmadani S, Zerfaoui M, Boulares HA, Palen DI, Matrougui K. Microvessel vascular smooth muscle cells contribute to collagen type I deposition through ERK1/2 MAP kinase, αvβ3-integrin, and TGF-β1 in response to ANG II and high glucose. Am J Physiol Heart Circ Physiol 295: H69–H76, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bender SB, Tune JD, Borbouse L, Long X, Sturek M, Laughlin MH. Altered mechanism of adenosine-induced coronary arteriolar dilation in early-stage metabolic syndrome. Exp Biol Med (Maywood) 234: 683–692, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berwick ZC, Dick GM, Moberly SP, Kohr MC, Sturek M, Tune JD. Contribution of voltage-dependent K channels to metabolic control of coronary blood flow. J Mol Cell Cardiol 52: 912–919, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berwick ZC, Dick GM, Tune JD. Heart of the matter: coronary dysfunction in metabolic syndrome. J Mol Cell Cardiol 52: 848–856, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berwick ZC, Moberly SP, Kohr MC, Morrical EB, Kurian MM, Dick GM, Tune JD. Contribution of voltage-dependent K+ and Ca2+ channels to coronary pressure-flow autoregulation. Basic Res Cardiol 107: 1–11, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Borbouse L, Dick GM, Asano S, Bender SB, Dincer UD, Payne GA, Neeb ZP, Bratz IN, Sturek M, Tune JD. Impaired function of coronary BKCa channels in metabolic syndrome. Am J Physiol Heart Circ Physiol 297: H1629–H1637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bratz IN, Dick GM, Tune JD, Edwards JM, Neeb ZP, Dincer UD, Sturek M. Impaired capsaicin-induced relaxation of coronary arteries in a porcine model of the metabolic syndrome. Am J Physiol Heart Circ Physiol 294: H2489–H2496, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Bu DX, Rai V, Shen X, Rosario R, Lu Y, D′Agati V, Yan SF, Friedman RA, Nuglozeh E, Schmidt AM. Activation of the ROCK1 branch of the transforming growth factor-β pathway contributes to RAGE-dependent acceleration of atherosclerosis in diabetic ApoE-null mice. Circ Res 106: 1040–1051, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carroll JA, Daniel JA, Keisler DH, Matteri RL. Non-surgical catheterization of the jugular vein in young pigs. Lab Anim 33: 129–134, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Celik T, Iyisoy A, Yuksel UC. Pediatric metabolic syndrome: a growing threat. Int J Cardiol 142: 302–303, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Choi WS, Mitsumoto A, Kochevar IE. Involvement of reactive oxygen species in TGF-β1-induced tropoelastin expression by human dermal fibroblasts. Photochem Photobiol 85: 1425–1433, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Dixon JL, Shen S, Vuchetich JP, Wysocka E, Sun GY, Sturek M. Increased atherosclerosis in diabetic dyslipidemic swine: protection by atorvastatin involves decreased VLDL triglycerides but minimal effects on the lipoprotein profile. J Lipid Res 43: 1618–1629, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Dixon JL, Stoops JD, Parker JL, Laughlin MH, Weisman GA, Sturek M. Dyslipidemia and vascular dysfunction in diabetic pigs fed an atherogenic diet. Arterioscler Thromb Vasc Biol 19: 2981–2992, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Dyson MC, Alloosh M, Vuchetich JP, Mokelke EA, Sturek M. Components of metabolic syndrome and coronary artery disease in female Ossabaw swine fed excess atherogenic diet. Comp Med 56: 35–45, 2006 [PubMed] [Google Scholar]
- 18. Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl Health Stat Report May 5: 1–7, 2009 [PubMed] [Google Scholar]
- 19. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112: 2735–2752, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Intengan HD, Deng LY, Li JS, Schiffrin EL. Mechanics and composition of human subcutaneous resistance arteries in essential hypertension. Hypertension 33: 569–574, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Kassab GS, Choy JS, Svendsen M, Sinha AK, Alloosh M, Sturek M, Huo Y, Sandusky GE, Hermiller J. A novel system for the reconstruction of a coronary artery lumen profile in real time: a preclinical validation. Am J Physiol Heart Circ Physiol 297: H485–H492, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Katz PS, Trask AJ, Souza-Smith FM, Hutchinson KR, Galantowicz ML, Lord KC, Stewart JA, Jr, Cismowski MJ, Varner KJ, Lucchesi PA. Coronary arterioles in type 2 diabetic (db/db) mice undergo a distinct pattern of remodeling associated with decreased vessel stiffness. Basic Res Cardiol 106: 1123–1134, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kern MJ, Lerman A, Bech JW, De Bruyne B, Eeckhout E, Fearon WF, Higano ST, Lim MJ, Meuwissen M, Piek JJ, Pijls NH, Siebes M, Spaan JA. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: a scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation 114: 1321–1341, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Knudson JD, Dincer UD, Bratz IN, Sturek M, Dick GM, Tune JD. Mechanisms of coronary dysfunction in obesity and insulin resistance. Microcirculation 14: 317–338, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Kucich U, Rosenbloom JC, Abrams WR, Rosenbloom J. Transforming growth factor-β stabilizes elastin mRNA by a pathway requiring active Smads, protein kinase Cδ, and p38. Am J Respir Cell Mol Biol 26: 183–188, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Kuzuya M, Asai T, Kanda S, Maeda K, Cheng XW, Iguchi A. Glycation cross-links inhibit matrix metalloproteinase-2 activation in vascular smooth muscle cells cultured on collagen lattice. Diabetologia 44: 433–436, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Levy BI, Schiffrin EL, Mourad JJ, Agostini D, Vicaut E, Safar ME, Struijker-Boudier HA. Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation 118: 968–976, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Marsh SA, Dell'italia LJ, Chatham JC. Interaction of diet and diabetes on cardiovascular function in rats. Am J Physiol Heart Circ Physiol 296: H282–H292, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meloche J, Paulin R, Courboulin A, Lambert C, Barrier M, Bonnet P, Bisserier M, Roy M, Sussman MA, Agharazii M, Bonnet S. RAGE-dependent activation of the oncoprotein Pim1 plays a critical role in systemic vascular remodeling processes. Arterioscler Thromb Vasc Biol 31: 2114–2124, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mokelke EA, Dietz NJ, Eckman DM, Nelson MT, Sturek M. Diabetic dyslipidemia and exercise affect coronary tone and differential regulation of conduit and microvessel K+ current. Am J Physiol Heart Circ Physiol 288: H1233–H1241, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Mulvany MJ, Baumbach GL, Aalkjaer C, Heagerty AM, Korsgaard N, Schiffrin EL, Heistad DD. Vascular remodeling. Hypertension 28: 505–506, 1996 [PubMed] [Google Scholar]
- 32. Naya M, Tsukamoto T, Morita K, Katoh C, Furumoto T, Fujii S, Tamaki N, Tsutsui H. Olmesartan, but not amlodipine, improves endothelium-dependent coronary dilation in hypertensive patients. J Am Coll Cardiol 50: 1144–1149, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Neeb ZP, Edwards JM, Alloosh M, Long X, Mokelke EA, Sturek M. Metabolic syndrome and coronary artery disease in Ossabaw compared with Yucatan swine. Comp Med 60: 300–315, 2010 [PMC free article] [PubMed] [Google Scholar]
- 34. Otis CR, Wamhoff BR, Sturek M. Hyperglycemia-induced insulin resistance in diabetic dyslipidemic Yucatan swine. Comp Med 53: 53–64, 2003 [PubMed] [Google Scholar]
- 35. Park Y, Yang J, Zhang H, Chen X, Zhang C. Effect of PAR2 in regulating TNF-α and NAD(P)H oxidase in coronary arterioles in type 2 diabetic mice. Basic Res Cardiol 106: 111–123, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Payne GA, Borbouse L, Kumar S, Neeb Z, Alloosh M, Sturek M, Tune JD. Epicardial perivascular adipose-derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase Cβ pathway. Arterioscler Thromb Vasc Biol 30: 1711–1717, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Prior JO, Quinones MJ, Hernandez-Pampaloni M, Facta AD, Schindler TH, Sayre JW, Hsueh WA, Schelbert HR. Coronary circulatory dysfunction in insulin resistance, impaired glucose tolerance, and type 2 diabetes mellitus. Circulation 111: 2291–2298, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 123: e18–e209, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schiffrin EL. Remodeling of resistance arteries in essential hypertension and effects of antihypertensive treatment. Am J Hypertens 17: 1192–1200, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3: 1101–1108, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Senador D, Kanakamedala K, Irigoyen MC, Morris M, Elased KM. Cardiovascular and autonomic phenotype of db/db diabetic mice. Exp Physiol 94: 648–658, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sims TJ, Rasmussen LM, Oxlund H, Bailey AJ. The role of glycation cross-links in diabetic vascular stiffening. Diabetologia 39: 946–951, 1996 [DOI] [PubMed] [Google Scholar]
- 43. Souza-Smith FM, Katz PS, Trask AJ, Stewart JA, Jr, Lord KC, Varner KJ, Vassallo DV, Lucchesi PA. Mesenteric resistance arteries in type 2 diabetic db/db mice undergo outward remodeling. PLos One 6: e23337, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Steinberger J, Daniels SR, Eckel RH, Hayman L, Lustig RH, McCrindle B, Mietus-Snyder ML. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation 119: 628–647, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Sturek MAM, Wenzel J, Byrd JP, Edwards JM, Lloyd PG, Tune JD, March KL, Miller MA, Mokelke EA, Brisbin IL., Jr Ossabaw Island miniature swine: cardiometabolic syndrome assessment. In: Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques, edited by Swindle M. Boca Raton, FL: CRC, 2007, p. 397–402 [Google Scholar]
- 46. Takano M, Mizuno K, Okamatsu K, Yokoyama S, Ohba T, Sakai S. Mechanical and structural characteristics of vulnerable plaques: analysis by coronary angioscopy and intravascular ultrasound. J Am Coll Cardiol 38: 99–104, 2001 [DOI] [PubMed] [Google Scholar]
- 47. Teragawa H, Mitsuba N, Nishioka K, Ueda K, Kono S, Higashi Y, Chayama K, Kihara Y. Impaired coronary microvascular endothelial function in men with metabolic syndrome. World J Cardiol 2: 205–210, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang R, Kudo M, Yokoyama M, Asano G. Roles of advanced glycation endproducts (AGE) and receptor for AGE on vascular smooth muscle cell growth. J Nihon Med Sch 68: 472–481, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Westerhof N, Boer C, Lamberts RR, Sipkema P. Cross-talk between cardiac muscle and coronary vasculature. Physiol Rev 86: 1263–1308, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Witczak CA, Mokelke EA, Boullion R, Wenzel J, Keisler DH, Sturek M. Noninvasive measures of body fat percentage in male Yucatan swine. Comp Med 55: 445–451, 2005 [PubMed] [Google Scholar]
- 51. Wolffenbuttel BH, Boulanger CM, Crijns FR, Huijberts MS, Poitevin P, Swennen GN, Vasan S, Egan JJ, Ulrich P, Cerami A, Levy BI. Breakers of advanced glycation end products restore large artery properties in experimental diabetes. Proc Natl Acad Sci USA 95: 4630–4634, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wong ND, Rozanski A, Gransar H, Miranda-Peats R, Kang X, Hayes S, Shaw L, Friedman J, Polk D, Berman DS. Metabolic syndrome and diabetes are associated with an increased likelihood of inducible myocardial ischemia among patients with subclinical atherosclerosis. Diabetes Care 28: 1445–1450, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Yoon YW, Kang TS, Lee BK, Chang W, Hwang KC, Rhee JH, Min PK, Hong BK, Rim SJ, Kwon HM. Pathobiological role of advanced glycation endproducts via mitogen-activated protein kinase dependent pathway in the diabetic vasculopathy. Exp Mol Med 40: 398–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zeiher AM, Drexler H, Wollschlager H, Just H. Endothelial dysfunction of the coronary microvasculature is associated with coronary blood flow regulation in patients with early atherosclerosis. Circulation 84: 1984–1992, 1991 [DOI] [PubMed] [Google Scholar]
- 55. Zhou F, Li GY, Gao ZZ, Liu J, Liu T, Li WR, Cui WS, Bai GY, Xin ZC. The TGF-β1/Smad/CTGF pathway and corpus cavernosum fibrous-muscular alterations in rats with streptozotocin-induced diabetes. J Androl 33: 651–659, 2012 [DOI] [PubMed] [Google Scholar]