Abstract
Myelodysplastic syndromes are associated with a risk of severe infections. While neutropenia is likely to be the main predisposing factor, several other immune defects have been reported, including impaired neutrophil function, B-, T- and NK-cell defects and the possible consequences of iron overload due to red blood cell transfusions. The advanced age of most patients, their frequent comorbidities, and the fact that drugs such as hypomethylating agents and lenalidomide, which are effective in myelodysplastic syndromes but can transiently worsen neutropenia, may increase the risk of infection and their severity in this context. The majority of infections in myelodysplastic syndromes are bacterial, while the incidence of fungal infections is not well known and viral infections seem to be rare. No prophylactic measures against infections have demonstrated efficacy in myelodysplastic syndromes. However, pending more data, we propose here some recommendations for the management of patients with myelodysplastic syndromes. In the future, an important contribution can be made by prospective trials testing the efficacy of prophylactic and therapeutic approaches to infection in these patients, especially in the context of the new drugs available for myelodysplastic syndromes.
Key words: myelodysplastic syndromes, infection, bacterial, prophylaxis, therapy
Introduction
Myelodysplastic syndromes (MDS) are clonal disorders of hematopoietic stem cells and are mostly observed in elderly patients. They are characterized by ineffective hematopoiesis resulting in blood cytopenias and by a high risk of progression to acute myeloid leukemia (AML).1 MDS may be triggered by previous chemotherapy (especially using alkylating agents), radiotherapy or by exposure to benzene derivatives, but they are mainly age-related. The frequency of MDS is approximately 4×103 per 100,000, being the third hematologic malignancy after non-Hodgkin's lymphoma and multiple myeloma. Up to the last decade, due to the lack of effective therapies and to the advanced age of many MDS patients, supportive care was the only therapeutic option proposed. Prognosis of MDS is routinely assessed by an international prognostic scoring system (IPSS), based on the number of blood cytopenias, the percentage of marrow blasts, and on karyotype.2 This system distinguishes between 4 groups of patients with MDS: low-, intermediate- 1, intermediate-2, and high-risk MDS. Patients with low-and intermediate-1 IPSS risk are often grouped into the category of “lower risk MDS”, and typically show a relatively low risk of progression to AML and prolonged survival. Patients with intermediate-2 and high IPSS risk are generally grouped as “higher risk MDS”, and often progress to AML and have a short survival.
Treatment of patients with lower risk MDS mainly aims at correcting cytopenias, especially anemia (which is generally the predominant or only cytopenia), using erythropoiesis-stimulating agents or, in the case of patients with lower risk MDS and chromosome 5q deletion (del 5q), lenalidomide. In patients with higher risk MDS, treatments aimed at modifying the disease are generally proposed, especially the hypomethylating agents azacitidine or decitabine which have recently been shown to improve survival in this category of patients.3,4 Less often, chemotherapy may also be proposed for these patients. Allogeneic hematopoietic stem cell transplant (HCT) remains the only curative approach for MDS, but is restricted to relatively younger patients (generally under 65 years of age) with an HLA-identical donor.5
Infection has long been recognized as a cause of morbidity and mortality in MDS, and has been attributed mainly to quantitative and qualitative defects of neutrophils.6-8 Until recently, and before the era of hypomethylating agents, in the absence of effective therapies, the long duration of the disease and the advanced age of the patients did not encourage the design of strategies aimed at reducing infectious risk in MDS. More effective treatments able to correct cytopenias, and especially neutropenia, have recently become available, although there is often a transient worsening of these complications. This has proved to be an incentive to review published data on infections in MDS, with the aim of proposing therapeutic strategies adapted to deal with these and making recommendations for the design of future prospective trials.
Morbidity and mortality due to infection in myelodysplastic syndromes
Relatively few precise data are available on the incidence and respective bacterial, fungal, and viral causes of infection in MDS. Most of these data are retrospective. Some are compromised by inconsistencies in the definition of the infectious events or, although taken from therapeutic trials, may be biased by patient eligibility criteria. In a recent US retrospective series of 273 untreated low-or intermediate-1 risk MDS patients who died in the period from 1980 to 2004, infection was the primary cause of death, accounting for 38% of the total, followed by AML transformation (15%) and hemorrhage (13%).9 Pneumonia was the most common infection, responsible for 40% of infectious deaths. Infection, mostly of a bacterial origin, was microbiologically documented in 30% of the cases of pneumonia in these MDS patients. As this study covered three decades, it was possible to show a significant decrease in the incidence of infectious deaths over time, likely attributable to improved supportive care. Another US survey of the Medicare population (an insurance covering more than 97% of all US citizens aged 65 years or over) indicated that, in a population of 1.3 million people over the age of 65 years followed between the years 2003 and 2005, patients with MDS had a higher prevalence of infections than the non-MDS Medicare population (22.5% vs. 6.1%; P<0.001). The difference was most pronounced in patients with MDS who had received transfusions, but there was also a clear difference for patients with MDS with various comorbidities, including diabetes, dyspnea, and hepatic diseases.10 This suggests that concomitant occurrence of multiple comorbidities, including those cases in which iron overload may be one of the causal factors, could contribute to infection in MDS.
Since hypomethylating agents have become a reference treatment in MDS, we have examined infectious morbidity and mortality in the main published prospective or observational studies using these agents (Table 1). The highly variable rate of infection of the patients studied, ranging from 2.7%14 to 57%,16 is probably largely accounted for by the variable proportion of patients with higher risk MDS, who are more prone to infection than patients with lower risk MDS. Again, most of these infections were accounted for by pneumonia, and were mostly considered to be of a bacterial origin, with a significant proportion of them occurring during neutropenic phases (i.e. febrile neutropenia).
Table 1.
Incidence of infectious complications, and of infectious deaths in the larger prospective or observational trials using hypomethylating agents in myelodysplastic syndromes.
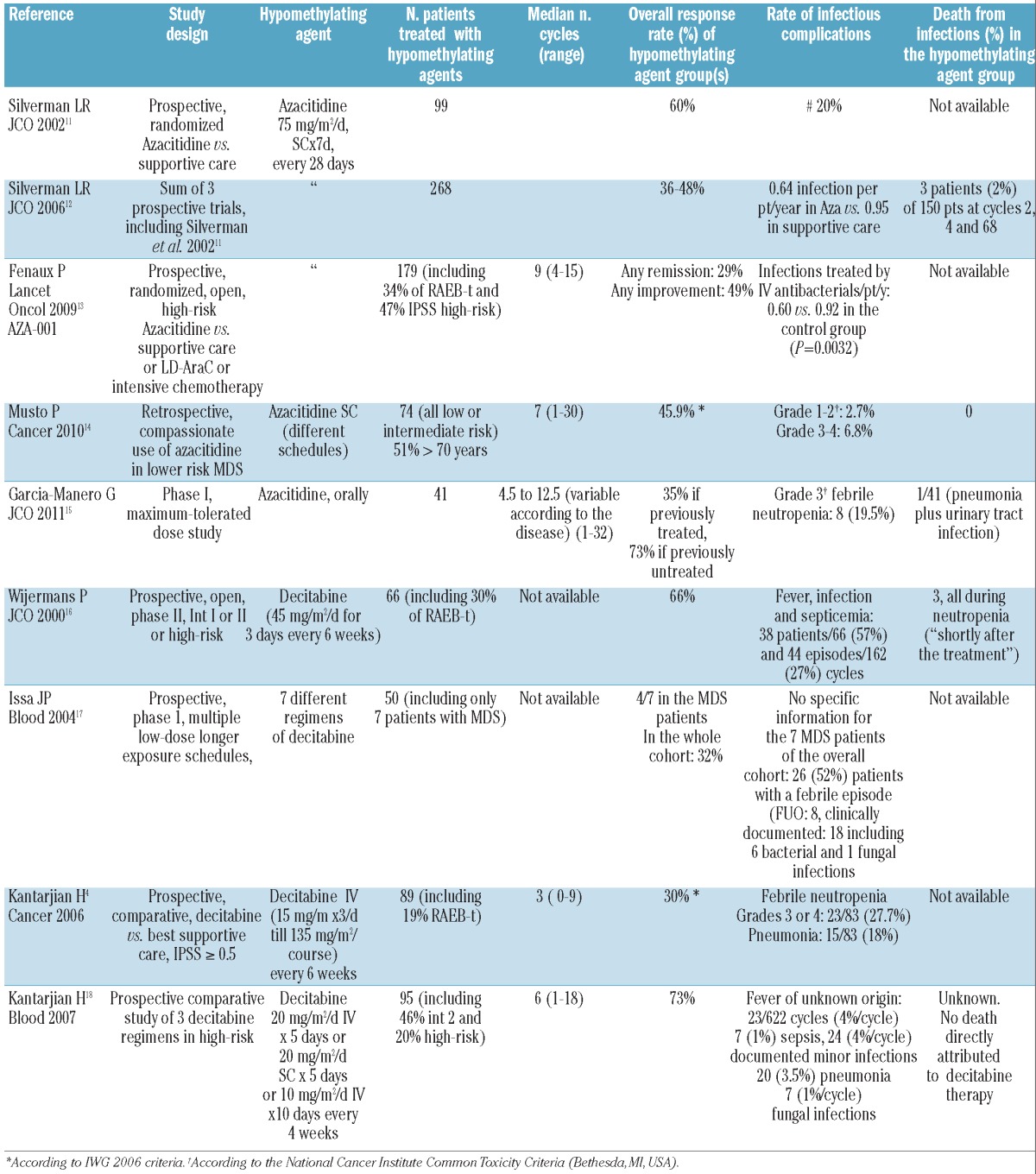
Similar variability was seen when considering infection as a cause of death, varying from 2%12 to 32%.19 This was probably due to the selection of patients in prospective trials in the former study, whereas the latter study was made up of patients in compassionate-use programs, many of whom would have been excluded from prospective trials.
Neutropenia, altered neutrophil functions and other spontaneous immune disorders predisposing to infections in myelodysplastic syndromes
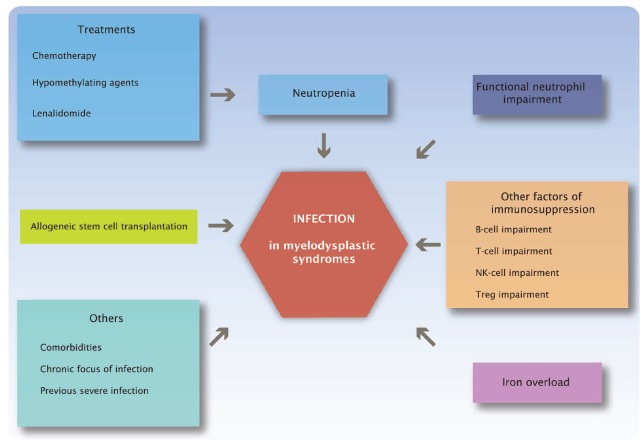
Neutropenia (spontaneous or transiently worsened by treatment) likely represents the major reason for increased risk of infection in MDS. However, qualitative neutrophil defects, other less well-known immune disorders and, in some situations, iron overload could also contribute to the risk of infection (Figure 1).
Figure 1.
Main risk factors of infection in myelodysplastic syndromes.
Neutropenia
Neutropenia occurs in nearly 50% of newly diagnosed patients with MDS, including 70-80% of higher-risk MDS patients, and 15-20% of lower-risk MDS patients. In advanced MDS, neutropenia is part of a more general process of bone marrow failure combining impaired differentiation, apoptosis resistance, and leukemic proliferation.20,21 In lower risk MDS, it appears to result mainly, like other cytopenias, from accelerated apoptosis of hematopoietic progenitor cells at all stages of maturation.22-24 Mechanisms underlying this increased apoptosis include activation of cell-death receptors and direct activation of mitochondrial apoptosis. While these apoptotic mechanisms have mainly been illustrated for the erythroid lineage, 25-29 few studies have focused on the accelerated neutrophil apoptosis which may explain neutropenia in early-stage MDS.30,31
Immune mechanisms may also be operative in some patients through a T-cell mediated inhibition of hematopoiesis or of autologous granulocytes. This T-cell mediated mechanism is probably associated with the observed increase in levels of plasma TNF-alpha and IFN-gamma, and is potentially reversible through administration of immunosuppressive treatment.32-35
However, while neutropenia is considered to be the main cause of infection in MDS, absolute neutrophil counts (ANC) were not found to relate to survival rates following infectious episodes, at least in one historical series of MDS patients in different risk categories, suggesting that other defects may also be important.6
Functional neutrophil impairment
Functional neutrophil defects, often associated with morphological abnormalities of neutrophils,36 include a marked reduction in phagocytosis and production of oxygen intermediates, 37 and a decrease in bactericidal and fungicidal activities,38 production of superoxide anions,39 and expression of CD11b, L-selectin, LFA1, and CD18 leading to defective granulocyte locomotion.40-42 MDS patients also have deficiencies in the contents of neutrophil granules, including quantitative and/or functional anomalies of myeloperoxidase, lactoferrin and antibiotic proteases such as elastase and cathepsin G,43-46 deficiencies in granule membrane glycoproteins,44 and matrix metalloproteinase dysfunction.47-49 Impaired activity of granule proteases in neutrophils, which can induce tissue injury through an inflammatory-mediated process, is potentially implicated in the increased susceptibility to infections even in the absence of neutropenia.50-52 However, these defects have still not been well defined in clinical studies.
Other spontaneous immune disorders potentially predisposing to infection independently of neutrophil impairment
B-cell and antibody production impairment
The impact of B-cell impairment in MDS on infectious risk has not been well defined. Absolute numbers of peripheral B cells were found to be reduced in patients with MDS compared to controls.53 Autoreactive B cells with or without detectable serum autoantibodies were observed.54 Furthermore, hypergammaglobulinemia was found in 39% and hypogammaglobulinemia in 8% of patients with MDS.54,55 Increased apoptosis of medullary B cells has also been described.56
T-cell impairment
Most patients with MDS have lymphocytopenia, mainly due to a decrease in T-helper lymphocyte counts.53,57 The balance between CD4+ TH1 cells and TH2 cells was found to be altered in patients with MDS, with a decrease in the number of TH1 cells and a decreased TH1:TH2 ratio.58,59 An increased proportion of CD8+ T cells, mainly of cytotoxic CD8+CD28− T cells, was observed.60 Whether such inbalances affect the risk of infections is unknown.
Anomalies of regulatory T (Treg) cells have also been described in patients with MDS, such as a decreased number of Treg cells in high-risk MDS, and impaired Treg function, despite a normal absolute count in low-risk MDS.61 The number of CD4+ T cells producing IL17 (TH17 cells) is higher in low-risk MDS compared to high-risk MDS, and inversely correlates with the number of Treg cells. The secretion of IFN gamma by the effector T cells is inhibited by Tregs, without modification of the secretion of IL17. The level of several inhibitory factors including IL2-receptor and IL10 are decreased in low-risk MDS.62
NK-cell impairment
The reduced expression of NK receptors such as NKG2D may contribute to the impairment of cytolytic function of NK cells in patients with MDS63,64 which persists after administration of activating cytokines such as IL-2.63 Furthermore, NK cells from patients with MDS show decreased levels of IL-32, which could be associated with the lower cytotoxic potential of NK cells observed in these patients.65
Role of iron overload in the risk of infection
Iron overload is common in MDS, mainly due to chronic red cell transfusions and, to a lesser extent, to increased gut absorption of iron as a consequence of ineffective erythropoiesis.66,67 Iron overload may increase the risk of infections68 through at least two mechanisms. First, this may be through a direct effect of free iron on bacterial and fungal growth.69,70 Some pathogens, such as Yersinia enterolitica,Y. pseudotuberculosis, or Legionella pneumophilia, have such an impaired ability to acquire iron that they may obtain the iron essential for their growth from their host; this would be dangerous mainly in hosts with excess iron loads.71 Second, excess free iron impairs the natural resistance to infection, through complex mechanisms including inhibition of IFN-gamma, TNF-alpha, IL-12, nitric oxide formation, and impairment of macrophage, neutrophil, and T-cell functions.68,72,73
However, there are no data evaluating the incidence of infectious episodes in patients with MDS with iron overload and who did not receive an allogeneic HCT. Likewise, the potential benefit of iron chelation for reducing the risk of infection in MDS patients, although suggested by retrospective studies, has not been demonstrated prospectively. A possible predisposing role of iron overload in infectious risk in MDS has not yet been documented outside the transplant setting.
The role of iron overload on the risk of infection has been more clearly demonstrated following allogeneic HCT, which remains the only curative treatment in MDS patients, even though it can only be performed in approximately 15% of cases. In a retrospective study of 190 recipients of myeloablative allogeneic HCT,74 elevated pre-transplant ferritin blood levels were associated with an increased incidence of bacteremia (60% vs. 44%; P=0.042). A prospective study in the early phase of allogeneic HCT also showed that non-transferrin-bound iron (NTBI) facilitates the growth of Staphylococcus epidermidis, and that the administration of plasma apotransferrin, by decreasing NTBI, can restore the inhibitory effect of patient's serum on this bacterial growth.75 Given that myeloablative chemotherapy may rapidly increase the serum levels of NTBI,76 chemotherapy could increase the risk of bacterial infection within the first few days of administration through its effect on iron metabolism, independently of a secondary decrease in ANC.67
Iron overload has also been associated with the risk of invasive fungal infections after allogeneic HCT.77-80 Finally, increasing infectious risk may be one of the mechanisms whereby iron overload negatively impacts on the prognosis of allogeneic HCT in patients with MDS.74,81,82
Whether iron chelation could decrease the risk of infection in MDS has not been demonstrated. Deferasirox and deferiprone decrease the levels of labile plasma iron (LPI), making these drugs possible candidates for dealing with this.83 On the other hand, deferoxamine acts as a siderophore to promote the growth of mucormycosis.84 Cases of mucormycosis have been reported in MDS patients and some, but not all, of these patients are known to have been treated with deferoxamine.85-87
Role of therapy in infectious susceptibility of myelodysplastic syndrome patients
In addition to the risk of infection posed by MDS itself, therapy may increase that risk, at least transiently until a hematologic response is achieved. High-dose chemotherapy is still administered to patients with higher risk MDS, especially in cases of transformation in AML, or in order to reduce the tumor load before allogeneic HCT. Hypomethylating agents and lenalidomide are being increasingly administered in specific conditions of MDS. The extensive experience with such drugs allows the merits of their use to be discussed separately for MDS patients in different risk categories. Allogeneic HCT represents a very specific condition in which the infectious risk is well-known and mostly anticipated.88,89 We review below whether MDS patients who are transplanted have additional risk factors for infection, when compared to other candidates for HCT, which may call for a specific approach.
High-dose chemotherapy
Due to the availability of hypomethylating agents, high-dose chemotherapy is used less now than it was ten years ago. When patients with advanced MDS were treated with high-dose chemotherapy, they were often analyzed together with de novo AML patients receiving the same regimen, with regards to chemotherapy90,91 or anti-infective drug trials.92-95 The infectious risk associated with chemotherapy-induced neutropenia in advanced MDS and de novo AML could not, therefore, be compared. However, the incidence of infectious events in advanced MDS patients treated with high-dose chemotherapy is probably comparable to that in AML patients of a similar age treated with the same regimens and experiencing comparable or longer duration of deep neutropenia.15
Hypomethylating agents
Several large trials performed in patients with high-risk MDS show that hypomethylating agents improve hematologic status4,13 and, in the case of azacitidine, overall survival.13 However, the effects of hypomethylating agents on the risk of infection seem poorly documented and heterogeneous. These agents usually worsen pre-existing cytopenias, including neutropenia, with nadir values of ANC occurring usually during the second to third weeks of each cycle.12,96 On the other hand, azacitidine has been shown to decrease the rate of infections when compared to supportive care or low-dose cytosine-arabinoside (LD-AraC).12,13,97 This is probably due to the fact that, in responders, neutropenia is less severe or resolved beginning with the third or fourth cycle of azacytidine, whereas neutropenia persists in patients receiving supportive care. In addition, (LD-AraC) appears to be more myelosuppressive than azacitidine.97On the other hand, in a randomized trial comparing decitabine and best supportive care, grade 3-4 febrile neutropenia was observed more frequently with decitabine.4 In a recent retrospective study of 82 high-risk MDS patients and 16 AML patients treated with azacitidine, multivariate analysis showed that the occurrence of infections during azacitidine therapy, but not neutropenia or age, was significantly associated with transfusion dependency prior to the first cycle and to platelet counts of less than 20×109/L prior to each cycle.98
Lenalidomide
Lenalidomide has been approved in the US and several other countries for the treatment of anemia in patients associating lower-risk MDS and del 5q.99 It also has some activity in high-risk patients with del 5q100 as well as in patients with lower-risk MDS with karyotypes other than del 5q.101 Grade 3-4 neutropenia is, along with thrombocytopenia, a major side-effect of lenalidomide, particularly in lower-risk MDS with del 5q where it was seen in 55%99 to 75%102 of patients. Induction of profound neutropenia (along with thrombocytopenia) was even found to be a favorable prognostic factor of subsequent erythroid response in the pivotal MDS 003 trial.99 Although febrile neutropenia was reported in only 1%102 to 4%99 of the patients, death associated with neutropenia occurred in 3 of the 146 patients included in the MDS 003 trial, and 2 of the 95 patients included in a French compassionate-use program of lenalidomide in lower-risk MDS with del 5q.103 Further use of lenalidomide may have improved the management of the risk of severe neutropenia and may have prevented deaths associated with neutropenia in del 5q low-risk MDS patients; a recent study showed that such patients had an overall poor prognosis.102
In uncommon indications of lenalidomide, such as high-risk patients with del 5q,100 or low-risk patients without del 5q,101 grade 3-4 neutropenia varied from 28%101 to 79% in patients with a baseline ANC count of less than 1.0×109/L.100 In high-risk patients, the worsening of baseline neutropenia by lenalidomide therapy may be a main side effect of the drug, leading to septic deaths.100
Allogeneic stem cell transplantation
Infection following allogeneic HCT to treat MDS is a well-recognized cause of death in MDS, accounting for 53% of overall mortality in a series of 109 patients.104 Like in acute leukemia patients, this risk was increased by previous infections and use of antibacterials leading to colonization and antibacterial resistance. However, available literature data do not indicate whether patients with MDS are at higher risk of severe infection than allogeneic HCT recipients transplanted for other diseases, and whether this justifies specific measures being taken in MDS patients. However, MDS patients are on average among the oldest patients referred for HCT, and this may explain a higher risk of post-HCT infection, especially of fungal origin, when compared to other patients with different underlying diseases.105,106 In addition, as mentioned above, iron overload increases the risk of both bacterial74,75 and fungal77-80 infections after HCT, and this may play a role in MDS patients who often experience iron overload during RBC transfusions. Finally, in a retrospective cohort of 291 consecutive patients transplanted for MDS, pre-transplant neutropenia (ANC<1.5×109/L) was associated with an increased infection-related mortality at three years post transplant (26% vs. 12.3%) mainly due to a more frequent occurrence of Gram positive bacterial and fungal infections.107 Therefore, MDS patients may be at particularly high risk of infections after allogeneic HCT, possibly justifying reinforced surveillance and prophylactic programs in this patient population.
Are specific infections observed in myelodysplastic syndromes?
Infection has not been a primary end point in prospective studies in MDS, and no detailed epidemiological data on infections in large cohorts of MDS patients were found. However, recent studies raise the possibility that specific infections are observed. We review the evidence for some of these, including common and uncommon bacterial infections, fungal diseases, as well as viral infections. Given that the impact of allogeneic HCT usually overwhelms that of the underlying disease on subsequent infectious complications, we will only consider infections occurring in patients with MDS outside the HCT setting.
Common bacteria
In the rare studies which looked at the bacterial causes of infections in MDS, the bacteria implicated were similar to those usually identified in febrile neutropenia in general, i.e. enterobacteriae and coagulase negative staphylococci.108
Several infections that are unusual because of their localization, 109,110 the responsible pathogen,109-118 or transfusionrelated infections14,116 were likely reported because of the rare occurrence of the clinical presentation rather than because of a specifically high prevalence in MDS.
Overall, these data suggest that, in the case of febrile neutropenia in patients with MDS, the type of first-line antibacterial therapy should be chosen on the basis of local epidemiological data and clinical presentation118 rather than on any specific consideration of the underlying disease.
Uncommon bacteria
Mycobacterial infections, including both tuberculosis and non-tuberculosis mycobacteria, have been reported in MDS patients over the last three decades,119-123 possibly with an increased incidence of extra-pulmonary involvement.120 Some patients had marrow involvement in the setting of disseminated infection.121-123 A large survey from China of 508 patients with MDS found 22 (4.3%) with tuberculosis.124 As most of these reports come from Asia, where the prevalence of mycobacterial infections is higher than in other continents, it is difficult to assess the respective roles of the natural exposure to mycobacteria in the community and of the underlying disease. However, such infections may be difficult to diagnose, and the clinician should be aware of their possible presence in the case of unexplained fever, pneumonia or lymphadenopathy, especially in geographical areas where tuberculosis is reappearing.
Invasive fungal infections
No published prospective data on the incidence of invasive fungal infections (IFI) in MDS is available. In the larger and more recent prospective therapeutic trials in MDS, the incidence of IFI was not mentioned.4,11-14,16,18,19,125 Dayyani et al. found that, among 273 deaths in lower-risk MDS, 23% were due to pneumonia of fungal origin, but no information was given on the fungal pathogen documented in these infections, or on the overall incidence of IFI in this low-risk MDS population.9
Large series of IFI usually include MDS as one of the diseases predisposing to these infections. In the report from the Italian IFI registry, covering ten years (1988-1997) in 14 centers, 391 patients with mold infections were identified; 12 of them had MDS (8 with aspergillus, 2 with mucormycosis, and 2 with unidentified filamentous fungi).126 Four of them had recently received steroids. These corresponded to 1.3% of the MDS cases seen by the centers during the observation period, i.e. a much lower incidence than that observed in AML.127 However, the study was performed before the era of hypomethylating agents.
While Aspergillus pneumonia is an expected complication of prolonged neutropenia, few cases have been reported in patients with MDS. Similarly, several cases of zygomycosis have been reported in MDS. Although the rarity of those infections may have encouraged reports being made.85-87,128-138 A relationship with the iron overload often seen in MDS cannot be ruled out, as iron overload is a known risk factor for zygomycosis infection (see above). In some cases, zygomycosis was observed at diagnosis of MDS.137,138 A significant number of patients had additional co-factors for zygomycosis, including diabetes,137,138 obstructive bronchial pneumonia,137 and treatment with deferoxamine.85,87 In some cases, the clinical presentation was atypical,139 with unusual features like spinal cord131,135,140 or coronary artery obstruction.141 In a recent, large European series of zygomycosis infections occurring between 2005 and 2007, MDS was the underlying disorder in only 6 of the 230 (2.6%) cases and in 6 of the 102 (6%) hematologic malignancies.139
Other unusual IFI have been reported, such as fusariosis, 142 Trichosporon beigelii infection.143 However, these occurred in the setting of acute transformation treated with intensive chemotherapy. Cryptococcal infection has also been reported with unusual presentations in MDS.144,145
Pneumocystis jirovecii pneumonia appears to be very uncommon in MDS patients. In two large series of P. jirovecii pneumonia in non-HIV patients, MDS was the underlying disease in 3 of 55 (5.4%)146 and 3 of 82 (4%)147 of the reported cases, respectively.
Overall, IFI is an expected complication in MDS patients, but it is likely that the risk varies greatly according to the severity of the underlying disease. No recent prospective data are available on the true incidence of, and risk factors for, IFI in MDS.
Viral infections
Severe viral infections rarely complicate the course of non-transplanted patients with MDS. In the series studied by Dayyani et al.,9 viruses were found in only 5% of the cases of lethal pneumonia. Recent large prospective trials do not mention viral infections as being a major complication of MDS4,12,13,125 However, this complication may be underestimated due to the lack of routine screening.
Parvovirus B19148-152 and HHV-6 infection,153 both in children and in adults, have been reported as mimicking MDS blood and marrow features, and these syndromes spontaneously disappeared in 1-2 months.
In HIV infection, marrow features of myelodysplasia have been reported in up to 69% of the patients, without evidence of any relationship between these features and HIV therapies.33
Comments on the current practices for prevention and management of infectious complications in myelodysplastic syndromes
As infection has rarely been an end point in therapeutic trials in MDS, it is impossible to propose evidence-based guidelines for the prevention and treatment of infection in these patients. However, a few recommendations can be made.
Prevention
Growth factors
Different studies have shown that GM-CSF154,155 (currently not available in Europe) and G-CSF156-158 at the conventional doses used after chemotherapy were found to improve neutropenia in 30-70% of neutropenic MDS, with better results in patients with no excess blasts and normal karyotype. Doses as low as 0.1 μg/kg/d of G-CSF159 and 0.3 μg/kg/d of GM-CSF160 have been evaluated and may yield slightly lower response rates. Interestingly, with conventional doses, a rapid increase in ANC may occur, probably due to mature neutrophil demargination.161 In contrast, response to low-dose GM-CSF was delayed.160
G-CSF induced a slight myeloid differentiating activity of MDS marrow cells that was greater than that induced by GM-CSF.162 G-or GM-CSF may also restore, in vitro and in vivo, several functions impaired in MDS neutrophils, such as chemotaxis and phagocytosis.159 Both may increase the myeloperoxidase granule content163 as the surface expression of CD11b molecules on both monocytes and granulocytes.163,164 On the other hand, the increase in ANC observed with GM-CSF treatment did not always correlate with improvement in neutrophil bactericidal functions.163 The lower number of GM-CSF receptors present on neutrophils could contribute to the observed impairment of response to GM-CSF.161
Two prospective randomized studies, one using GM-CSF165 and one using G-CSF,166 were performed around two decades ago in neutropenic MDS patients. The study, using G-CSF doses ranging from 0.5 to 10 μg/kg/d in high-risk patients, did not show any difference in the rate of infections or in overall survival between the 2 treatment arms.166 However, the overall survival of the subgroup of refractory anemia with an excess of blasts had a shorter survival in the G-CSF group when compared to the controls. This study was never published as a full paper. In the study with GM-CSF, the dose of 3 μg/kg/d of GM-CSF was compared to supportive care and was shown to decrease the rate of infections from 33% in the supportive care group to 15% in the treated group. However, no benefit in survival or in risk of AML transformation was observed.165
G and GM-CSF have also been tested after chemotherapy in higher-risk MDS, to determine whether they could reduce the impact of neutropenia. One prospective trial compared LD-AraC either alone or combined with either Gm-CSF or IL-3.167 Surprisingly, the study showed that infection rates were higher in the GM-CSF or IL3-containing arms. In a trial from the HOVON group, G-CSF was compared to no G-CSF following chemotherapy with daunomycin and cytosine-arabinoside in patients with high-risk MDS.168 Despite a significant reduction in the duration of neutropenia (from 35 to 23 days), there was no significant effect on the infection rate or on overall survival. Due to the lack of precise data on the use of growth factors during studies examining hypomethylating agents, it is not possible to draw any recommendation for the use of G-or GM-CSF under those conditions4,12-14,125
In patients with low-risk MDS and del 5q who receive lenalidomide, neutropenia will generally occur and is often profound, as mentioned above. In order to avoid potentially fatal infections (as seen in the first trials with this agent), a group of experts has recommended adding G-CSF whenever ANC drops below 1.0×109/L.169 This approach could also avoid the dose reductions that appear to be associated with lower cytogenetic responses to lenalidomide, as shown by a combined analysis of the MDS 003 and MDS 004 trials in lower-risk MDS with del 5q. On the other hand, it still has to be clearly demonstrated that the use of G-CSF, in this context, can help maintain a higher dose of lenalidomide, especially given that this agent also causes thrombocytopenia.
Therefore, no clear recommendation can be made for the use of G-or GM-CSF as routine infection prophylaxis in MDS patients with neutropenia who are not receiving myelosuppressive treatment.154,170,171 Likewise, in patients receiving myelosuppressive treatment, no indication for G-or GM-CSF has been clearly established, especially in higher-risk patients in whom these agents could potentially increase the risk of AML progression.
Antibacterial prophylaxis
Whether antibacterial prophylaxis may benefit patients with MDS receiving myelosuppressive treatment (mainly hypomethylating agents or chemotherapy) has not been established. A recent retrospective study suggested some benefit from prophylactic antibiotics in decreasing the incidence of febrile episodes in MDS patients treated with decitabine.108 However, the conclusions drawn from that study should be interpreted with caution since the administration of antibacterial drugs was left to the discretion of the physicians, three different antibacterials were used, and many of the infections did not occur in the setting of neutropenia. The risk-benefit analysis of prophylactic antibacterials should be considered carefully in the context of both increasing bacterial resistance and the risk of a decrease in the availability of new antibacterials over the next decade.172,173 Prospective randomized trials will be important in resolving this issue and may also provide data on the actual risk of selecting resistant bacteria.
Antifungal prophylaxis
As for IFI, prospective controlled data are only available for patients with MDS receiving intensive AML chemotherapy. In this setting, posaconazole significantly reduced the risk of proven and probable IFI when compared to itraconazole or fluconazole in a cohort of 602 patients in whom the mean duration of chemotherapy-induced severe neutropenia was 24 days.93 In that cohort, however, only 14.5% of the patients' MDS transformed into AML, whereas the other patients had de novo AML. In addition, most patients with higher-risk MDS now receive hypomethylating agents and whether antifungal prophylaxis is effective in these patients is unknown. If the incidence of IFI in MDS is comparable to that shown before the era of hypomethylating agents (2% in the Italian experience126), primary fungal prophylaxis is not a recommended treatment. This is because, unlike in AML or allogeneic HCT recipients, this incidence in MDS patients is lower than the rate that is usually considered to be that justifying primary prophylaxis (typically at least 5%).174 Furthermore, MDS patients may have prolonged neutropenia, requiring prolonged prophylactic triazoles, a situation which has been associated with the risk of acquired resistance to those drugs.175,176 Thus, antifungal prophylaxis with triazoles cannot currently be recommended for MDS patients receiving hypomethylating agents outside controlled trials.
Iron chelation
Repairing organ damage, especially in liver and heart, due to iron overload has been the main goal of iron chelation, and little attention has been paid to another potential benefit, i.e. reducing the risk of infection.177 Whether iron chelation can reduce the risk of infection is still not known. Iron chelation is currently recommended before transplant in iron overloaded patients who are candidates for HCT, including patients with MDS.74,81 The mechanisms of its beneficial effects in this situation remain largely unknown, but may include reducing the risk of infection. A small randomized trial assessing the addition of deferasirox to liposomal amphotericin B in the treatment of mucormycosis unfortunately failed to show any benefit from iron chelation.178
Treatment of infectious episodes
Patients with MDS should be educated about neutropenia and the risk of infection. The risk of infection may not change much over time in patients with supportive care only or may worsen for variable periods in patients receiving hypomethylating agents, chemotherapy or lenalidomide. Neutropenic episodes associated with use of lenalidomide are particularly important to monitor, as they occur in patients who were generally not neutropenic at baseline, and induced-neutropenia may be profound. In cases of fever, patients should immediately undergo tests, including blood cultures, and urgently require broad-spectrum empirical antibacterials.118
Cases of febrile neutropenia, given the usually more advanced age of these patients, should be admitted to hospital to avoid severe complications. The choice of the antibacterials should be driven by clinical presentation, local epidemiology and severity of the infection.118 The general practitioner should also be keenly aware of the risks of infection.
Treatment recommendations according to type of myelodysplastic syndrome therapy and individual risk
Prophylactic measures should be considered in the context of specific clinical situations. In patients who only receive supportive care, it is generally accepted that neutropenia per se does not warrant the administration of prophylactic anti-infectives. The main reason is that the duration of neutropenia would necessitate continuous use of antibacterials or antifungals for months or years. This would likely lead to an unacceptable risk of induced resistance, well-illustrated in the case of long-term treatments with quinolones179,180 and antifungal triazoles,175,176 and also a risk of drug-induced adverse effects.
More than half the patients receiving lenalidomide develop grade 3-4 neutropenia during the first course of therapy, and ANC counts of patients receiving this drug should, therefore, be regularly monitored. There are, however, no data to support the routine administration of prophylactic antibacterials or antifungals. Since neutropenia is a limiting side-effect of lenalidomide,99 treatment adjustment is critical, and a group of experts recommended administration of G-CSF in patients with ANC counts of less than 1.0×109/L at baseline or during treatment.169
In patients receiving hypomethylating agents, neutropenia mainly occurs during the first one or two treatment courses, and mostly in patients who are neutropenic at baseline.181 However, in the absence of data, there are no established indications for primary or secondary prophylactic anti-infectives or for the use of G-CSF. The administration of G-CSF may additionally be a concern for patients with a significant proportion of blasts in the marrow at baseline, and should probably be restricted to neutropenic patients with overt and severe infection.182 Recent data from Israel suggest that transfusion dependency and platelet counts less than 20×109/L prior to each cycle could be an indication of an increased risk of infection during azacitidine therapy.98
Two categories of patients deserve specific consideration: those with severe comorbidities known to increase their risk of infection, i.e. chronic obstructive bronchitis or any chronic focus of infection which may reactivate during neutropenia, and those who developed a previous severe infection during a prior course of treatment. Antibacterial prophylaxis should be seriously considered in these patients, especially with the use of quinolones. However, the duration of prophylaxis when administered should be as short as possible, covering only the nadir time of the risk. Consensus guidelines for antibacterial and growth factor prophylaxis in MDS urgently require large, prospective trials addressing their benefits regarding infection prevention, infection-related mortality, overall survival, and assessing their cost-efficacy.
Conclusion
Patients with MDS mainly develop common bacterial infections, especially when they are profoundly neutropenic. However, the large variety of other infections reported in these patients suggests that, in addition to neutropenia, other mechanisms may contribute to immune suppression, at least in some patients. In those rare patients with MDS who still receive intensive AML-type chemotherapy, measures to prevent and treat infection are similar to those recommended in AML patients who receive intensive therapy. While demethylating agents now provide a real benefit to many of them, solid epidemiological data and controlled studies of infectious complications are urgently needed in order to develop optimal strategies for preventing severe infection in these patients.
Supplementary Material
Acknowledgments
the authors thank Brandon J. Farley for editorial assistance.
Footnotes
Authorship and Disclosures: The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361(19):1872-85 [DOI] [PubMed] [Google Scholar]
- 2.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079-88 [PubMed] [Google Scholar]
- 3.Fenaux P, Rose C. Impact of iron overload in myelodysplastic syndromes. Blood Rev. 2009;23(Suppl 1):S15-9 [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H, Issa JPJ, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, et al. Decitabine improves patient outcomes in myelodysplastic syndromes. Cancer. 2006;106(8):1794-80 [DOI] [PubMed] [Google Scholar]
- 5.Kindwall-Keller T, Isola LM. The evolution of hematopoietic SCT in myelodysplastic syndrome. Bone Marrow Transplant. 2009;43(8):597-609 [DOI] [PubMed] [Google Scholar]
- 6.Cunningham I, MacCalum SJ, Nicholls MD, Byth K, Hewson JW, Arnold B, et al. The myelodysplastic syndromes: an analysis of prognostic factors in 226 cases from a single institution. Br J Haematol. 1995;90(3):602-6 [DOI] [PubMed] [Google Scholar]
- 7.Ganser A, Hoelzer D. Clinical course of myelodysplastic syndromes. Hematol Oncol Clin North Am. 1992;6(3):607-18 [PubMed] [Google Scholar]
- 8.Mufti GJ, Galton DAG. Myelodyslastic syndrome natural history and features of prognostic significance. Clin Haematol. 1986;15(4):953-69 [PubMed] [Google Scholar]
- 9.Dayyani F, Conley AP, Strom SS, Stevenson W, Cortes JE, Borthakur G, et al. Cause of death in patients with lower-risk myelodysplastic syndrome. Cancer. 2010;116(9):2174-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldberg SL, Chen E, Corral M, Guo A, Mody-Patel N, Pecora AL, et al. Incidence and clinical complications of myelodysplastic syndromes among United States Medicare beneficiaries. J Clin Oncol. 2010;10(28):2847-52 [DOI] [PubMed] [Google Scholar]
- 11.Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Ochimar-Reissig R, et al. Randomized controlled trial of azacytidine in patients with the myelodysplastic syndrome: A study of the Cancer and Leukemia Group B. J CIin Oncol. 2002;20(10):2429-40 [DOI] [PubMed] [Google Scholar]
- 12.Silverman LR, McKenzie DR, Peterson BL, Holland JC, Backstrom JT, Beach CL, et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: Studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006;24(24):3895-903 [DOI] [PubMed] [Google Scholar]
- 13.Fenaux P, Mufti JG, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musto P, Maurillo L, Spagnoli A, Gozzini A, Rivellini F, Lunghi M, et al. Azacitidine for the treatment of lower risk myelodysplastic syndromes. Cancer. 2010;116(6):1485-94 [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Manero G, Fenaux P. Hypomethylating agents and other novel strategies in myelodysplastic syndromes. J Clin Oncol. 2011;29(5):516-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wijermans P, Lubbert M, Verhoef G, Bosly A, Ravoet C, Andre M, et al. Low-dose 5-Aza-2'-Deoxycytidine, a DNA hypomethylating agent, for the treatment of high-risk myelodysplastic syndrome: A multicenter phase II study in elderly patients. J CIin Oncol. 2000;18(5):956-62 [DOI] [PubMed] [Google Scholar]
- 17.Issa JP, Garcia-Manero G, Giles FJ, et al. Phase I study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2'-deoxycytidine (decitabine) in hematopoietic malignancies. Blood. 2004;103(5):1635-40 [DOI] [PubMed] [Google Scholar]
- 18.Kantarjian H, Oki Y, Garcia-Manero G, Huang X, O'Brien S, Cortes J, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109(1):52-7 [DOI] [PubMed] [Google Scholar]
- 19.Itzykson R, Thépot S, Quesnel B, Dreyfus F, Beyne-Rauzy O, Turlure P, et al. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood. 2011;117(2):403-11 [DOI] [PubMed] [Google Scholar]
- 20.Acquaviva C, Gelsi-Boyer V, Birnbaum D. Myelodysplastic syndromes: lost between two states?. Leukemia. 2010;24(1):1-5 [DOI] [PubMed] [Google Scholar]
- 21.Vardiman J, Thiele J, Arber D, Brunning R, Borowitz M, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-51 [DOI] [PubMed] [Google Scholar]
- 22.Briggs R, Shults K, Flye L, McClintock-Treep S, Jagasia M, Goodman S, et al. Dysregulated human myeloid nuclear differentiation antigen expression in myelodysplastic syndromes: evidence for a role in apoptosis. Cancer Res. 2006;66(9):4645-51 [DOI] [PubMed] [Google Scholar]
- 23.Mundle S, Venugopal P, Cartlidge J, Pandav D, Broady-Robinson L, Gezer S, et al. Indication of an involvement of interleukin-1 beta converting enzyme-like protease in intramedullary apoptotic cell death in the bone marrow of patients with myelodysplastic syndromes. Blood. 1996;88(7):2640-7 [PubMed] [Google Scholar]
- 24.Shetty V, Hussaini S, Broady-Robinson L, Allampallam K, Mundle S, Borok R, et al. Intramedullary apoptosis of hematopoietic cells in myelodysplastic syndrome patients can be massive: apoptotic cells recovered from high-density fraction of bone marrow aspirates. Blood. 2000;96(4):1388-92 [PubMed] [Google Scholar]
- 25.Claessens Y, Bouscary D, Dupont J, Picard F, Melle J, Gisselbrecht S, et al. In Vitro Proliferation and Differentiation of Erythroid Progenitors from Patients with Myelodysplastic Syndromes: Evidence for Fas – Dependent Apoptosis. Blood. 2002;99(5):1594-601 [DOI] [PubMed] [Google Scholar]
- 26.Hellström-Lindberg E, Kanter-Lewensohn L, Øst A. Morphological Changes and Apoptosis in Bone Marrow from Patients with Myelodysplastic Syndromes Treated with Granulocyte-CSF and Erythropoietin. Leuk Res. 1997;21:415-25 [DOI] [PubMed] [Google Scholar]
- 27.Hellström-Lindberg E, Schmidt Mende J, Forsblom A, Christensson B, Fadeel B, Zhlvotovsky B. Apoptosis in Refractory Anaemia with Ringed Sideroblasts is Initiated at the Stem Cell Level and Associated with Increased Activation of Caspases. Br J Haematol. 2001;112(3):714-26 [DOI] [PubMed] [Google Scholar]
- 28.Tehranchi R, Fadeel B, Forsblom A, Christensson B, Samuelsson J, Zhivotovsky B, et al. Granulocyte Colony - Stimulating Factor Inhibits Spontaneous Cytochrome C Release and Mitochondria – Dependent Apoptosis of Myelodysplastic Syndrome Hematopoietic Progenitors. Blood. 2003;101(3):1080-6 [DOI] [PubMed] [Google Scholar]
- 29.Tehranchi R, Fadeel B, Schmidt-Mende J, Forsblom A, Emanuelsson E, Jadersten M, et al. Antiapoptotic Role of Growth Factors in the Myelodysplastic Syndromes: Concordance Between in Vitro and in Vivo Observations. Clin Cancer Res. 2005;11(17):6291-9 [DOI] [PubMed] [Google Scholar]
- 30.Raza A, Gezer S, Mundle S, Gao X, Alvi S, Borok R, et al. Apoptosis in bone marrow biopsy samples involving stromal and hematopoietic cells in 50 patients with myelodysplastic syndrome. Blood. 1995;86(1):268-75 [PubMed] [Google Scholar]
- 31.Rajapaksa R, Ginzton N, Rott L, Greenberg P. Altered oncoprotein expression and apoptosis in myelodysplastic syndrome marrow cells. Leukemia. 2010;24(1):1-520068572 [Google Scholar]
- 32.Barrett A, Saunthararajah Y, Molldrem J. Myelodysplastic syndrome and aplastic anemia; distinct entities or diseases linked by a common pathophysiology? Semin Hematol.2000;37(1):15-29 [DOI] [PubMed] [Google Scholar]
- 33.Karcher D, Frost A. The bone marrow in human immunodeficiency virus (HIV)-related disease. Morphology and clinical correlation. Am J Clin Pathol. 1991;95(1):63-71 [DOI] [PubMed] [Google Scholar]
- 34.Smith M, Smith J. The occurrence subtype and significance of hematopoietic inhibitory T cells (HIT cells) in myelodysplasia: an in vitro study. Leuk Res. 1991;15(7):597-601 [DOI] [PubMed] [Google Scholar]
- 35.Voulgarelis M, Giannouli S, Ritis K, Tzioufas A. Myelodysplasia-associated autoimmunity: clinical and pathophysiologic concepts. Eur J Clin Invest. 2004;34(10):690-700 [DOI] [PubMed] [Google Scholar]
- 36.Shetty V, Mundle S, Raza A. Pseudo Pelger-Huet anomaly in myelodysplastic syndrome: hyposegmented or apoptotic neutrophil? Blood 2001;98(4):1273-5 [DOI] [PubMed] [Google Scholar]
- 37.Prodan M, Tulissi P, Perticarari S, Presani G, Franzin F, Pussini E, et al. Flow cytometric assay for the evaluation of phagocytosis and oxidative burst of polymorphonuclear leukocytes and monocytes in myelodysplastic disorders. Haematologica. 1995;80(3):212-8 [PubMed] [Google Scholar]
- 38.Fianchi L, Leone G, Posteraro B, Sanguinetti M, Guidi F, Valentini C, et al. Impaired bactericidal and fungicidal activities of neutrophils in patients with myelodysplastic syndrome. Leuk Res. 2012;36(3):331-3 [DOI] [PubMed] [Google Scholar]
- 39.Itoh Y, Kuratsuji K, Aizawa S, Sai M, Ohyashiki K, Toyama K. Superoxide anion production and expression of cytochrome b 558 by neutrophils are impaired in some patients with myelodysplastic syndrome. Ann Hematol. 1991;63(5):270-5 [DOI] [PubMed] [Google Scholar]
- 40.Mazzone A, Porta C, Fossati G, Gritti D, Mazzucchelli I, Ricevuti G. Granulocyte dysplasia and dysfunction, and CD11/CD18 defects in myelodysplastic syndromes. Leuk Lymphoma. 1996;23(3-4):267-75 [DOI] [PubMed] [Google Scholar]
- 41.Ricevuti G, Mazzone A, Pasotti D, Fossati G, Mazzucchelli I, Notario A. The role of integrins in granulocyte dysfunction in myelodysplastic syndrome. Leuk Res. 1993;17(7):609-19 [DOI] [PubMed] [Google Scholar]
- 42.Ohsaka A, Saionji K, Igari J, Watanabe N, Iwabuchi K, Nagaoka I. Altered surface expression of effector cell molecules on neutrophils in myelodysplastic syndromes. Br J Haematol. 1997;98:108-13 [DOI] [PubMed] [Google Scholar]
- 43.Elghetany M. Surface marker abnormalities in myelodysplastic syndromes. Haematologica. 1998;83(12):1104-15 [PubMed] [Google Scholar]
- 44.Elghetany M, Peterson B, MacCallum J, Nelson D, Varney J, Sullivan A, et al. Deficiency of neutrophilic granule membrane glycoproteins in the myelodysplastic syndromes: a common deficiency in 216 patients studied by the Cancer and Leukemia Group B. Leuk Res. 1997;21(9):801-6 [DOI] [PubMed] [Google Scholar]
- 45.Ito Y, Kawanishi Y, Shoji N, Ohyashiki K. Decline in antibiotic enzyme activity of neutrophils is a prognostic factor for infection in patients with myelodysplastic syndrome. Clin Infect Dis. 2000;31(5):1292-5 [DOI] [PubMed] [Google Scholar]
- 46.Moretti S, Lanza F, Spisani S, Latorraca A, Rigolin G, Giuliani A, et al. Neutrophils from patients with myelodysplastic syndromes: relationship between impairment of granular contents, complement receptors, functional activities and disease status. Leuk Lymphoma. 1994;13(5-6):471-7 [DOI] [PubMed] [Google Scholar]
- 47.Bottcher T, Spreer A, Azeh I, Nau R, Gerber J. Matrix metalloproteinase-9 deficiency impairs host defense mechanisms against Streptococcus pneumoniae in a mouse model of bacterial meningitis. Neurosci Lett. 2003;338(3):201-4 [DOI] [PubMed] [Google Scholar]
- 48.Dubois B, Starckx S, Pagenstecher A, Oord J, Arnold B, Opdenakker G. Gelatinase B deficiency protects against endotoxin shock. Eur J Immunol. 2002;32(2):2163-71 [DOI] [PubMed] [Google Scholar]
- 49.Yamada K, Yoshino K, Sekikawa K, Madarame H, Yagita H, Nakane A. Effect of a matrix metalloproteinase inhibitor on host resistance against Listeria monocytogenes infection. FEMS Immunol Med Microbiol. 2000;29(3):187-94 [DOI] [PubMed] [Google Scholar]
- 50.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss D, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532-5 [DOI] [PubMed] [Google Scholar]
- 51.Travaglino E, Benatti C, Malcovati L, Della Porta M, Gallì A, Bonetti E, et al. Biological and clinical relevance of matrix metalloproteinases 2 and 9 in acute myeloid leukaemias and myelodysplastic syndromes. Eur J Haematol. 2008;80(3):216-26 [DOI] [PubMed] [Google Scholar]
- 52.Yamaguchi N, Ito Y, Ohyashiki K. Increased intracellular activity of matrix metallopro-teinases in neutrophils may be associated with delayed healing of infection without neutropenia in myelodysplastic syndromes. Ann Hematol. 2005;84(6):383-8 [DOI] [PubMed] [Google Scholar]
- 53.Marisavljević D, Kraguljac N, Rolović Z. Immunologic abnormalities in myelodysplastic syndromes: clinical features and characteristics of the lymphoid population. Med Oncol. 2006;23(3):385-91 [DOI] [PubMed] [Google Scholar]
- 54.Katsuki K, Shinohara K, Kameda N, Yamada T, Takeda K, Kamei T. Two cases of myelodysplastic syndrome with extramedullary polyclonal plasma cell proliferation and autoantibody production: possible role of soluble Fas antigen for production of excessive self-reactive B cells. Intern Med. 1998;37(11):973-7 [DOI] [PubMed] [Google Scholar]
- 55.Okamoto T, Okada M, Mori A, Saheki K, Takatsuka H, Wada H, et al. Correlation between immunological abnormalities and prognosis in myelodysplastic syndrome patients. Int J Hematol. 1997;66(3):345-51 [DOI] [PubMed] [Google Scholar]
- 56.Amin H, Jilani I, Estey E, Keating M, Dey A, Manshouri T, et al. Increased apoptosis in bone marrow B lymphocytes but not T lymphocytes in myelodysplastic syndrome. Blood. 2003;102(5):1866-8 [DOI] [PubMed] [Google Scholar]
- 57.Shioi Y, Tamura H, Yokose N, Satoh C, Dan K, Ogata K. Increased apoptosis of circulating T cells in myelodysplastic syndromes. Leuk Res. 2007;31(12):1641-8 [DOI] [PubMed] [Google Scholar]
- 58.Hamdi W, Ogawara H, Handa H, Tsukamoto N, Murakami H. Clinical significance of Th1/Th2 ratio in patients with myelodysplastic syndrome. Int J Lab Hematol. 2009;31(6):630-8 [DOI] [PubMed] [Google Scholar]
- 59.Melenhorst J, Eniafe R, Follmann D, Nakamura R, Kirby M, Barrett A. Molecular and flow cytometric characterization of the CD4 and CD8 T-cell repertoire in patients with myelodysplastic syndrome. Br J Haematol. 2002;119(1):97-105 [DOI] [PubMed] [Google Scholar]
- 60.Kook H, Zeng W, Guibin C, Kirby M, Young N, Maciejewski J. Increased cytotoxic T cells with effector phenotype in aplastic anemia and myelodysplasia. Exp Hematol. 2001;29(11):1270-7 [DOI] [PubMed] [Google Scholar]
- 61.Kotsianidis I, Bouchliou I, Nakou E, Spanoudakis E, Margaritis D, Christophoridou A, et al. Kinetics, function and bone marrow trafficking of CD4+CD25+FOXP3+ regulatory T cells in myelodysplastic syndromes. Leukemia. 2009;23(3):510-8 [DOI] [PubMed] [Google Scholar]
- 62.Kordasti S, Afzali B, Lim Z, Ingram W, Hayden J, Barber L, et al. IL-17-producing CD4(+) T cells, pro-inflammatory cytokines and apoptosis are increased in low risk myelodysplastic syndrome. Br J Haematol. 2009;145(1):64-72 [DOI] [PubMed] [Google Scholar]
- 63.Kiladjian J, Bourgeois E, Lobe I, Braun T, Visentin G, Bourhis J, et al. Cytolytic function and survival of natural killer cells are severely altered in myelodysplastic syndromes. Leukemia. 2006;20(3):463-70 [DOI] [PubMed] [Google Scholar]
- 64.Epling-Burnette P, Bai F, Painter J, Rollison D, Salih H, Krusch M, et al. Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood. 2007;109(11):4816-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marcondes A, Mhyre A, Stirewalt D, Kim S, Dinarello C, Deeg H. Dysregulation of IL-32 in myelodysplastic syndrome and chronic myelomonocytic leukemia modulates apoptosis and impairs NK function. Proc Natl Acad Sci USA. 2008;105(8):2865-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cortelezzi A, Cattaneo C, Cristiani S, Duca L, Sarina B, Deliliers GL, et al. Non-transferrin-bound iron in myelodysplastic syndromes: a marker of ineffective erythropoiesis? Hematol J 2000;1(3):153-8 [DOI] [PubMed] [Google Scholar]
- 67.Pullarkat V. Objectives of iron chelation in myelodysplastic syndromes: more than meets the eye? Blood 2009;114(26):5251-5 [DOI] [PubMed] [Google Scholar]
- 68.Bullen JJ, Rogers HJ, Spalding PB, Ward CG. Natural resistance, iron and infection: a challenge for clinical medicine. J Med Microbiol. 2006;55(3):251-8 [DOI] [PubMed] [Google Scholar]
- 69.Lounis N, Truffot-Pernot C, Grosset J, Gordeuk VR, Boelaert JR. Iron and Mycobacterium tuberculosis infection. J Clin Virol. 2001;20(3):123-6 [DOI] [PubMed] [Google Scholar]
- 70.Schaible UE, Kaufmann SH. Iron and microbial infection. Nat Rev Microbiol. 2004;2(12):946-53 [DOI] [PubMed] [Google Scholar]
- 71.Weinberg ED. Microbial pathogens with impaired ability to acquire host iron. Biometals. 2000;13(1):85-9 [DOI] [PubMed] [Google Scholar]
- 72.Boelaert JR, Vandecasteele SJ, Appelberg R, Gordeuk VR. The effect of the host's iron status on tuberculosis. J Infect Dis. 2007;195(12):1745-53 [DOI] [PubMed] [Google Scholar]
- 73.Pieracci FM, Barie PS. Iron and the risk of infection. Surg Infect (Larchmt). 2005;6(Suppl 1):S41-6 [DOI] [PubMed] [Google Scholar]
- 74.Pullarkat V, Blanchard S, Tegtmeier B, Dagis A, Patane K, Ito J, et al. Iron overload adversely affects outcome of allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2008;42(12):799-805 [DOI] [PubMed] [Google Scholar]
- 75.von Bonsdorff L, Sahlstedt L, Ebeling F, Ruutu T, Parkkinen J. Apotransferrin administration prevents growth of Staphylococcus epidermidis in serum of stem cell transplant patients by binding of free iron. FEMS Immunol Med Microbiol. 2003;37(1):45-51 [DOI] [PubMed] [Google Scholar]
- 76.Bradley SJ, Gosriwitana I, Srichairatanakool S, Hider RC, Porter JB. Non-transferrin-bound iron induced by myeloablative chemotherapy. Br J Haematol. 1997;99(2):337-43 [DOI] [PubMed] [Google Scholar]
- 77.Altes A, Remacha AF, Sarda P, Sancho FJ, Sureda A, Martino R, et al. Frequent severe liver iron overload after stem cell transplantation and its possible association with invasive aspergillosis. Bone Marrow Transplant. 2004;34(6):505-9 [DOI] [PubMed] [Google Scholar]
- 78.Busca A, Falda M, Manzini P, D'Antico S, Valfre A, Locatelli F, et al. Iron overload in patients receiving allogeneic hematopoietic stem cell transplantation: Quantification or iron burden by a superconducting quantum interference device (SQUID) and therapeutic effectiveness of phlebotomy. Biol Blood Marrow Transplant. 2010;16(1):115-22 [DOI] [PubMed] [Google Scholar]
- 7.Kontoyiannis DP, Chamilos G, Lewis RE, Giralt S, Cortes J, Raad II, et al. Increased bone marrow iron stores is an independent risk factor for invasive aspergillosis in patients with high-risk hematologic malignancies and recipients of allogeneic hematopoietic stem cell transplantation. Cancer. 2007;110(6):1303-6 [DOI] [PubMed] [Google Scholar]
- 80.Maertens J, Demuynck H, Verbeken EK, Zachée P, Verhoef GE, Vandenberghe P, et al. Mucormycosis in allogeneic bone marrow transplant recipients: report of five cases and review of the role of iron overload in the pathogenesis. Bone Marrow Transplant. 1999;24(3):307-12 [DOI] [PubMed] [Google Scholar]
- 81.Armand P, Kim HT, Cutler CS, Ho VT, Koreth J, Alyea EP, et al. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood. 2007;109(10):4586-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Platzbecker U, Bornhäuser M, Germing U, Stumpf J, Scott BL, Kröger N, et al. Red blood cell transfusion dependence and outcome after allogeneic peripheral blood stem cell transplantation in patients with de novo myelodysplastic syndrome Biol Blood Marrow Transplant. 2008;14(11):1217-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Greenberg P, Koller C, Cabantchik Z, Warsi G, Glynos T, Paley C, et al. Prospective assessment of effects on iron-overload parameters of deferasirox therapy in patients with myelodysplastic syndromes. Leukemia Res. 2010;34(12):1560-5 [DOI] [PubMed] [Google Scholar]
- 84.Boelaert JR, de Locht M, Van Cutsem J, Kerrels V, Cantinieaux B, Verdonck A, et al. Mucormycosis during deferoxamine therapy is a siderophore-mediated infection. In vitro and in vivo animal studies. J Clin Invest. 1993;91(5):1979-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Keijzer A, van der Valk P, Ossenkoppele GJ, van de Loosdrecht AA. Mucormycosis in a patient with low risk myelodysplasia treated with anti-tnf-alpha. Haematologica. 2006;91(10):139-40 [PubMed] [Google Scholar]
- 86.Prokopowicz GP, Bradley SF, Kauffman CA. Indolent zygomycosis associated with deferoxamine chelation therapy. Mycoses. 1994;37(11-12):427-31 [DOI] [PubMed] [Google Scholar]
- 87.Reyes HM, Tingle EJ, Fenves AZ, Spiegel J, Burton EC. Pulmonary invasive mucormycosis in a patient with secondary iron overload following deferoxamine therapy. Proc (Bayl Univ Med Cent). 2008;21(4):378-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cordonnier C. Management of infectious complications in Hematological patients. In: Dunitz, (ed.) Text Book on Malignant Haematology. 2nd ed. London, 2005 [Google Scholar]
- 89.Cordonnier C, Maury S. Epidemiology and risk factors for serious infections in the stem cell transplant setting. Blood Rev. 2007; (Suppl 1)S39-42 [Google Scholar]
- 90.Chauncey T, Gundacker H, Shadman M, List A, Dakhil S, Erba H, et al. Sequential phase II Southwest Oncology Group studies (S0012 and S0301) of daunorubicin and cytarabine by continuous infusion, without and with cyclosporine, in older patients with previously untreated acute myeloid leukemia. Br J Haematol. 2010;148(1):48-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gardin C, Turlure P, Fagot T, Thomas X, Terre C, Contentin N, et al. Postremission treatment of elderly patients with acute myeloid leukemia in first complete remission after intensive induction chemotherapy: results of the multicenter randomized Acute Leukemia French Association (ALFA) 9803 trial. Blood. 2007;109(12):5129-35 [DOI] [PubMed] [Google Scholar]
- 92.Boogaerts M, Winston DJ, Bow EJ, Garber G, Reboli AC, Schwarer AP, et al. Intravenous and oral itraconazole versus intravenous amphotericin B deoxycholate as empirical antifungal therapy for persistent fever in neutropenic patients with cancer who are receiving broad-spectrum antibacterial therapy. Ann Intern Med. 2001;135(6):412-22 [DOI] [PubMed] [Google Scholar]
- 93.Cornely OA, Maertens J, Winston DJ, Perfect JR, Ullmann AJ, Walsh TJ, et al. Posaconazole vs. Fluconazole or Itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356(4):348-59 [DOI] [PubMed] [Google Scholar]
- 94.Mattiuzzi GN, Cortes J, Alvarado G, Verstovsek S, Koller C, Pierce S, et al. Efficacy and safety of intravenous voriconazole and intravenous itraconazole for antifungal prophylaxis in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome. Support Care Cancer. 2011;19(1):19-26 [DOI] [PubMed] [Google Scholar]
- 95.Pagano L, Girmenia C, Mele L, Ricci P, Tosti ME, Nosari A, et al. Infections caused by filamentous fungi in patients with hematologic malignancies. A report of 391 cases by GIMEMA Infection Program. Haematologica. 2001;86(8):862-70 [PubMed] [Google Scholar]
- 96.Santini V, Fenaux P, Mufti G, Hellström-Lindberg E, Silverman L, List A, et al. Management and supportive care measures for adverse events in patients with myelodysplastic syndromes treated with azacitidine. Eur J Haematol. 2010;85(2):130-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fenaux P, Gattermann N, Seymour J, Hellström-Lindberg E, Mufti G, Duehrsen U, et al. Prolonged survival with improved tolerability in higher-risk myelodysplastic syndromes: azacitidine compared with low dose ara-C. Br J Haematol. 2010;149(2):244-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Merkel D, Filanovsky K, Aviv A, Gatt M, Herishanu Y, Arad A, et al. Predictive Parameters for Infections During Azacitidine Therapy in High Risk MDS Patients American Society for Hematology Annual Meeting 2011; San Diego. 2011. [Google Scholar]
- 99.List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355(14):1456-65 [DOI] [PubMed] [Google Scholar]
- 100.Adès L, Boehrer S, Prebet T, Beyne-Rauzy O, Legros L, Ravoet C, et al. Efficacy and safety of lenalidomide in intermediate-2 or high-risk myelodysplastic syndromes with 5q deletion: results of a phase 2 study. Blood. 2009;113(17):3947-52 [DOI] [PubMed] [Google Scholar]
- 101.Raza A, Reeves JA, Feldman EJ, Dewald GW, Bennett JM, Deeg JH, et al. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1 -risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood. 2008;111(1):86-93 [DOI] [PubMed] [Google Scholar]
- 102.Fenaux P, Giagounidis A, Selleslag D, Beyne-Rauzy O, Mufti G, Mittleman M, et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with Low-/Intermediate-1-risk myelodysplastic syndromes with del5q. Blood. 2011;118(14):3765-76 [DOI] [PubMed] [Google Scholar]
- 103.Le Bras F, Sebert M, Kelaidi C, Lamy T, Dreyfus F, Delaunay J, et al. Treatment by Lenalidomide in lower risk myelodysplastic syndrome with 5q deletion–The GFM experience. Leukemia Res. 2011;35(11):1444-8 [DOI] [PubMed] [Google Scholar]
- 104.Deeg HJ, Storer B, Slattery JT, Anasetti C, Doney KC, Hansen JA, et al. Conditioning with targeted busulfan and cyclophosphamide for hemopoietic stem cell transplantation from related and unrelated donors in patients with myelodysplastic syndrome. Blood. 2002;100(4):1201-7 [DOI] [PubMed] [Google Scholar]
- 105.Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood. 2002;100(13):4358-66 [DOI] [PubMed] [Google Scholar]
- 106.Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and Outcome of Mould Infections in Hematopoietic Stem Cell Transplant Recipients. Clin Infect Dis. 2002;34(7):909-17 [DOI] [PubMed] [Google Scholar]
- 107.Scott BL, Park JY, Deeg HJ, Marr KA, Boeckh M, Chauncey TR, et al. Pretransplant neutropenia is associated with poor-risk cytogenetic features and increased infection-related mortality in patients with myelodysplastic syndromes. Biol Blood Marrow Transplant. 2008;14(7):799-806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee JH, Lee KH, Lee JH, Kim DY, Kim SH, Lim SN, et al. Decreased incidence of febrile episodes with antibiotic prophylaxis in the treatment of decitabine for myelodysplastic syndrome. Leuk Res. 2011;35(4):499-503 [DOI] [PubMed] [Google Scholar]
- 109.Monselise A, Blickstein D, Ostfeld I, Segal R, Weinberger M. A case of cellulitis complicating Campylobacter jejuni subspecies jejuni bacteremia and review of the literature. Eur J Clin Microbiol Infect Dis. 2004;23(9):718-21 [DOI] [PubMed] [Google Scholar]
- 110.Soravia-Dunant V, Loo V, Salit I. Aortitis due to Salmonella: report of 10 cases and comprehensive review of the literature. Clin Infect Dis. 1999;29(4):862-8 [DOI] [PubMed] [Google Scholar]
- 111.Olson J, Nguyen V, Yoo J, Kuechie M. Cutaneous manifestations of Corynebacterium jeikeium sepsis. Int J Dermatol. 2009;48(8):886-8 [DOI] [PubMed] [Google Scholar]
- 112.Ortin X, Jean-Martinez J, Rodriguez-Luaces M, Alvaro T, Font L. Fatal pulmonary hemorrhage in a patient with myelodysplastic syndrome and fulminant pneumonia caused by Stenotrophomonas maltophilia. Infection. 2007;35(3):201-2 [DOI] [PubMed] [Google Scholar]
- 113.Fry N, Duncan J, Edwards M, Tilley R, Chitnavis D, Harman R, et al. A UK clinical isolate of Bordetella hinzii from a patient with myelodysplastic syndrome. J Med Microbiol. 2007;56(12):1700-3 [DOI] [PubMed] [Google Scholar]
- 114.Zhu X, Xu J, Xiang L, Kang K. Cutaneous infectious granuloma caused by Phenylobacterium in an adult with myelodysplastic syndrome: a first case report. Am J Clin Dermatol. 2010;11(5):363-6 [DOI] [PubMed] [Google Scholar]
- 115.Bay J, Tournilhac O, Ducher E, Romaszko JP, Ergani A, Bouvet A, et al. A near fatal septic transfusion reaction due to Streptococcus dysgalactiae supspecies equisimilis calls for novel safety measures. Vox Sang. 2009;96(3):271. [DOI] [PubMed] [Google Scholar]
- 116.Coutinho H, Galloway A, Ajdukiewicz K, Cleeve V. A case of Staphylococcus aureus septicaemia following platelet transfusion. J Clin Pathol. 2010;63(3):262-3 [DOI] [PubMed] [Google Scholar]
- 117.Diamantidis M, Ionanidou-Papagiannnaki E, Kountouras J, Mandala E, Taspournas G, Frida-Michailidou I, et al. High prevalence of Helicobacter pylori infection in Greek patients with myelodysplastic syndromes. Acta Haematol. 2010;124(3):141-9 [DOI] [PubMed] [Google Scholar]
- 118.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guidelines for the use of antiicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56-93 [DOI] [PubMed] [Google Scholar]
- 119.Benton N, Musaad S, Vaughan R, McLean L. Recurrent multifocal Mycobacterium kansasii infection in an immunosuppressed patient with myelodysplasia and relapsing polychondritis. Rheumatology (Oxford). 2004;43(11):1453-4 [DOI] [PubMed] [Google Scholar]
- 120.Kim H, Goo J, Kim H, Lee J, Seo J, Im J. Tuberculosis in patients with myelodysplastic syndromes. Clin Radiol. 2002;57(5):408-14 [DOI] [PubMed] [Google Scholar]
- 121.Komeno T, Itoh T, Ohtani K, Kamoshita M, Hasegawa Y, Hori M, et al. Disseminated nontuberculous mycobacteriosis caused by mycobacterium kansasii in a patient with myelodysplastic syndrome. Intern Med. 1996;35(4):323-6 [DOI] [PubMed] [Google Scholar]
- 122.Nakada S, Sekikawa T, Takahara S, Yamazaki Y, Yamada J, Yamada H, et al. Nontuberculous atypical mycobacterial infection with progressive pancytopenia in a patient with myelodysplastic syndrome. Runsho Ketsueki. 2001;42(7):543-8 [PubMed] [Google Scholar]
- 123.Tsukada H, Chou T, Ishizuka Y, Ogawa O, Saeki T, Ito S, et al. Disseminated mycobacterium avium-intracellulare infection in a patient with myelodysplastic syndrome (refractory anemia). Am J Hematol. 1994;45(4):325-9 [DOI] [PubMed] [Google Scholar]
- 124.Chen B, Zhao WL, Jin J, Xue YQ, Cheng X, Chen XT, et al. Clinical and cytogenetic features of 508 Chinese patients with myelodysplastic syndrome and comparison with those in Western countries. Leukemia. 2005;19(5):767-75 [DOI] [PubMed] [Google Scholar]
- 125.Garcia-Manero G, Gore SD, Cogle C, Ward R, Shi T, MacBeth KJ, et al. Phase I Study of Oral Azacitidine in Myelodysplastic Syndromes, Chronic Myelomonocytic Leukemia, and Acute Myeloid Leukemia. J Clin Oncol. 2011;29(18):2521-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mele L, Ricci P, Nosari A, Tonso A, Fianci L, Cudillo L, et al. Invasive fungal infection in patients with myelodysplastic syndrome: a report of twelve cases. Leuk Lymphoma. 2002;43(8):1613-7 [DOI] [PubMed] [Google Scholar]
- 127.Pagano L, Caira M, Candoni A, Offidani M, Martino B, Specchia G, et al. Invasive aspergillosis in patients with acute myeloid leukemia: SEIFEM-2008 registry study. Haematologica. 2010;95(4):644-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chandra S, Woodgyer A. Primary cutaneous zygomycosis due to Mucor circinelloides. Australas J Dermatol. 2002;43(1):39-42 [DOI] [PubMed] [Google Scholar]
- 129.García-Bustínduy M, Guimerá-Martín-Neda F, Noda A, Lecuona M, Sánchez-González R, González de Mesa MJ, et al. Primary cutaneous mucormycosis: a diagnosis to consider. J Eur Acad Dermatol Venereol. 1999;12(3):258-62 [DOI] [PubMed] [Google Scholar]
- 130.Kalayjian RC, Herzig RH, Cohen AM, Hutton MC. Thrombosis of the aorta caused by mucormycosis. South Med. 1988;81(9):1180-2 [DOI] [PubMed] [Google Scholar]
- 131.Machida U, Kami M, Uozaki H, Makimura K, Yamaguchi H, Hirai H. Subacute spinal cord infarction due to zygomycotic thrombosis in a patient with myelodysplastic syndrome. Haematologica. 2000;85(9):1004-6 [PubMed] [Google Scholar]
- 132.Moses AE, Rahav G, Barenholz Y, Elidan J, Azaz B, Gillis S, et al. Rhinocerebral mucormycosis treated with amphotericin B colloidal dispersion in three patients. Clin Infect Dis. 1998;26(6):1430-3 [DOI] [PubMed] [Google Scholar]
- 133.Nakazato T, Nagasaki A, Nakayama T, Shinhama A, Taira N, Takasu N. Sinonasal zygomycosis in a patient with myelodysplastic syndrome following non-myeloablative allogeneic peripheral blood stem cell transplantation. Intern Med. 2007;46(22):1881-2 [DOI] [PubMed] [Google Scholar]
- 134.Ng TT, Campbell CK, Rothera M, Houghton JB, Hughes D, Denning DW. Successful treatment of sinusitis caused by Cunninghamella bertholletiae. Clin Infect Dis. 1994;19(2):313-6 [DOI] [PubMed] [Google Scholar]
- 135.Rozich J, Holley HJ, Henderson F, Gardner J, Nelson F. Cauda equina syndrome secondary to disseminated zygomycosis. JAMA. 1988;260(24):3638-40 [PubMed] [Google Scholar]
- 136.Takashima R, Odaka M, Watanabe Y, Hirat K, Yoshida A. Case of basilar artery occlusion caused by mucormycotic embolism in the course of myelodysplastic syndrome. Brain Nerve. 2009;61(9):1079-82 [PubMed] [Google Scholar]
- 137.Wohlrab JL, Anderson ED, Read CA. A patient with myelodyplastic syndrome, pulmonary nodules, and worsening infiltrates. Chest. 2001;120(3):1014-7 [DOI] [PubMed] [Google Scholar]
- 138.Wüppenhorst N, Lee MK, Rappold E, Kayser G, Beckervordersandforth J, de With K, et al. Rhino-orbitocerebral zygomycosis caused by Conidiobolus incongruus in an immunocompromised patient in Germany. J Clin Microbiol. 2010;48(11):4322-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Skiada A, Pagano L, Groll A, Zimmerli S, Dupont B, Lagrou K, et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect. 2011;17(12):1859-67 [DOI] [PubMed] [Google Scholar]
- 140.De Pasqual A, Deprez M, Ghaye B, Frère P, Kaschten B, Hayette MP, et al. Invasive pulmonary mucormycosis with invasion of the thoracic spine in a patient with myelodysplastic syndrome. Rev Med Liege. 2008;63(12):702-6 [PubMed] [Google Scholar]
- 141.Joshita S, Kitano K, Nagaya T, Kamijo A, Nakazawa K, Ishida F. Zygomycosis presenting as acute myocardial infarction during hematological malignancies. Intern Med. 2008;47(9):839-42 [DOI] [PubMed] [Google Scholar]
- 142.Patterson TF, Mackool B, Gilman M, Piris A. Case 22-2009: A 59 year-old man with skin and pulmonary lesions after chemotherapy for leukemia. N Engl J Med. 2009;36(13):287-96 [DOI] [PubMed] [Google Scholar]
- 143.Kim J, Kim Y, Park C, Kang J, Kim B, Woo J, et al. A case of disseminated Trichosporon beigelii infection in a patient with myelodyspalstic syndrome after chemotherapy. J Korean Med Sci. 2001;16(4):505-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Inoue Y, Onoda M, Aihara M, Koiwa K, Yamane Y, Ikezawa Z. Cryptococcal cellulitis in a patient with myelodysplastic syndrome. Acta Derm Venerol. 2011;91(2):199-200 [DOI] [PubMed] [Google Scholar]
- 145.Liu P, Yang Y, Shi Z. Cryptococcal liver abscess: a case report of successful treatment with amphotericin-B and literature review. Jpn J Infect Dis. 2009;62(1):59-60 [PubMed] [Google Scholar]
- 146.Pagano L, Fianchi L, Mele L, Girmenia C, Offidani M, Ricci P, et al. Pneumocystis carinii pneumonia in patients with malignant haematological diseases: 10 years' experience of infection in GIMENA centres. Br J Haematol. 2002;117(2):379-86 [DOI] [PubMed] [Google Scholar]
- 147.Matsumura Y, Shindo Y, Iinuma Y, Yamamoto M, Shirano M, Matsushima A, et al. Clinical characteristics of Pneumocystis pneumonia in non-HIV patients and prognostic factors including micriobiological genotypes. BMC Infect Dis. 2011;11:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Baurmann H, Schwarz T, Oertel J, Serke S, Roggendorf M, Huhn D. Acute parvovirus B19 infection mimicking myelodysplastic syndrome of the marrow. Ann Hematol. 1992;64(1):43-5 [DOI] [PubMed] [Google Scholar]
- 149.Hasle H, Heegaard E, Kerndrup G, Jensen I, Peterslund N, Hornsleth A. Parvovirus B19 infection infrequently involved in children and adults with myelodysplastic syndrome. Leuk Res. 1996;20(1):81-3 [DOI] [PubMed] [Google Scholar]
- 150.Hasle H, Kerndrup G, Jacobsen BB, Heegaard E, Horsnleth A, Lillevang S. Chronic parvovirus infection mimicking myelodysplastic syndrome in a child with subclinical immunodeficiency. Am J Pediatr Hematol Oncol. 1994;16(4):329-33 [PubMed] [Google Scholar]
- 151.Yetgin S, Cetin M, Yenicesu I, Ozaltin F, Uckan D. Acute parvovirus B19 infection mimicking juvenile myelomonocytic leukemia. Eur J Haematol. 2000;65(4):276-8 [DOI] [PubMed] [Google Scholar]
- 152.Yarali N, Duru F, Sipahi T, Kara A, Tezic T. Parvovirus B19 infection reminiscent of myelodysplastic syndrome in three children with chronic hemolytic anemia. Pediatr Hematol Oncol. 2000;17(6):475-82 [DOI] [PubMed] [Google Scholar]
- 153.Kagialis-Girard S, Durand B, Mialou V, Pagès M, Galambrun C, Bertrand Y, et al. Human herpesvirus 6 infection and transient acquired myelodysplasia in children. Pediatr Blood Cancer. 2006;47(5):543-8 [DOI] [PubMed] [Google Scholar]
- 154.Steensma D. Hematopoietic growth factors in myelodysplastic syndromes. Semin Oncol. 2011;38(5):635-47 [DOI] [PubMed] [Google Scholar]
- 155.Thompson JA, Gilliland DG, Prchal JT, Bennett JM, Larholt K, Nelson RA, et al. Effect of recombinant human erythropoietin combined with granulocyte/macrophage colony-stimulating factor in the treatment of patients with myelodysplastic syndrome. Blood. 2000;95(4):1175-9 [PubMed] [Google Scholar]
- 156.Hellström-Lindberg E, Ahigren T, Beguin Y, Carlsson M, Carneskog J, Dahl IM, et al. Treatment of anemia in myelodysplastic syndromes with granulocyte colony-stimulating factor plus erythropoietin: Results from a randomized phase II study and long-term follow-up of 71 patients. Blood. 1998;92(1):68-75 [PubMed] [Google Scholar]
- 157.Negrin RS, Stein R, Doherty K, Cornwell J, Vardiman J, Krantz S, et al. Maintenance treatment of the anemia of myelodysplastic syndromes with recombinant human granulocyte colony-stimulating factor and erythropoietin: Evidence for in vivo synergy. Blood. 1996;87(10):4076-81 [PubMed] [Google Scholar]
- 158.Negrin RS, Stein R, Vardiman J, Doherty K, Cornwell J, Krantz S, et al. Treatment of the anemia of myelodysplastic syndromes using recombinant human granulocyte colony-stimulating factor in combination with erythropoietin. Blood. 1993;82(3):737-43 [PubMed] [Google Scholar]
- 159.Negrin R, Haeuber D, Nagler A, Kobayashi Y, Sklar J, Donlon T, et al. Maintenance treatment of patients with myelodysplastic syndromes using recombinant human granulocyte colony-stimulating factor. Blood. 1990;76(1):36-43 [PubMed] [Google Scholar]
- 160.Rose C, Wattel E, Bastion Y, Berger E, Bauters F, Coiffier B, et al. Treatment with very lowdose GM-CSF in myelodysplastic syndromes with neutropenia. A report on 28 cases. Leukemia. 1994;8(9):1458-62 [PubMed] [Google Scholar]
- 161.Shikama Y, Shichishima T, Ohto H, Jubinski PT, Maryuma Y. Neutrophil-specific reduction in the expresion of granulocyte-macrophage colony-stimulating factor receptor subunits in myelodysplastic syndromes. Br J Haematol. 2000;111(3):863-72 [PubMed] [Google Scholar]
- 162.Nagler A, Binet C, Mackichan M, Negrin R, Bangs C, Donlon T, et al. Impact of marrow cytogenetics and morphology on in vitro hematopoiesis in the myelodysplastic syndromes: comparison between recombinant human granulocyte colony-stimulating factor (CSF) and granulocyte-monocyte CSF. Blood. 1990;76(7):1299-307 [PubMed] [Google Scholar]
- 163.Verhoef G, Boogaerts M. In vivo administration of granulocyte-macrophage colony stimulating factor enhances neutrophil function in patients with myelodysplastic syndromes. Br J Haematol. 1991;79(2):177-84 [DOI] [PubMed] [Google Scholar]
- 164.Felzmann T, Gisslinger H, Ludwig H. Immunological findings in patients with myelodysplastic syndrome. Leuk Lymphoma. 1994;15(3-4):201-8 [DOI] [PubMed] [Google Scholar]
- 165.Schuster M, Thompson J, Larson R, et al. Randomized trial of subcutaneous granulocyte-macrophage colon-stimulating factor versus observation in patients with myelodysplastic syndrome. J Cancer Res Clin Oncol. 1990;116:1079a [Google Scholar]
- 166.Greenberg P, Taylor K, Larson R, et al. Phase III randomized multicenter trial of recombinant human G-CSF in MDS. American Society of Hematology Annual Meeting; 1993: Blood;1993. p.196a [Google Scholar]
- 167.Zwierzina H, Suciu S, Loeffler-Ragg J, Neuwirtova R, Fenaux P, Beksac M, et al. Low-dose cytosine-arabinoside (LD-AraC) vs LD-AraC plus granulocyte-macrophage colony stiumating factor vs LD-AraC plus Interleukin-3 for myelodysplastic syndrome patients with a high risk of developing acute leukemia: final results of a randomized phase III study (06903) of the EORTC Leukemia Cooperative Group. Leukemia. 2005;19(11):1929-33 [DOI] [PubMed] [Google Scholar]
- 168.Ossenkoppele G, van der Holt B, Verhoef G, Daenen S, Verdonck L, Sonneveld P, et al. A randomized study of granulocyte colony-stimulating factor applied during and after chemotherapy in patients with poor risk myelodysplastic syndromes: a report from the HOVON Cooperative Group. Dutch-Belgian Hemato-Oncology Cooperative Group. Leukemia. 1999;13(8):1207-13 [DOI] [PubMed] [Google Scholar]
- 169.Giagounidis A, Fenaux P, Mufti G, Muus P, Platzbecker U, Sanz G, et al. Practical recommendations on the use of lenalidomide in the management of myelodysplastic syndromes. Ann Hematol. 2008;87(5):345-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Levenga TH, Timmer-Bonte NH. Review of the value of colony stimulating factors for prophylaxis of febrile neutropenic episodes in adult patients treated for haematological malignancies. Br J Haematol. 2007;138(2):146-52 [DOI] [PubMed] [Google Scholar]
- 171.Scott BL, Estey E. Management of myelodysplastic syndromes: 2008 update. Oncology. 2008;22(12):1344-68 [PMC free article] [PubMed] [Google Scholar]
- 172.Carlet J, Collignon P, Goldmann D, Goossens H, Gyssens IC, Harbath S, et al. Society's failure to protect a precious resource: antibiotics. The Lancet. 2011;378(9788):369-71 [DOI] [PubMed] [Google Scholar]
- 173.Hughes J. Preserving the lifesaving power of antimicrobial agents. JAMA. 2011;305(10):1027-8 [DOI] [PubMed] [Google Scholar]
- 174.Maertens J, Groll A, Cordonnier C, De la Camara R, Roilides E, Marchetti O. Treatment and timing in invasive mould disease. J Antimicrob Chemother. 2011;66(Suppl 1):i37-43 [DOI] [PubMed] [Google Scholar]
- 175.Alanio A, Cordonnier C, Bretagne S. Azole Resistance in Aspergillus fumigatus – Current Epidemiology and Future Perspectives. Curr Fungal Infect Rep. 2011;5:168-78 [Google Scholar]
- 176.Howard S, Cerar D, Anderson M, Albarrag A, Fisher M, Pasqualotto A, et al. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis. 2009;15(7):1068-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Leitch HA. Iron overload in the infectious risk of MDS. Drugs. 2011;71(2):155-77 [DOI] [PubMed] [Google Scholar]
- 178.Spellberg B, Ibrahim A, Chin-Hong P, Kontoyannis D, Morris M, Perfect J, et al. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012;67(3):715-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Almyroudis N, Segal BH. Antibacterial prophylaxis in patients with cancer and neutropenia. N Engl J Med. 2006;354(1):90-4 [PubMed] [Google Scholar]
- 180.Baden L. Prophylactic antimicrobial agents and the importance of fitness. N Engl J Med. 2005;353(10):1052-4 [DOI] [PubMed] [Google Scholar]
- 181.Lyons R, Cosgriff T, Modi S, Gersh R, Hainsworth J, Cohn A, et al. Hematologic response to three alternative dosing schedules of azacitidine in patients with myelodysplastic syndromes. J Clin Oncol. 2009;27(11):1850-6 [DOI] [PubMed] [Google Scholar]
- 182.Fenaux P, Bowen D, Gattermann N, Hellström-Lindberg E, Hofmanne W, Pfeilstöcker M, et al. Practical use of azacitidine in higher-risk myelodysplastic syndromes: An expert panel opinion. Leukemia Res. 2010;34(11):1410-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.