Abstract
Vascular remodeling has been implicated in many inflammation-involved diseases. This study aims to investigate the microvascular remodeling-associated alterations in cell-cell adhesion and cytoskeleton reactions to inflammatory stimuli and their impact on microvessel permeability. Experiments were conducted in individually perfused rat mesenteric venules. Microvessel permeability was determined by measuring hydraulic conductivity (Lp), and endothelial intracellular calcium concentration, [Ca2+]i, was measured in fura-2-perfused vessels. Alterations in VE-cadherin and F-actin arrangement were examined by confocal imaging. Vascular wall cellular composition and structural changes were evaluated by electron microscopy. Vessels exposed to platelet activating factor (PAF) on day 1 were reevaluated 3 days later in rats that had undergone survival surgery. Initial PAF exposure and surgical disturbance increased microvascular wall thickness along with perivascular cell proliferation and altered F-actin arrangement. Although basal permeability was not changed, upon reexposure to PAF, peak endothelial [Ca2+]i was augmented and the peak Lp was 9.3 ± 1.7 times higher than that of day 1. In contrast to patterns of PAF-induced stress fiber formation and VE-cadherin redistribution observed in day 1 vessels, the day 4 vessels at the potentiated Lp peak exhibited wide separations of VE-cadherin between endothelial cells and striking stress fibers throughout the vascular walls. Confocal images and ultrastructural micrographs also revealed that the largely separated VE-cadherin and endothelial gaps were completely covered by F-actin bundles in extended pericyte processes at the PAF-induced Lp peak. These results indicate that inflammation-induced vascular remodeling increased endothelial susceptibility to inflammatory stimuli with augmented Ca2+ response resulting in upregulated contractility and potentiated permeability increase. Weakened adhesions between the endothelial cells and contractile mechanisms are both involved in increasing permeability in the intact microvessels and are aggravated during remodeling. The perivascular cells play important roles in stabilizing the microvessel wall, while lessening an otherwise much greater magnitude of leakage during cytoskeletal contraction.
Keywords: inflammation, VE-cadherin, stress fibers, microvessel permeability, pericyte
vascular remodeling involves structural alterations in the vascular walls including the changes in size and cellular compositions. Under pathological conditions, vascular remodeling usually occurs in response to locally generated mediators, injury, inflammatory cell infiltration, or changes in hemodynamic conditions (14). Currently, most of the vascular remodeling studies focus on large arteries and arterioles but only a few have investigated chronic inflammation-associated remodeling in microvessels, a site that allows macromolecules to escape, resulting in tissue edema and organ dysfunction. Identifying the inflammation-induced structural changes in microvascular walls and their impact on microvessel permeability will be crucial for a better understanding of the pathogenesis of vascular diseases and thereby benefit the development of targeted therapies.
Studies on the tracheal microvasculature of mice showed that following airway infection, tracheal microvessels manifested endothelial cell proliferation, new vessel growth, and phenotypic alteration in existing capillaries (13, 31). Although some focal leaky regions were found in microvessels at the peak of the endothelial cell proliferation period in infected mice, the overall amount of plasma leakage measured with Evans blue under basal conditions was not significantly different from that of pathogen-free mice up to 14 days after the infection (13, 31). Higher plasma leakage was found when angiogenic blood vessels were exposed to substance P (5). A study using a single-vessel perfusion technique in rat mesenteric venules detected enlargements of endothelial cells and vessel diameters 24 h after an initial perfusion or exposure to the platelet activating factor (PAF) (8). Most interestingly, microvessels that had no response to an initial thrombin exposure showed increased permeability responses 24 h after the inflammatory stimulation, suggesting that prior endothelial injury or exposure to inflammatory mediators can alter the endothelial responses to inflammatory stimuli in 24 h (8). Although these studies characterized some of the inflammation-induced phenotypic changes in endothelial cells of the microvessel walls (5, 8, 13, 31), the vascular remodeling-associated changes in cell-cell adhesion and cytoskeleton contractile responses to inflammatory stimuli have not been explored. Furthermore, beyond the reported changes in endothelial cell plasticity, the changes in the perivascular cells of the microvascular wall during vascular remodeling remain to be revealed.
The objectives of our present study are to characterize the impact of early stage microvascular remodeling on vascular wall structure, cellular adhesion, and cytoskeleton responses to inflammatory stimuli with correlated measurements of microvessel permeability. Our survival-surgery animal model permitted the same mesenteric venule that was cannulated and perfused on day 1 to be reevaluated days after. Because the physical and phenotypic changes of endothelial cells occurred as early as 24 h (8) and reached a plateau 7 days after an initial inflammatory exposure (13), our study focused on the effect of vascular remodeling over a 3-day period by directly comparing the cellular structure and permeability responses to PAF observed between day 1 and day 4. Experiments were conducted in individually perfused rat mesenteric venules. Microvessel permeability was assessed by measuring hydraulic conductivity (Lp), and endothelial intracellular calcium concentration, [Ca2+]i, was measured by fura-2 AM in individually perfused microvessels. The changes in VE-cadherin, F-actin, and the ultrastructure of the remodeled microvascular walls were investigated under the same experimental conditions in which permeability is measured by confocal imaging and electron microscopy.
METHODS AND MATERIALS
Animal preparation.
Experiments were carried out on female Sprague-Dawley rats (2–3 mo old, 220 to 250 g; Hilltop Laboratory Animal, Scottdale, PA). All procedures and animal use were approved by the Animal Care and Use Committee at West Virginia University. Pentobarbital sodium was used for anesthesia, given subcutaneously. The initial dosage was 65 mg/kg body wt with an additional 3 mg/dose given as needed. A midline surgical incision (1.5 to 2 cm) was made in the abdominal wall. The mesentery was gently moved out of the abdominal cavity and spread over a pillar for Lp measurements or over a glass coverslip attached to an animal tray for confocal imaging and measurement of endothelial [Ca2+]i. The upper surface of the mesentery was continuously superfused with mammalian Ringer solution at 37°C. Venular microvessels initially free of firmly attached leukocytes and with brisk blood flow were selected for the day 1 experiments. Details of the acute experiment have been described (40).
For the animals that underwent survival surgery, the procedures on day 1 were the same as those described above, except they were conducted under aseptic conditions. After the experiment, the perfusion pipette was removed to allow blood flow to resume in the perfused vessel. The mesentery was then returned to the abdominal cavity and the incision was closed with sutures. The rat then recovered from anesthesia and rested for 3 days. Buprenorphine HCl (0.1 mg/kg) was given pre- and postsurgery to reduce pain and was repeated at 6 and 12 h during the recovery period. On day 4, the rat that went through the experiment on day 1 was anesthetized again following the day 1 procedures.
Measurement of Lp in individually perfused rat mesenteric microvessels.
A modified Landis technique was used to measure Lp in individually perfused microvessels. The methods have been evaluated in detail (10, 23). Briefly, a single microvessel was cannulated with a micropipette and perfused with albumin-Ringer solution (control) containing red blood cells (1% vol/vol) as markers under a known hydrostatic pressure ranging from 40 to 60 cmH2O. For each measurement, the perfused vessel was briefly occluded downstream with a glass rod for 5–7 s. The initial water flux per unit area of microvessel wall was calculated from the velocity of the marker cell after vessel occlusion, the vessel radius, and the distance between the marker cell and the occlusion site. Lp was calculated as the slope of the relationship between the initial water flow per unit area of vessel wall and the pressure difference across the vessel wall. In each experiment, the baseline Lp and the Lp after application of PAF were measured in the same vessel. The changes in Lp were expressed as the ratio of Lptest:Lpcontrol. In the survival-surgery experiments, the vessel that was perfused and exposed to PAF on day 1 was identified in the mesentery 3 days after and recannulated with a micropipette to measure the changes in baseline Lp and the Lp response to PAF on day 4.
Measurements of endothelial [Ca2+]i.
Endothelial [Ca2+]i was measured in individually perfused microvessels using the fluorescent Ca2+ indicator fura-2 AM and a Nikon photometry system. Details have been described (19). In brief, a venular microvessel was cannulated and perfused first with albumin-Ringer solution that contained 10 μM of fura-2 AM for 45 min. The vessel was then recannulated and perfused with albumin-Ringer solution for 10 min to remove fura-2 AM from the vessel lumen. A segment of fura-2 AM-loaded vessel at least 100 μm away from the cannulation site was then positioned within the measuring window, which covered ∼50 endothelial cells forming the vessel wall. The excitation wavelengths for fura-2 AM were selected by two narrow-band interference filters (340 ± 5 and 380 ± 5 nm; Oriel Instruments, Stratford, CT), and the emission was separated with a dichroic mirror (DM400) and a wide-band interference filter (500 ± 35 nm; Oriel). The corresponding fluorescence intensity (FI) values (FI340 and FI380, respectively) were collected with a 0.25-s exposure at each wavelength. At the end of the experiment, the microvessel was superfused with a modified Ringer solution (5 mM of Mn2+ without Ca2+) and perfused with the same solution that contained ionomycin (10 μM) to bleach the Ca2+-sensitive form of fura-2. The background FI due to unconverted fura-2 AM and other Ca2+-insensitive forms of fura-2 were subtracted from FI340 and FI380 values. The ratios of the two FI values were converted to Ca2+ concentrations using an in vitro calibration curve (19).
Confocal imaging.
A Leica TCS SL confocal microscope attached to a single-vessel perfusion rig was used for collecting images. A stack of confocal images was obtained from each vessel by optical sectioning at successive X-Y focal planes with vertical steps (Z-axis) at 0.5 and 0.2 μm using a Leica objective ×20 (HC PL APO, NA 0.7) with ×3 electronic zoom and ×63 objective(HCX PL APO, NA 1.2), respectively, with a 1,024 × 1,024 pixel scanning format. The selected pinhole diameter (one airy disk) and the vertical step at the Z-axis were within the effective resolution limitations calibrated for the objective, the wavelength, and the electronic scanning format used in the Leica confocal system and comply with Nyquist criterion. Comparable image acquisition parameters were applied to each group of studies. Stacks of images were processed and projections were made using Leica confocal software.
Fluorescent immunostaining.
Actin staining was performed after each vessel was fixed with paraformaldehyde and treated with Triton X-100 during albumin-Ringer perfusion or at PAF-induced Lp peak (about 7 min of PAF perfusion). Then each vessel was perfused with Alexa Fluor 488- or tetramethylrhodamine isothiocyanate (TRITC)-labeled phalloidin for 5–10 min in the absence or presence of nuclei dye, DRAQ5. Single- or dual-channel confocal images were acquired after washing away the unbound dye(s) by albumin-Ringer perfusate. VE-cadherin staining was performed after the rat mesentery bearing the perfused venule was fixed and removed from the animal. The tissue was then exposed to the primary antibody against VE-cadherin overnight followed by the secondary antibody staining with or without DRAQ5.
Electron microscopy.
Electron microscopy was conducted under control conditions in both day 1 and day 4 vessels and at PAF-induced peak Lp in day 4 vessels. Each vessel was fixed during perfusion by superfusion of the upper surface of the mesentery with a fixative solution (1% paraformaldehyde, 1.25% glutaraldehyde, and 5% sucrose in 0.1 M phosphate buffer) for 20 min. Following fixation, a small panel of mesentery that contained the perfused microvessel was dissected and placed in the same fixative at 4°C overnight. The specimens were then rinsed in 0.1 M phosphate buffer and permeabilized with 50% ethanol in H2O. The samples were postfixed in osmium tetroxide (1%), dehydrated in a graded ethanol series, transferred into propylene oxide, and embedded in Epon (Ladd LX112; Ladd Research, Williston, VT). Thin sections were stained with alcoholic uranyl acetate and Reynolds citrate. Photomicrographs were taken on a JEOL 1220 transmission electron microscope.
Solutions and reagents.
Mammalian Ringer solution was used for the experiments (19). The composition of the mammalian Ringer solution was (in mM) 132 NaCl, 4.6 KCl, 2 CaCl2, 1.2 MgSO4, 5.5 glucose, 5.0 NaHCO3, 20 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid and Na-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid. All perfusates contained 1 g/dl BSA. Alexa Fluor 488 (Molecular Probes, Eugene, OR) and TRITC-labeled phalloidin and the primary and secondary VE-cadherin antibodies were all from Invitrogen. DRAQ5 was from Biostatus Limited (UK). PAF (1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine; Sigma, St. Louis MO) was initially dissolved in 95% ethyl alcohol (5 mM) and further diluted to a final concentration of 10 nM with albumin-Ringer solution. All of the perfusates containing the test agents were freshly prepared before each cannulation.
Data analysis and statistics.
All values are means ± SE. A paired t-test was used for paired data analysis. ANOVA was used to compare data between groups. A probability value of P < 0.05 was considered statistically significant. In the figures, * indicates a significant increase from the control within a group and † indicates a significant increase between groups with the same treatment.
RESULTS
Potentiated permeability and enhanced endothelial calcium responses to PAF in day 4 vessels.
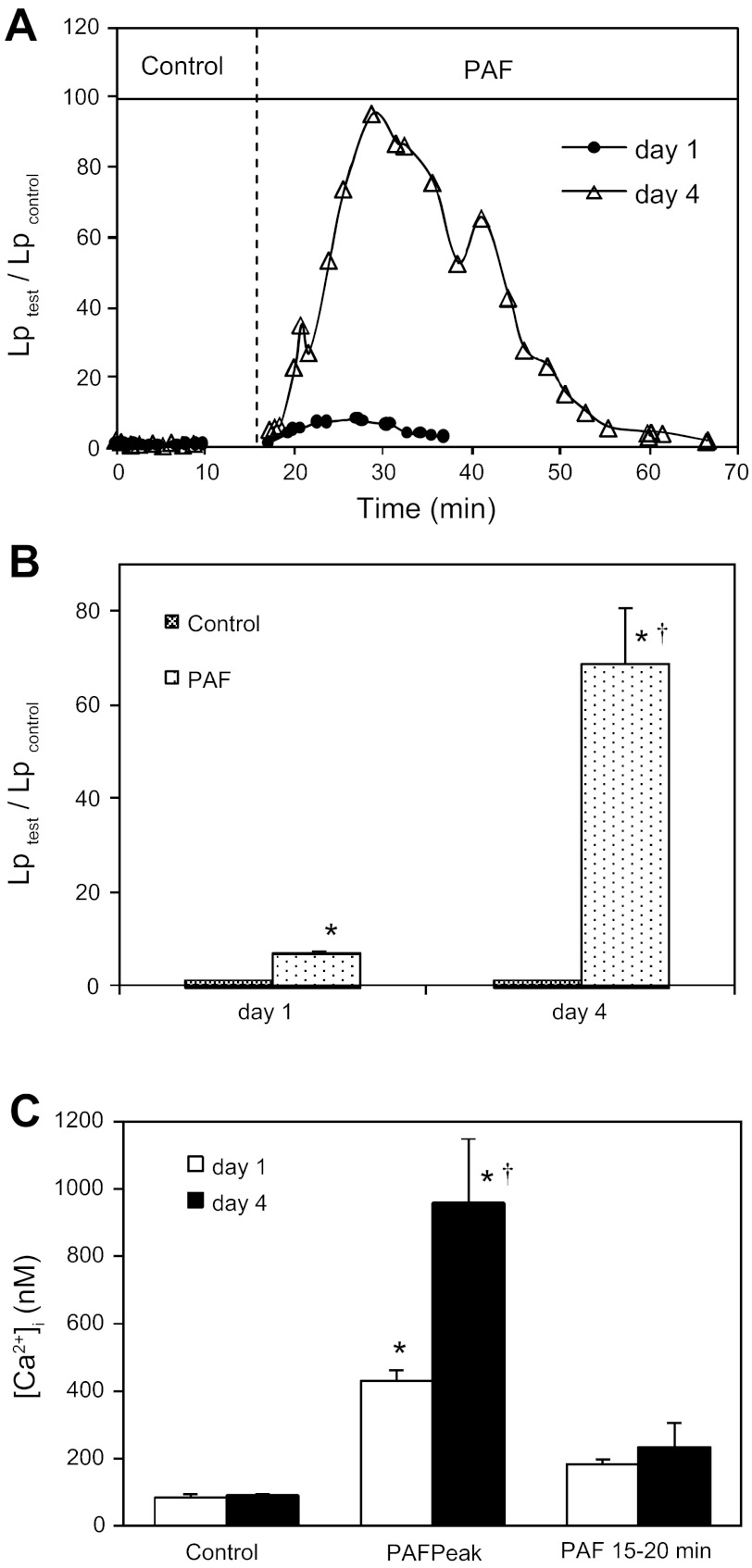
Permeability measurements were conducted in seven microvessels. On day 1, the mean baseline Lp of seven vessels was 1.6 ± 0.1 × 10−7cm·s−1·cmH2O−1. Perfusion of PAF (10 nM) induced a transient increase in Lp. The mean peak Lp that occurred at 7.2 ± 1.3 min of PAF perfusion was 11.3 ± 1.7 × 10−7cm·s−1·cmH2O−1. On day 4, the same vessel that was perfused on day 1 showed significant increases in vessel diameter and leukocyte adhesion. The mean vessel diameter of seven vessels increased from 37 ± 1.0 μm on day 1 to 61 ± 8 μm on day 4 (62% ± 6% increase). Albumin-Ringer perfusion at a pressure of 50 cmH2O did not significantly change the vessel diameter of either day 1 or day 4 vessels. Whereas the initial mean number of adherent leukocytes on day 4 vessels was 32 ± 1 per 100 μm of vessel length, about 80% of the adherent leukocytes quickly detached from the vessel wall during the albumin-Ringer perfusion. Despite abundant adherent leukocytes, the mean baseline Lp was 1.7 ± 0.1 × 10−7 cm·s−1·cmH2O−1, not significantly different from that of day 1. However, when PAF was applied to each of the day 4 vessels, the mean peak Lp reached 104.6 ± 17.6 × 10−7 cm·s−1·cmH2O−1, which was 69.1 ± 11.7 times that of the day 4 control and 9.3 times the mean day 1 peak response to PAF. The time to Lp peak on day 4 (6.3 ± 0.9 min) was slightly shorter, but not statistically different from that on day 1 (7.2 ± 1.3 min, P = 0.53). However, due to the markedly potentiated peak Lp on day 4, the rate of the Lp increase on day 4 was about 12 times faster. Figure 1A shows the differences in magnitude and time course of the Lp response to PAF measured on day 1 and day 4 from the same vessel. Figure 1B summarizes the results of seven vessels.
Fig. 1.
Potentiated permeability and endothelial calcium responses to platelet activating factor (PAF) in day 4 vessels. A: paired hydraulic conductivity (Lp) measurements in the same vessel on day 1 and day 4. The day 1 baseline Lp (●) was 2.1 × 10−7cm·s−1·cmH2O−1 and the PAF (10 nM)-induced Lp peak was 17.6 × 10−7cm·s−1·cmH2O−1. The baseline Lp measured on day 4 (△) was 1.9 × 10−7 cm·s−1·cmH2O−1 and the Lp peak upon PAF perfusion was 187.5 × 10−7 cm·s−1·cmH2O−1. B: summary of seven vessels shows that the mean baseline Lp on day 4 was not significantly different from that on day 1, whereas the PAF-induced mean Lp peak on day 4 was 9.3 ± 1.7 times the value on day 1. C: comparisons of PAF-induced increases in endothelial [Ca2+]i between the day 1 and day 4 vessels (n = 4 per group). The PAF-induced peak endothelial [Ca2+]i of the day 4 vessels (filled bars) was more than double the values on day 1 (open bars). *Significant increase from the control within a group; †significant increase between groups with the same treatment.
The changes in endothelial [Ca2+]i were measured in both day 1 and day 4 vessels (n = 4 for each group). Consistent with the Lp changes, the basal endothelial [Ca2+]i was not significantly different between day 1 and day 4 vessels (84 ± 10 and 92 ± 4 nM, respectively). However, when day 4 vessels were exposed to PAF, endothelial [Ca2+]i reached a mean peak level of 953 ± 189 nM, which is much higher than 430 ± 32 nM measured in the day 1 vessels. Figure 1C summarizes the results.
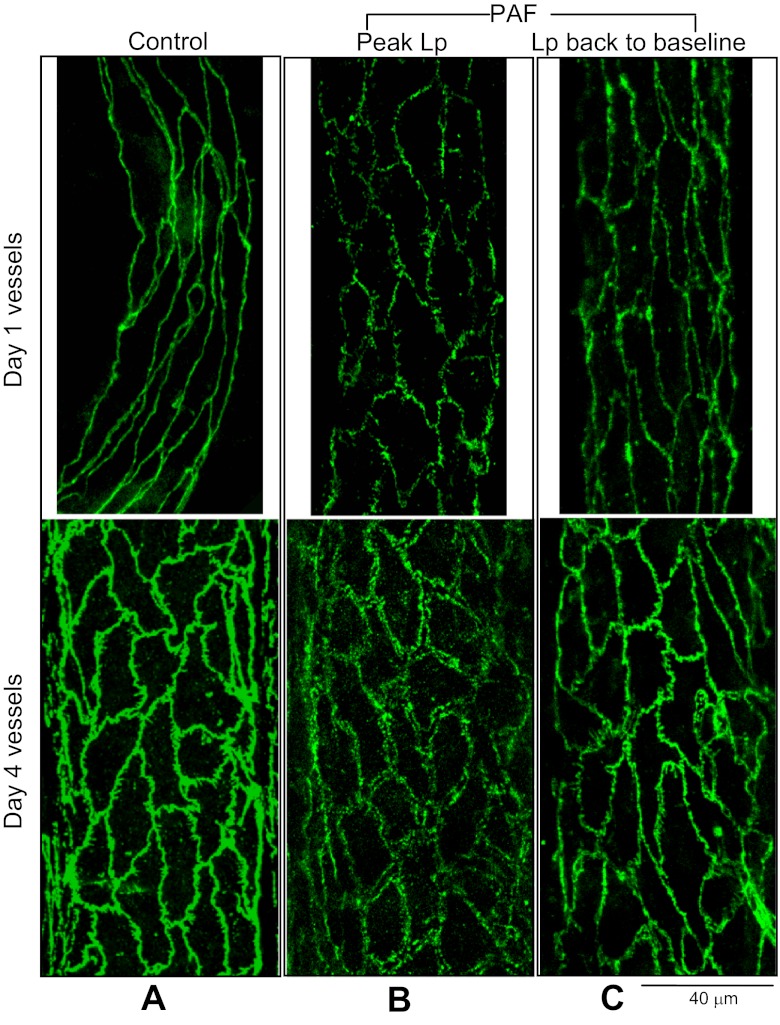
Day 4 microvessels exhibited largely separated VE-cadherin between endothelial cells at the PAF-induced potentiated Lp peak.
Endothelial VE-cadherin was imaged with confocal microscopy in 24 vessels. Each vessel was fixed either under the albumin-Ringer perfusion or after exposure to PAF at both the Lp peak and recovery phase in day 1 and day 4 vessels (n = 4 per group). All of the day 4 vessels experienced the same day 1 treatment as that stated in materials and methods. All of the vessels chosen for experimentation had vessel diameters between 38 and 42 μm on day 1. Figure 2A shows the representative images of VE-cadherin with the albumin-Ringer perfusion on day 1 and day 4 vessels, respectively. The day 4 control image outlines the changes in endothelial cell shape. Although VE-cadherin distribution became tortuous with increased spikes on day 4 compared with day 1, it maintained its continuity between the endothelial cells under basal conditions. The mean basal Lp measured right before fixation was 2.1 ± 0.1 × 10−7 cm·s−1·cmH2O−1, not significantly different from 2.3 ± 0.2 × 10−7 cm·s−1·cmH2O−1 measured on day 1 from the same four vessels (P > 0.05). When the vessels were fixed at the PAF-induced Lp peak, the day 4 vessels showed a distinct VE-cadherin pattern from that on day 1. VE-cadherin in day 1 vessels showed a single profile staining between endothelial cells but with frequent breaks, whereas the day 4 vessel VE-cadherin exhibited large separations between adjacent endothelial cells with double outline (Fig. 2B). Most importantly, the separated VE-cadherin appeared in almost all of the endothelial junctions of the vessel segment. These two distinct patterns of VE-cadherin redistribution correlated with a ninefold difference in the PAF-induced peak Lp. The images in Fig. 2C demonstrate that the changes in VE-cadherin at the Lp peak recovered close to the control state after the increased Lp returned to baseline in both day 1 and day 4 vessels.
Fig. 2.
VE-cadherin distributions in day 1 and day 4 vessels. A: under control conditions, the day 4 VE-cadherin distribution (bottom panel) was not as smooth as that shown on day 1 (top panel), but remained as a single profile with relative continuity. B: at the platelet activating factor (PAF)-induced hydraulic conductivity (Lp) peak, VE-cadherin displays frequent breaks on day 1 (top panel), but wide separations between endothelial cells on day 4 (bottom panel). C: redistributed VE-cadherin at the PAF-induced Lp peak returned close to the control state in both day 1 and day 4 vessels when the increased Lp returned to the baseline.
Although the day 4 vessels increased vessel diameter from 40 ± 0.4 to 72 ± 2.8 μm, the number of endothelial junctions per cross section along the vessel wall as that outlined by VE-cadherin staining was not significantly different from that of the day 1 vessels. The mean number of endothelial junctions per cross section was 11.0 ± 0.2 on day 1 (14 cross sections from 4 vessels) and 11.6 ± 0.3 on day 4 (13 cross sections from another 4 vessels). This observation indicates that the increased diameter in day 4 vessels was the result of the enlargement of the existing endothelial cells and not the endothelial cell proliferation.
Perivascular cell proliferation with altered F-actin arrangements in day 4 microvessels.
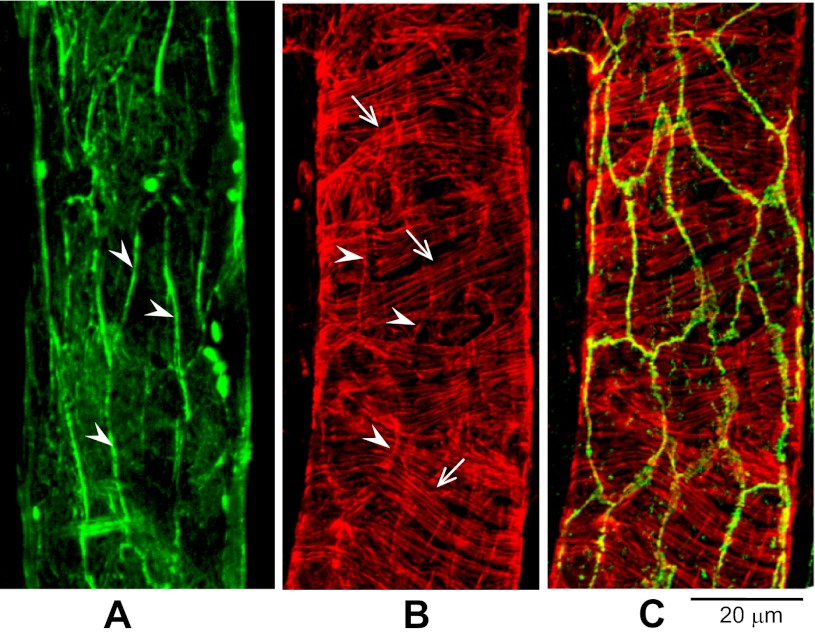
The distribution of cytoplasmic F-actin under unstimulated conditions was examined in both day 1 and day 4 vessels (four vessels per group). Each vessel was fixed during albumin-Ringer perfusion and followed by the perfusion of Alexa Fluor 488- or TRITC-labeled phalloidin for 5–10 min. Confocal images were acquired after washing away unbound phalloidin by albumin-Ringer perfusate. In day 1 vessels, the endothelial F-actin was located mainly at the cell periphery overlapping with VE-cadherin (Fig. 3), and pericyte F-actin was circumferentially oriented in a parallel pattern (Fig. 3B). In day 4 vessels, the endothelial peripheral F-actin was less complete than that observed on day 1, but could still be located at the cell borders (Fig. 4, A and B), whereas the pericyte F-actin changed from an organized parallel pattern to disorganized loose bundles (Fig. 4C). Of interest, extra layers of thin actin filaments were observed outside the pericyte actin (Fig. 4D), suggesting the formation of an additional layer of perivascular cells. On the basis of detected F-actin fluorescence during confocal optical sectioning, we observed a significant increase in wall thickness in day 4 vessels. Using identical instrumental settings, the mean thickness of the vessel wall was 1.9 ± 0.4 (SD) μm (nuclei areas were avoided) in day 1 vessels and 3.4 ± 0.3 (SD) μm in day 4 vessels. A stack of confocal images at the X-Y plane from a day 4 vessel is presented as a video in supplemental material, available with the on-line version of this paper, which illustrates actin filament distribution throughout different cell layers of the vessel wall.
Fig. 3.
Peripheral F-actin colocalizes with VE-cadherin under control conditions in day 1 vessels. A: endothelial cell peripheral F-actin (green, arrowheads). B: F-actin staining (red) in both endothelial cells (arrowheads) and pericyte (arrows). C: the same vessel segment shown in B, but with double staining for F-actin (red) and VE-cadherin (green), demonstrating the overlap of VE-cadherin with endothelial F-actin peripheral bands.
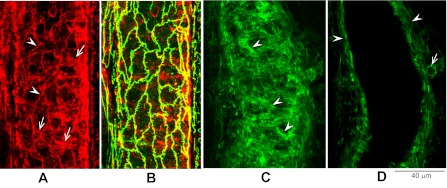
Fig. 4.
Endothelial and perivascular F-actin distribution in unstimulated day 4 vessels. A: a slight distortion of endothelial peripheral band of F-actin (arrowheads) from that observed in a day 1 vessel. Arrows indicate pericyte F-actin. B: overlap between endothelial F-actin (red shown in A) with VE-cadherin (green) upon double staining. C and D: another day 4 vessel with staining focused on perivascular cells. Pericyte F-actin appeared as disorganized loose bundles (arrowheads). D: a single image near the center plane of the vessel lumen, demonstrating extra layers of actin surrounding the vessel wall (arrowheads). The arrow indicates a migrating leukocyte. A stack of images is presented in the supplemental material, which is available with the on-line version of this article.
De novo appearance of stress fibers at PAF-induced Lp peak in day 4 vessels.
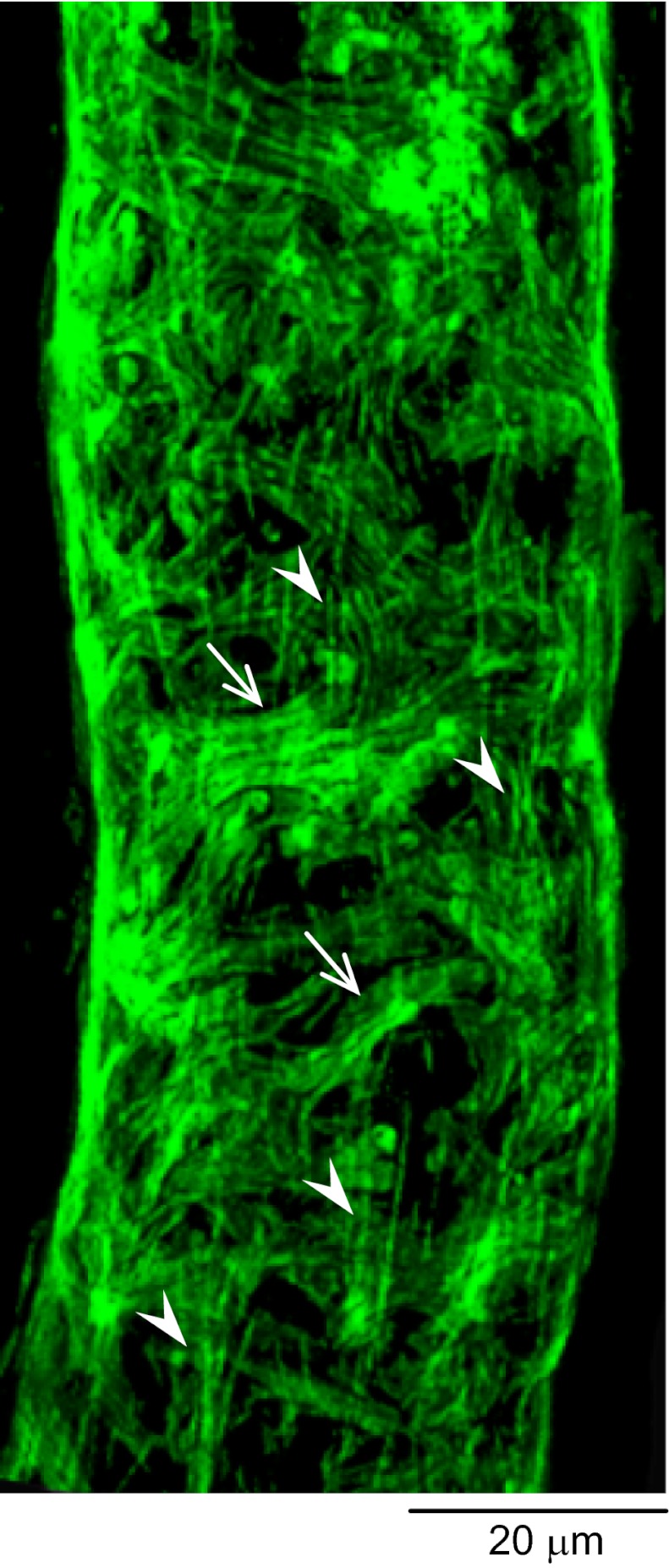
Actin-cytoskeleton assembly in response to PAF was investigated in eight microvessels. Both day 1 and day 4 vessels were fixed at the peak of PAF-induced increase in microvessel Lp. Figure 5 shows a representative image of the F-actin rearrangements in response to acutely applied PAF in a day 1 vessel. The confocal image shows cytoplasmic stress fiber formation and the disappearance of peripheral F-actin around endothelial cells and the formation of pericyte F-actin bundles. These F-actin responses correlated with a sevenfold Lp increase relative to control. The F-actin response to PAF was much more prominent in day 4 vessels than that observed on day 1. In addition to increased stress fibers in endothelial cells compared with day 1, the pericyte F-actin was aggregated, forming thick bundles. We also observed striking long stress fibers that extended beyond the pericyte layer. Some long stress fibers appeared to have traversed the cell body to connect adjacent cells within and between cell layers. No visible changes were found in mesothelial cells. Figure 6A demonstrates the F-actin distribution at different cell layers of the vessel wall through confocal optical sectioning in the Z-dimension. Figure 6C presents a reconstructed X-Z sectional image and a schematic illustration of the cell layers of the vessel wall. These cytoskeleton rearrangements in day 4 vessels correlated with a ninefold higher peak Lp response to PAF compared with that in day 1 vessels.
Fig. 5.
Platelet activating factor (PAF)-induced changes in endothelial and pericyte F-actin in a day 1 vessel. At PAF-induced peak hydraulic conductivity, endothelial peripheral F-actin disappeared and was replaced by radiating stress fibers (vertically oriented, arrowheads). Pericyte F-actin formed bundles (horizontally oriented, arrows).
Fig. 6.
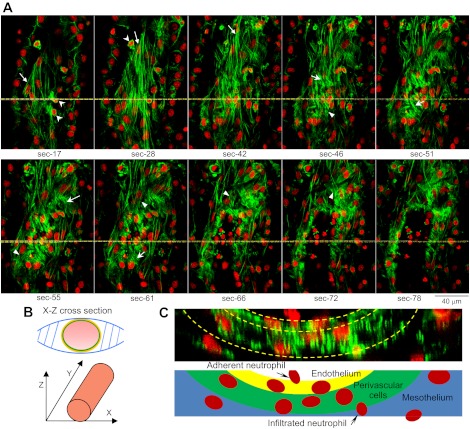
Platelet activating factor (PAF)-induced stress fiber formation in endothelial and perivascular cells in a day 4 vessel. The vessel was fixed at the PAF-induced peak hydraulic conductivity and double labeled for F-actin (phalloidin, green) and nuclei (DRAQ5, red). A: confocal images collected on the X-Y plane through sequential optical sectioning from the bottom (section 0) toward the center of the vessel with 0.2-μm vertical steps along the Z-axis. Selected individual images are presented (section number indicates image position). The vessel is slightly slanted, so different cell layers are visible within a single image. The long stress fibers with elongated nuclei in sections 17–42 (arrows) are at or beyond the pericyte layer and fit the characterization of fibroblasts/myofibroblasts. Three migrating leukocytes appeared in sections 17–28 (arrowheads). The pericyte actin formed short bundles (arrows in sections 46–61) and was different in appearance from fibroblasts. Endothelial stress fibers are indicated by arrowheads in sections 46–72. Some of the stress fibers appeared to have traversed the cell body connecting adjacent cells (sections 55–61, top). These changes correlated with a ninefold higher permeability response to PAF than the day 1 vessels. B: schematic drawing shows the image orientation in relation to vessel position. C: a reconstructed X-Z cross-sectional image from the image stack shown in A, illustrating a different view of the stress fiber distribution at different cell layers of the microvessel wall. The yellow lines on A indicate the cross-section position.
To investigate the potential functional roles of perivascular cells when endothelial barrier impaired and permeability is increased, we further examined the pericyte location in relation to the largely separated VE-cadherin at the PAF-induced Lp peak by dual staining of VE-cadherin and F-actin in day 4 vessels. Confocal images revealed that at the PAF-induced Lp peak, nearly all of the separated VE-cadherin regions were covered by the pericyte F-actin bundles. Images in Fig. 7 provide details.
Fig. 7.
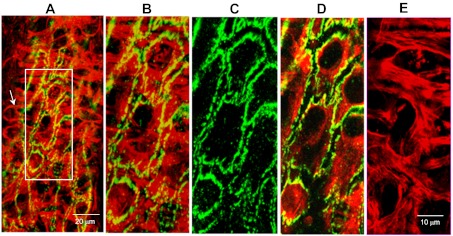
Confocal images of a day 4 vessel demonstrating the positioning of pericyte processes in relation to open endothelial junctions at the platelet activating factor-induced hydraulic conductivity peak. A: a partial projection of the lower half of the vessel wall with dual staining of F-actin (red) and VE-cadherin (green). An arrow indicates a typical pericyte structure: a single nucleus with multiple cellular processes. Details of a local region of the vessel wall (rectangular box) are displayed in B to E at higher magnification. B: a dual-channel image projection of both endothelial and pericyte layers demonstrating that all of the open VE-cadherin is covered by aggregated pericyte actin bundles. C: large separations of endothelial VE-cadherin. D: a dual-channel image projection at the endothelial cell layer showing the changes in endothelial F-actin and VE-cadherin. E: aggregated pericyte F-actin bundles (a projection of image sections below endothelial cells).
Remodeling-associated ultrastructural changes of vascular walls under basal and stimulated states.
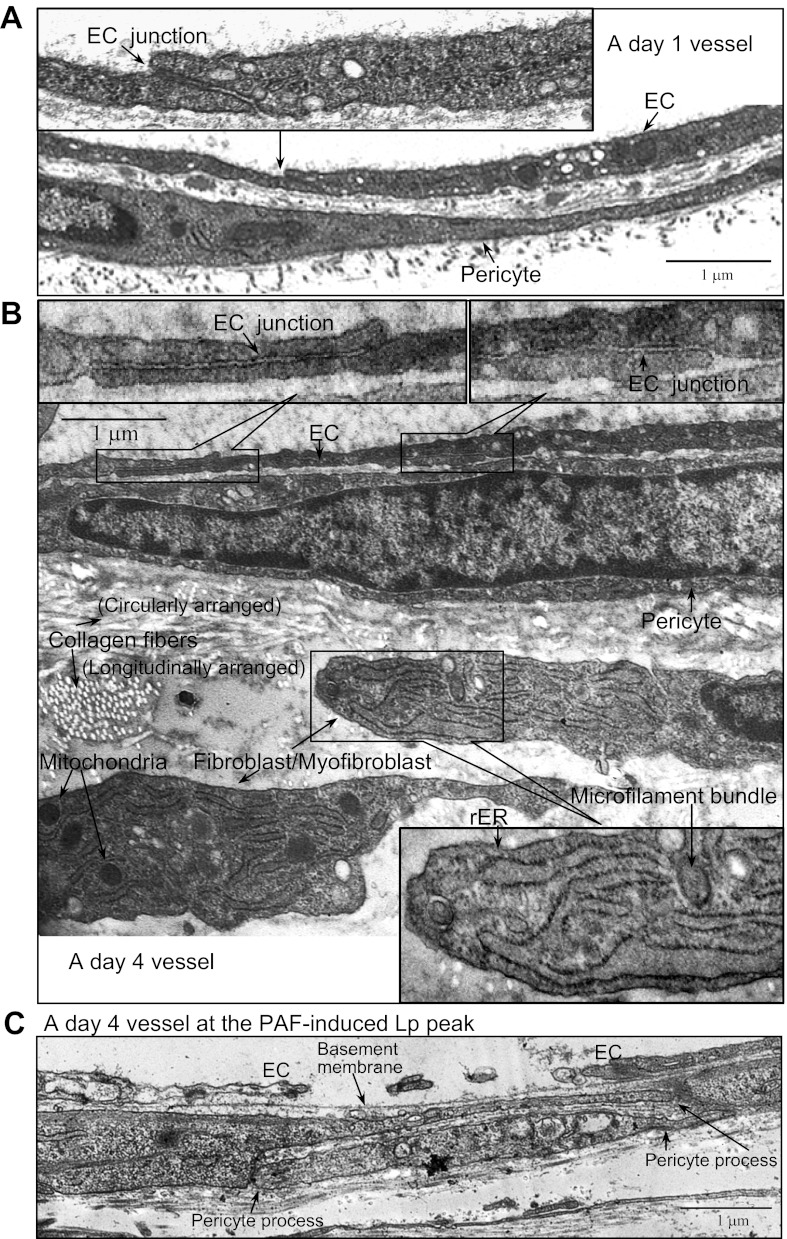
The ultrastructural changes in the vascular wall during the 3-day remodeling process were examined by electron microscopy in six vessels (10–15 sections per vessel segment) under basal conditions and at PAF-induced Lp peak (two vessels per group). Figure 8, A and B, shows representative micrographs of a day 1 and a day 4 vessel fixed during the Ringer-albumin perfusion, respectively. In general, the endothelial cells of the day 4 vessels appeared thinner than those observed in day 1 vessels, especially at junctional areas. However, the junctions between endothelial cells remain intact, which is consistent with the lack of significantly increased basal Lp measured in day 4 vessels. Compared with the day 1 vessel wall structure, the day 4 vessel wall showed markedly increased extracellular matrix, with filled collagen fibers in both longitudinal and circumferential arrangements. Most importantly, we observed an extra layer of cells adjacent to the pericytes having morphology distinct from either endothelial cells or pericytes. These cells showed an abundant rough endoplasmic reticulum, were rich in mitochondria, and contained cytoplasmic microfilaments, which are defining features of fibroblast or myofibroblast (12, 15). Figure 8C shows a representative micrograph demonstrating the ultrastructural changes between endothelial cells at the PAF-induced peak Lp on day 4 vessels. The gaps formed between adjacent endothelial cells were much larger than those observed in day 1 vessels (3, 21). However, despite the large gaps, the basement membrane remained intact. Most interestingly, the extended pericyte processes were found right beneath the opened endothelial gaps and the intact basement membrane.
Fig. 8.
Electron micrographs of rat mesenteric venules under control conditions and at platelet activating factor (PAF)-induced hydraulic conductivity (Lp) peak. Although intact junctions between endothelial cells (EC) are observed in both day 1 (A) and day 4 (B) vessels, the day 4 vessel EC junctional area is thinner than that in day 1. The day 4 vessel also shows significantly expanded perivascular components, demonstrating increased collagen fibers and additional layers of cells characteristic of fibroblast/myofibroblast. These cells are rich in mitochondria, have abundant rough endoplasmic reticulum (rER), and contain microfilament bundles, indicating active protein synthesis. Ultrastructural details can be seen in the inserted higher magnification images, which are the local regions indicated in micrographs A and B. C: structural changes in vascular wall at the PAF-induced Lp peak in a day 4 vessel displaying a wide-open endothelial gap with intact basement membrane. Two extended overlapping pericyte processes provide a complete coverage of the endothelial gap.
DISCUSSION
Our study demonstrated the phenotypic changes in both endothelial and perivascular cells and their impact on microvessel permeability in venules that have undergone remodeling. Our main new findings derived from these studies are that 3 days after the single vessel perfusion and PAF exposure, endothelial cells maintained their intact junctions and normal basal permeability in the absence of additional stimuli but exhibited augmented increases in endothelial [Ca2+]i and marked stress fiber formation upon PAF exposure resulting in widely separated VE-cadherin, largely formed endothelial gaps, and potentiated increases in microvessel permeability. In addition to the changes observed in endothelial cells, we also identified perivascular cell proliferation, expanded extracellular matrix, and increased vascular wall thickness. Most importantly, our study revealed the physical relationship between pericytes and the widely opened endothelial gaps at the Lp peak, suggesting important roles of pericytes not only in lessening a potentially higher degree of leakage in place of impaired endothelium, but also in stabilizing the microvessel wall during stimulation-induced cytoskeletal contractions. These observations might resemble the vascular changes at an early stage of inflammation or disease conditions.
Vascular remodeling and microvessel permeability.
Our study demonstrates the impact of vascular remodeling on microvessel permeability by direct comparison of basal permeability and the permeability responses to PAF before and after remodeling from the same vessel. Three days after exposure to PAF and surgical perturbation, despite notable changes in endothelial cell shape, vessel diameter, and abundant adherent leukocytes on the microvessel wall, the microvessels were able to maintain normal basal permeability. These results are consistent with those of the Evans blue measurements in tracheal microvasculature following airway infection in mice (13). The augmented increases in the endothelial [Ca2+]i and Lp when day 4 vessels were exposed to PAF indicate that the altered cellular phenotype at an early stage of vascular remodeling is highly susceptible to additional stimuli, and capable of resulting in exacerbated vascular dysfunction. Comparing day 4 vessels that were perfused with BSA-Ringer alone with vessels having additional PAF exposure on day 1, the basal Lp, vessel diameter enlargement, and leukocyte adhesion were similar, but the PAF-induced peak Lp was about 50% lower than vessels having PAF perfusion on day 1 (data not shown). Therefore, both surgical procedure and prior PAF exposure-induced inflammation contributes to the observed alterations.
Our previous studies in individually perfused intact microvessels demonstrated that the magnitude of agonist-induced peak endothelia [Ca2+]i determines the magnitude of permeability increases (17, 18). In permeabilized endothelial monolayers, the extent of the cell retraction and phosphorylation of myosin light chains were dependent on Ca2+ and agonist concentrations, which could account for the formation of gaps between adjacent cells (35). We consider that the enhanced endothelial Ca2+ response to PAF in day 4 vessels is the key signaling molecule that initiated the enhanced cytoskeleton contractile activity, as well as the increased rate and magnitude of the permeability response. The similar numbers of cross-sectional endothelial cell junctions shown by VE-cadherin staining in day 1 and day 4 vessels suggest that the enlarged existing endothelial cells are responsible for the augmented PAF responses in day 4 vessels. The wide separations of VE-cadherin between adjacent endothelial cells (Fig. 2B) observed in the entire day 4 vessel segment at peak response to PAF indicate that the enhanced permeability was not contributed by only a few large leaky sites. Instead, nearly all of the endothelial cells are involved, even though the magnitude of the changes may vary. The recovery of the largely separated VE-cadherin after the increased Lp was returned to the control level demonstrates that the dynamic changes in VE-cadherin distribution is directly correlated with the time course of PAF-induced increases in Lp. The intact basement membrane (Fig. 8) might be an important structural basis for the recovery of junctional proteins and the high-permeability state.
Adherent leukocytes are commonly observed in inflammation-induced remodeled microvessels (8, 13). Consistent with those observations, all our day 4 vessels showed a significant number of adherent leukocytes, indicating an upregulated expression of adhesion molecules on remodeled endothelial cells. However, in the presence of adherent and migrating leukocytes, there was no significant increase in the basal Lp measured on day 4 vessels or plasma leakage measured with Evans blue up to 14 days after infection of the mice (13). Dissociation of adherent and migrating leukocytes from permeability increases has been reported by multiple studies (16, 20, 25, 26, 38, 39). The mechanisms that initiate leukocyte adhesion have been demonstrated to be independent from those that trigger leukocyte oxidative burst, wherein the released reactive oxygen species are mainly responsible for increased permeability (16, 39). Remodeled microvessels with abundant adherent leukocytes without notable changes in VE-cadherin distribution, endothelial junction, and basal permeability further support this notion.
Structural and compositional changes of the remodeled vascular wall.
Our study observed similar enlargements in endothelial cells and vessel diameters to those reported by others (8, 13). The important new findings of this study are the alterations of endothelial junctions and the actin cytoskeleton arrangements in both endothelial and perivascular cells of the venular walls over a 3-day remodeling period. The less noticeable changes in endothelial VE-cadherin peripheral F-actin but marked changes in the perivascular cells under basal conditions suggest that pericytes are highly susceptible to inflammation and play an important role in the early stage of vascular remodeling. Cellular transformation to a myofibroblastic phenotype commonly occurs during wound healing and tissue injury (32). Pericytes have been indicated to function as progenitor cells capable of differentiating into a variety of cell types and synthesizing collagen during inflammation, injury repair, or angiogenesis (6, 11). In particular, PAF, normally released by activated leukocytes and endothelial cells during inflammation, has been reported to be able to act as a direct cytokine and stimulate lung pericyte growth in vitro (24). Our electron micrograph ultrastructurally revealed the compositional changes of extracellular matrix in remodeled microvascular walls. The identified perivascular myofibroblasts in day 4 vessels could be either phenotypically converted or differentiated from pericytes during the process of vascular wall remodeling. Matrix metalloproteinases have been implicated to play important roles in vascular remodeling, especially in arterial wall thickening and smooth muscle cell proliferation (14, 30). Although abundant adherent leukocytes did not increase basal permeability of day 4 vessels, we predict that the proteolytic enzymes released by leukocytes and inflamed vascular cells might play essential roles in initiating vascular wall remodeling.
Altered cell-cell adhesion and cytoskeleton contractile responses to PAF in vessels that have undergone remodeling.
Inflammation-associated permeability increases are dominated by the paracellular pathway, a result of gap formation between endothelial cells, and have been hypothesized to be regulated by the forces of cell-cell adhesion and the tension generated by actin-myosin interaction (9, 27, 28). Although this hypothesis has been supported by cultured cell studies, whether it applies to intact microvessels remains controversial (1–3, 9, 28, 33, 34). Some recent studies reported that the reduced adhesion at endothelial clefts could be sufficient to open local gaps and that the contractile mechanisms do not apply to increased permeability in intact microvessels (1–3, 9, 34). These conclusions were based on the results that both myosin light chain kinase (MLCK) and Rho-kinase inhibitors failed to inhibit the increased permeability in intact microvessels (1, 3). Although some central actin fibers have been identified during histamine exposure in an early in vivo study, they were not considered to be associated with increasing permeability (4). Even though studies using isolated vessel segments support the involvement of a contractile mechanism in increasing microvessel permeability, it was also solely based on the effects of the manipulating MLCK function using either ML-7 or peptide on increased permeability (36, 37). To our knowledge, stress fiber formation, the direct evidence of active myosin-actin interaction, so far has not been demonstrated to be directly involved in permeability increase in intact microvessels.
Our confocal images provided the first direct evidence of the presence of radiated stress fibers and the disappearance of endothelial peripheral bands of F-actin at the PAF-induced Lp peak in both day 1 and day 4 vessels. Unlike the cultured endothelial cells, the stress fibers were not present in the vessels under basal conditions, suggesting that they are not constitutively formed. The stress fiber formation and VE-cadherin redistribution identified at the PAF-induced Lp peak in intact venules suggest that both weakened adhesion between endothelial cells and the forces generated by the actin-myosin interaction contribute to increased permeability. The largely separated VE-cadherin at the PAF-induced Lp peak in day 4 vessels is a different pattern from the frequent breaks observed in day 1 vessels. The de novo appearance of the stress fibers displayed at the peak Lp in day 4 vessels resembled stress fiber manifestation that is usually found in cultured endothelial cells and often associated with a proinflammatory phenotype (9). These observations suggest that the endothelial contractile machinery is upregulated when vessels undergo remodeling and phenotypic alterations. With enhanced endothelial Ca2+ responses to PAF, the greater tensions generated by the upregulated endothelial contraction may play a significant role in the large endothelial gap formation and enhanced permeability response to PAF.
Roles of perivascular cells in increased microvessel permeability of day 4 vessels.
PAF-induced transient increase in microvessel permeability in normal vessels has been demonstrated to be directly correlated with the dynamic opening and closing of endothelial gaps (21), indicating that the endothelium is the crucial barrier for fluid and macromolecule transport. The widely separated VE-cadherin observed throughout the day 4 vessel segment together with the ultrastructural evidence of the formation of large gaps between the endothelial cells suggest that the PAF-induced augmented permeability increase involved the opening of all endothelial clefts along the vessel wall. However, based on the calculation by Clough and Michel (7), if fluid filtration is proportional to the third power of the width of the cleft (Poiseuille's law for a parallel-walled slit), a gap that is 10 times wider than a normal cleft may result in a 1,000-fold increase in Lp. Then the widely formed gaps in all endothelial junctions would predict a much greater magnitude of Lp increase than the measured value. This disparity strongly suggests that other components of the vascular wall might play a role that lessens a greater magnitude of leakage during inflammatory exposure.
Currently, the roles of perivascular cells in microvessel permeability, especially under stimulated conditions, remain to be determined. Based on the highly contractile nature of pericytes, some investigators consider pericyte contraction to be responsible for the formation of interendothelial gaps (22). In contrast, some early in vivo studies suggest that pericytes tend to be localized over endothelial gaps during histamine treatment, therefore protecting junctions from being overstretched (29). The formation of a pericyte F-actin bundle at the peak Lp indicates the contraction or mobilization of pericytes upon PAF stimulation in intact microvessels. Most importantly, the complete coverage of largely separated VE-cadherin and open endothelial gaps by pericyte F-actin bundles and pericyte processes at the Lp peak as demonstrated by confocal images and ultrastructural evidence suggest that perivascular cells, instead of promoting endothelial gap formation, play an important role in lessening a potentially much higher degree of leakage while the endothelial barrier is impaired. This may also explain the wide separations in endothelial adhesion with less than expected Lp increase upon PAF exposure in day 4 vessels. The long stress fibers from proliferated fibroblasts/myofibroblasts could be crucial in protecting the structural integrity of the vascular wall during the stimulation-induced endothelial contraction with significantly weakened adhesion force.
In summary, our study illustrated the remodeling-associated changes in cell-cell adhesion and cytoskeleton reactions to inflammatory stimuli with correlative permeability measurements in intact microvessels. The stress fiber formation revealed at the peak of PAF-induced permeability increases in both day 1 and day 4 vessels provides the first direct evidence that contractile mechanisms contribute to the increased permeability in intact microvessels and that the enhanced Ca2+ responses to stimuli with upregulated contractile machinery of remodeled endothelial cells are responsible for the potentiated permeability response. The proliferated perivascular cells and expanded extracellular matrix might serve as secondary barriers that lessen a potentially higher degree of leakage while serving as the supporting structure to stabilize the microvessel wall during stimulation-induced cytoskeleton contractions. This study provides a structural basis for the future investigation of the interdependent roles of endothelial cells and perivascular cells in the regulation of microvessel permeability in normal and remodeled microvessels and the involved signaling pathways.
GRANTS
This study was supported by the National Heart, Lung, and Blood Institute, Grants HL56237 and HL084338.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.Y. performed experiments; D.Y. and P.H. analyzed data; D.Y. and P.H. prepared figures; D.Y. and P.H. drafted manuscript; P.H. conception and design of research; P.H. interpreted results of experiments; P.H. edited and revised manuscript; P.H. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Bernard Schreur for his contribution to the electron micrographs, technical assistance from Paul Harton and Xueping Zhou, and Dr. Christian Stork for proofreading the manuscript.
REFERENCES
- 1. Adamson RH, Curry FE, Adamson G, Liu B, Jiang Y, Aktories K, Barth H, Daigeler A, Golenhofen N, Ness W, Drenckhahn D. Rho and rho kinase modulation of barrier properties: cultured endothelial cells and intact microvessels of rats and mice. J Physiol 539: 295–308, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adamson RH, Ly JC, Sarai RK, Lenz JF, Altangerel A, Drenckhahn D, Curry FE. Epac/Rap1 pathway regulates microvascular hyperpermeability induced by PAF in rat mesentery. Am J Physiol Heart Circ Physiol 294: H1188–H1196, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Adamson RH, Zeng M, Adamson GN, Lenz JF, Curry FE. PAF- and bradykinin-induced hyperpermeability of rat venules is independent of actin-myosin contraction. Am J Physiol Heart Circ Physiol 285: H406–H417, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Baldwin AL, Thurston G. Changes in endothelial actin cytoskeleton in venules with time after histamine treatment. Am J Physiol Heart Circ Physiol 269: H1528–H1537, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Baluk P, Bowden JJ, Lefevre PM, McDonald DM. Upregulation of substance P receptors in angiogenesis associated with chronic airway inflammation in rats. Am J Physiol Lung Cell Mol Physiol 273: L565–L571, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Canfield AE, Doherty MJ, Wood AC, Farrington C, Ashton B, Begum N, Harvey B, Poole A, Grant ME, Boot-Handford RP. Role of pericytes in vascular calcification: a review. Z Kardiol 89 Suppl 2: 20–27, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Clough G, Michel CC. Quantitative comparisons of hydraulic permeability and endothelial intercellular cleft dimensions in single frog capillaries. J Physiol 405: 563–576, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Curry FE, Zeng M, Adamson RH. Thrombin increases permeability only in venules exposed to inflammatory conditions. Am J Physiol Heart Circ Physiol 285: H2446–H2453, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Curry FR, Adamson RH. Vascular permeability modulation at the cell, microvessel, or whole organ level: towards closing gaps in our knowledge. Cardiovasc Res 87: 218–229, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Curry PE, Huxley VH, Sarelius IH. Techniques in microcirculation: measurement of permeability, pressure and flow. In: Cardiovascular Physiology. Techniques in the Life Sciences. New York: Elsevier, 1983, p. 1–34 [Google Scholar]
- 11. Diaz-Flores L, Gutierrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martin-Vasallo P, Diaz-Flores L., Jr Pericytes morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol 24: 909–969, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Eyden B. The myofibroblast: an assessment of controversial issues and a definition useful in diagnosis and research. Ultrastruct Pathol 25: 39–50, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Ezaki T, Baluk P, Thurston G, La Barbara A, Woo C, McDonald DM. Time course of endothelial cell proliferation and microvascular remodeling in chronic inflammation. Am J Pathol 158: 2043–2055, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res 90: 251–262, 2002 [PubMed] [Google Scholar]
- 15. Gandorfer A, Rohleder M, Charteris D, Kampik A, Luthert P. Ultrastructure of vitreomacular traction syndrome associated with persistent hyaloid artery. Eye (Lond) 19: 333–336, 2005 [DOI] [PubMed] [Google Scholar]
- 16. He P. Leucocyte/endothelium interactions and microvessel permeability: coupled or uncoupled? Cardiovasc Res 87: 281–290, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. He P, Curry FE. Depolarization modulates endothelial cell calcium influx and microvessel permeability. Am J Physiol Heart Circ Physiol 261: H1246–H1254, 1991 [DOI] [PubMed] [Google Scholar]
- 18. He P, Curry FE. Endothelial cell hyperpolarization increases [Ca2+]i and venular microvessel permeability. J Appl Physiol 76: 2288–2297, 1994 [DOI] [PubMed] [Google Scholar]
- 19. He P, Zhang X, Curry FE. Ca2+ entry through conductive pathway modulates receptor-mediated increase in microvessel permeability. Am J Physiol Heart Circ Physiol 271: H2377–H2387, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Hurley JV. Acute inflammation: The effect of concurrent leucocytic emigration and increased vascular permeability on particle retention by the vascular wall. Br J Exp Pathol 45: 627–633, 1964 [PMC free article] [PubMed] [Google Scholar]
- 21. Jiang Y, Wen K, Zhou X, Schwegler-Berry D, Castranova V, He P. Three-dimensional localization and quantification of PAF-induced gap formation in intact venular microvessels. Am J Physiol Heart Circ Physiol 295: H898–H906, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kelley C, D'Amore P, Hechtman HB, Shepro D. Vasoactive hormones and cAMP affect pericyte contraction and stress fibres in vitro. J Muscle Res Cell Motil 9: 184–194, 1988 [DOI] [PubMed] [Google Scholar]
- 23. Kendall S, Michel CC. The measurement of permeability in single rat venules using the red cell microperfusion technique. Exp Physiol 80: 359–372, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Khoury J, Langleben D. Platelet-activating factor stimulates lung pericyte growth in vitro. Am J Physiol Lung Cell Mol Physiol 270: L298–L304, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Kim MH, Curry FR, Simon SI. Dynamics of neutrophil extravasation and vascular permeability are uncoupled during aseptic cutaneous wounding. Am J Physiol Cell Physiol 296: C848–C856, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McDonald DM. Endothelial gaps and permeability of venules in rat tracheas exposed to inflammatory stimuli. Am J Physiol Lung Cell Mol Physiol 266: L61–L83, 1994 [DOI] [PubMed] [Google Scholar]
- 27. Michel CC, Curry FE. Microvascular permeability. Physiol Rev 79: 703–761, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Shen Q, Rigor RR, Pivetti CD, Wu MH, Yuan SY. Myosin light chain kinase in microvascular endothelial barrier function. Cardiovasc Res 87: 272–280, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sims DE, Miller FN, Donald A, Perricone MA. Ultrastructure of pericytes in early stages of histamine-induced inflammation. J Morphol 206: 333–342, 1990 [DOI] [PubMed] [Google Scholar]
- 30. Steed MM, Tyagi SC. Mechanisms of cardiovascular remodeling in hyperhomocysteinemia. Antioxid Redox Signal 15: 1927–1943, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thurston G, Murphy TJ, Baluk P, Lindsey JR, McDonald DM. Angiogenesis in mice with chronic airway inflammation: strain-dependent differences. Am J Pathol 153: 1099–1112, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3: 349–363, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Valeski JE, Baldwin AL. Role of the actin cytoskeleton in regulating endothelial permeability in venules. Microcirculation 10: 411–420, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Waschke J, Drenckhahn D, Adamson RH, Curry FE. Role of adhesion and contraction in Rac 1-regulated endothelial barrier function in vivo and in vitro. Am J Physiol Heart Circ Physiol 287: H704–H711, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Wysolmerski RB, Lagunoff D. Involvement of myosin light-chain kinase in endothelial cell retraction. Proc Natl Acad Sci USA 87: 16–20, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yuan SY, Wu MH, Ustinova EE, Guo M, Tinsley JH, De Lanerolle P, Xu W. Myosin light chain phosphorylation in neutrophil-stimulated coronary microvascular leakage. Circ Res 90: 1214–1221, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Yuan Y, Huang Q, Wu HM. Myosin light chain phosphorylation: modulation of basal and agonist-stimulated venular permeability. Am J Physiol Heart Circ Physiol 272: H1437–H1443, 1997 [DOI] [PubMed] [Google Scholar]
- 38. Zeng M, Zhang H, Lowell C, He P. Tumor necrosis factor-alpha-induced leukocyte adhesion and microvessel permeability. Am J Physiol Heart Circ Physiol 283: H2420–H2430, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Zhu L, He P. fMLP-stimulated release of reactive oxygen species from adherent leukocytes increases microvessel permeability. Am J Physiol Heart Circ Physiol 290: H365–H372, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Zhu L, He P. Platelet-activating factor increases endothelial [Ca2+]i and NO production in individually perfused intact microvessels. Am J Physiol Heart Circ Physiol 288: H2869–H2877, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.