Abstract
Background
Nucleus free red blood cells are unique to mammals. During their terminal stage of differentiation, mammalian erythroblasts exit the cell cycle and enucleate. We previously found that survivin, a member of the chromosomal passenger complex that is required for cytokinesis, is highly expressed in late non-dividing cells. The role of survivin in enucleating erythroblasts is not known.
Design and Methods
In order to identify the role of survivin in these late erythroblasts, we performed proteomic analysis on survivin-bound protein complexes purified from murine erythroleukemia cells. Various molecular and cell biological techniques were used to confirm the presence and function of this novel complex. Furthermore, we used survivinfl/fl mice to study the effect of loss of survivin in enucleating erythroblasts.
Results
We found that survivin failed to co-localize with its known partners' inner centromere protein or Aurora-B in enucleating erythroblasts but rather exists in a multi-protein complex with epidermal growth factor receptor substrate15 and clathrin, two proteins that mediate endocytic vesicle trafficking. As evidence for a direct role of this latter complex in enucleation, we found that knockdown of the genes reduced the efficiency of enucleation of primary human erythroblasts. We also observed that loss of survivin in murine erythroblasts inhibited enucleation and that survivin-deficient cells harbored smaller cytoplasmic vacuoles. Interestingly, vacuolin-1, a small molecule that induces vacuole fusion, rescued the defective enucleation caused by survivin deficiency.
Conclusions
This study identified a novel role for survivin in erythroblast enucleation through previously unknown protein partners.
Key words: survivin, erythroblast enucleation, protein role
Introduction
Survivin, a 16.5 kDa member of the inhibitor of apoptosis (IAP) family of proteins, plays an essential role in cell division as a component of the chromosome passenger complex (CPC).1 The CPC, which comprises survivin, aurora B kinase, INCENP (inner centromere protein) and borealin, has essential functions at multiple points and multiple locations during mitosis.2 This complex first binds along the length of chromosomes, re-localizes to centromeres, then migrates to the central spindle in anaphase, and resides in the midbody and cleavage furrow during cytokinesis. Loss of any of the CPC proteins results in delocalization of the partner proteins and multiple cell division defects. For example, knockdown or knockout of survivin leads to G1 arrest, polyploidy, and death by mitotic catastrophe.3 Apart from in normal proliferating cells, survivin is also highly expressed in cancer cells where it likely serves as an inhibitor of apoptosis.4 This high expression level in tumors has encouraged researchers to consider survivin as a molecular target to treat cancer cells.
We previously reported that survivin is abundantly expressed in orthochromatic erythroblasts that have exited the cell cycle and are undergoing nuclear condensation on the way towards enucleation.5 We were surprised to find that survivin is highly expressed in these non-cancerous, non-dividing cells. As evidence for a functional role of survivin in red blood cell maturation, we observed that a subset of survivin heterozygous mice exhibited a decrease in the percentage of enucleated cells as compared to wild-type littermates.6 Together, these findings led us to hypothesize that survivin plays a novel role in enucleation of erythroblasts.
We recently showed that vesicle trafficking, specifically the formation, movement and subsequent coalescence of vacuoles at the junction of the nucleus and the cytoplasm of erythroblast, contributes to enucleation.7 Here, we show that survivin is required for erythroblast enucleation, but instead of acting on cytokinesis via the CPC, survivin contributes to enucleation through an interaction with EPS15 and clathrin, two proteins that mediate endocytic vesicle trafficking. We also demonstrate that survivin overexpression is sufficient to induce enucleation of MEL (murine erythroleukemia) cells, and that in primary erythroblasts it influences the formation of large cytoplasmic vacuoles, which in turn promote the separation of the nascent reticulocyte from the nucleus.
Design and Methods
Materials
For Western blot assays, rabbit polyclonal anti-survivin (R&D Systems and Novus Biologicals), mouse monoclonal anti-EPS15 (BD Transduction Laboratories), mouse monoclonal anti-clathrin heavy chain (clone TD.1 from Covance), mouse anti-HSC-70 (Santa Cruz), and rabbit polyclonal anti-INCENP (Sigma) antibodies were used. For immunofluorescence, mouse monoclonal anti-survivin (Cell Signaling), rabbit polyclonal anti-survivin (Novus Biologicals), rabbit polyclonal anti-EPS15 (Covance), mouse monoclonal anti-clathrin heavy chain (clone X22 from Abcam), rabbit polyclonal anti-Aurora B kinase (Abcam), goat polyclonal anti-lamin B (clone M-20 from Santa Cruz), and rabbit and mouse isotype IgG (Abcam) antibodies were used as primary antibodies. Secondary antibodies used in immunofluorescence included Cy2-conjugated F(ab')2 fragment donkey anti-mouse and anti-rabbit, rhodamine red X-conjugated F(ab')2 fragment donkey anti-mouse and anti-rabbit, and Cy5-conjugated F(ab')2 fragment donkey anti-goat (all purchased from Jackson Immuno Research Laboratories). Rhodamine phalloidin, Syto 16, Syto 64, SytoX blue, SytoX red and Slowfade Gold anti-fade reagents were purchased from Molecular Probes. Annexin V-Cy5 was purchased from BioVision. pEF1αBiotag and pEF1αBirA vectors were kind gifts of Dr. Alan Cantor. Blebbistatin and vacuolin-1 were purchased from Calbiochem (EMD Biosciences). Monensin was provided by Dr. Piers Nash.
Further detailed information concerning study design and methods is available in the Online Supplementary Appendix.
Results
Survivin overexpression promotes enucleation of differentiating erythroblasts
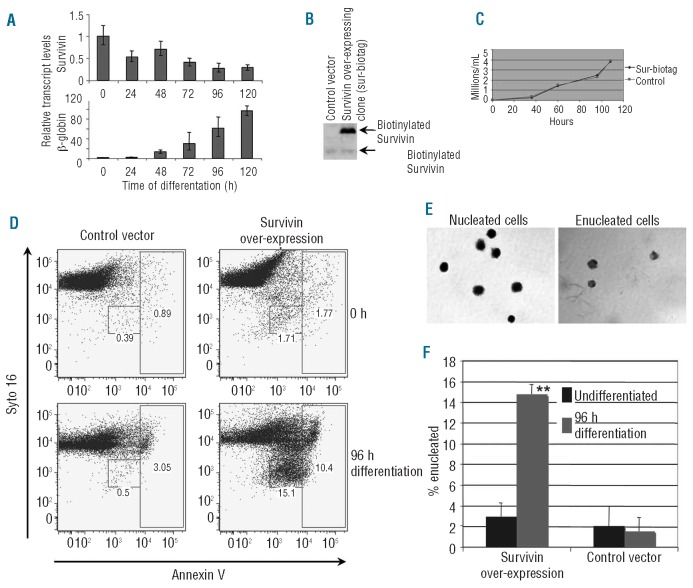
In order to test our hypothesis that survivin plays a role in enucleation, we utilized the murine erythroleukemia (MEL) cell line to examine whether high survivin expression induced enucleation. MEL cells proliferate as proerythroblasts, but can be induced to differentiate through the orthochromatic stage by the addition of DMSO. This differentiation is accompanied by proliferation arrest and upregulation of erythroid genes such as β-globin (Figure 3A). Compared to primary human cells, which show persistent survivin expression through late stages, MEL cells show a striking reduction in survivin mRNA following DMSO treatment (Figure 3A). Since the reduction in survivin expression is accompanied by a failure of DMSO-induced MEL cells to enucleate, we asked whether ectopic expression of survivin would be sufficient to promote enucleation. We utilized an in vivo biotinylation strategy to stably over-express biotin-conjugated survivin in MEL cells. Multiple clones that over-expressed biotinylated survivin, or control clones that only expressed the biotag, were selected (Figure 3B and Online Supplementary Figure S2A) and subjected to differentiation. In the absence of DMSO, both control and survivin over-expressing clones failed to enucleate to an appreciable extent (Figure 3D and F). In contrast, four days after the addition of DMSO, nearly 15% of the survivin over-expressing clones, but less than 2% of the control infected clones, underwent enucleation (Figure 3D-F). Even though survivin is known to play a role in mitosis, its overexpression did not increase or block proliferation of MEL cells (Figure 3C). We observed a similar phenotype in multiple MEL cell clones engineered to express untagged survivin or HA-tagged survivin (Online Supplementary Figure S2B and C and data not shown). These results reveal that overexpression of survivin promotes enucleation of erythroblasts in a differentiation dependent fashion.
Figure 3.
Survivin overexpression promotes enucleation of murine erythroleukemia cells. (A) RT-qPCR analysis of survivin and β-globin expression in MEL cells induced to differentiate with DMSO; 18S rRNA is used as reference control between samples. (B) Expression of biotinylated and endogenous survivin in control and survivin over-expressing MEL clones. (C) Survivin over-expressing and control MEL clones were counted for live cells by trypan blue exclusion and plotted. (D) Enucleation of MEL cells was measured by flow cytometry of Syto 16 and Annexin V stained cultures. Enucleated viable cells are contained within the Syto 16low/Annexin Vlow gate (small box), whereas dying cells or expelled nuclei reside within the Syto 16high/Annexin Vhigh gate (large box). (E) Enucleated cells (small box) and nucleated cells shown were collected by fluorescent activated cell sorting (FACS), cytospun onto glass slides, stained by May-Grunwald-Wright Giemsa and imaged by light microscopy. (F) Overexpression of survivin resulted in a significant increase in enucleation of MEL cells. Bars show mean±standard deviation (SD) for 3 independent experiments.
EPS15 and clathrin are novel partners of survivin
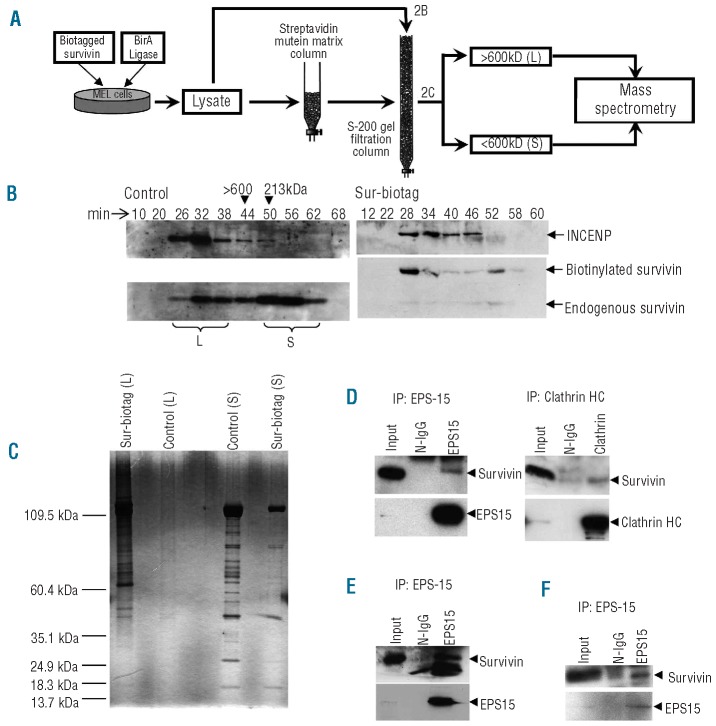
Next, to gain insights into the mechanism of action of survivin in late erythroblasts, we tried to identify its protein partners in erythroid cells. We utilized an in vivo biotinylation strategy to stably over-express biotin-conjugated survivin in MEL cells and a proteomic approach to identify survivin interacting proteins (Figure 2A). Prior to large-scale purification of biotinylated protein complexes from MEL clones that over-expressed biotinylated survivin or biotag-control, we first confirmed that biotinylated survivin formed the same complexes as endogenous survivin. Whole cell extracts from these clones were separated by S-200 gel filtration chromatography and subjected to Western blot analysis or silver staining. This analysis revealed that the endogenous and biotinylated forms of survivin eluted in the same manner (Figure 2B, Online Supplementary Figure S1), indicating that the biotin conjugation did not alter the ability of survivin to enter into its endogenous complexes.
Figure 2.
EPS15 and clathrin, two proteins involved in vesicle trafficking, are novel binding partners of survivin. (A) A schema showing the methodology used to identify survivin binding partner proteins. (B) Whole cell lysates of MEL cells expressing in vivo biotinylated survivin and of MEL cells expressing only the biotag ligase were passed through a Superdex s200 gel filtration column. Fractions of eluted proteins were analyzed for INCENP and survivin by Western blotting. Note that biotinylated survivin and endogenous survivin co-fractionate in two complexes: a large (L) complex (>600 kDa), which contains the majority of endogenous INCENP, and a small (S) complex (<600 kDa). (C) To purify the survivin containing complexes, eluates from the streptavidin mutein matrix were separated by gel filtration chromatography. Two sets of survivin containing fractions, including a large (>600 kD) complex and a small (<600 kD) complex, were collected and pooled. Aliquots from these two fractions were loaded onto a SDS-PAGE and stained with silver. (D) Immunoprecipitation of endogenous EPS15 or clathrin heavy chain from 72 h differentiated human erythroleukemia cells co-precipitated endogenous survivin. Extracts from twenty million cells were used in the IP. N-IgG, normal IgG control. (E) Endogenous EPS15 immunoprecipitates endogenous survivin in uninduced K562 cells. Note that this co-immunoprecipitation was performed from lysates of 50 million cells. (F) Survivin co-precipitates with endogenous EPS15 in lysates of 10 million Day 12 primary human erythroblasts.
To identify survivin partners in erythroid cells, we purified biotinylated survivin and its associated proteins from MEL cell extracts by streptavidin affinity chromatography followed by gel filtration chromatography. In order to minimize the background, and to identify the specific proteins that interact with survivin forming a supernumerary complex of more than 600 kDa, we used a novel approach of passing the purified survivin protein complexes through S-200 gel filtration chromatography and pooled the eluted protein that coincided with large and small molecular weight survivin complexes. We then performed mass spectrometry analysis on those fractions (Figure 2C) and analyzed the proteomic data with Spectrum Mill and Mascot (Online Supplementary Table S1). Our analysis revealed that the top scoring peptides were derived from EGFR-pathway-substrate-15 (EPS15), a protein that is involved in endocytic vesicle formation, progression and trafficking.8-11 Furthermore, our proteomic data analysis showed that peptides derived from clathrin were also present in the purified fractions. Neither of these proteins was isolated from the control samples. Given that EPS15 interacts with clathrin adaptors, such as AP-1 and AP-2, and is a component of clathrin-coated pits,9,11,12 our results suggest that survivin associates with both EPS15 and clathrin in erythroid cells.
To confirm an endogenous interaction among survivin, EPS15 and clathrin in human cells, we performed coimmunoprecipitation studies in the K562 cell line and in primary human erythroblasts. We found that antibodies against EPS15 or clathrin heavy chain co-immunoprecipitated endogenous survivin in human K562 cells that were differentiated 72 h with 1 μM ara-C (Figure 2D). We also detected an interaction between survivin and EPS15 in undifferentiated K562 cells, but only when we used 2.5 times as much input lysates for the immunoprecipitation (Figure 2E). Even though the interaction is less abundant, it is important to note that survivin-EPS15 interaction also exists in undifferentiated proliferating erythroblasts (Figure 2E). It is worth mentioning here that an intact vesicle trafficking pathway is essential for the completion step of cytokinesis in addition to providing required membrane for the dividing daughter cells,13,14 and that a role for survivin- EPS15-clathrin complex in this stage is possible in dividing cells. Next, with respect to primary cells, we confirmed the survivin-EPS15 interaction by performing endogenous EPS15 immunoprecipitation in lysates from Day 12 primary human enucleating erythroblasts, a time point at which more than 95% of the erythroblasts have exited the cell cycle and are in a stage prior to enucleation (Figure 2F). We therefore demonstrate that EPS15 and clathrin are novel binding partners of survivin in erythroblasts.
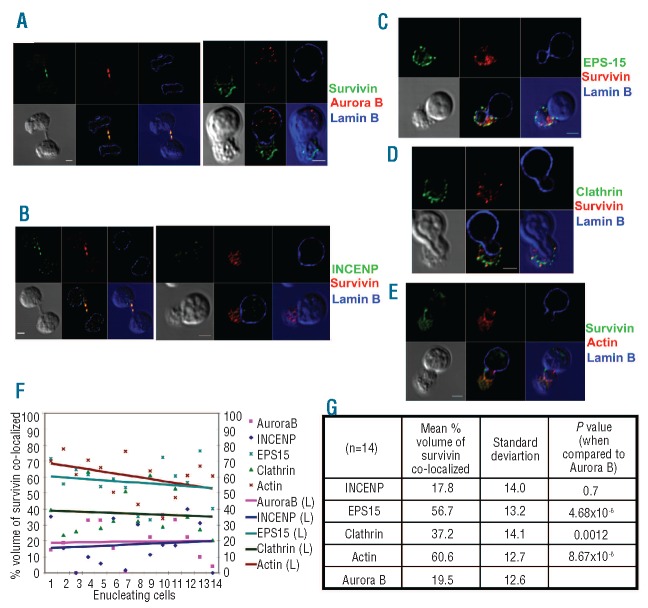
Survivin co-localizes with EPS15 but fails to co-localize with CPC proteins in primary human enucleating cells
Since we did not detect aurora B kinase or inner centromere proteins (INCENP, proteins that partner with survivin in CPC) in the survivin-containing protein complexes by proteomic analysis, and also since multiple attempts of immunoprecipitation of endogenous aurora B kinase did not co-precipitate survivin in erythroblasts (data not shown), we decided to compare the localization patterns of survivin with respect to aurora B kinase, INCENP, EPS15, clathrin and actin in enucleating erythroblasts. To do this, we cultured human primary CD34+ progenitors ex vivo under conditions to generate enucleated reticulocytes.15 After 14 days of culture, after which 30-40% of the cells are reticulocytes, we stained cells with various fluorescently labeled antibodies (against survivin, lamin B, aurora B kinase, INCENP, EPS15 and/or clathrin) or phalloidin (that stains actin) and studied the cells under confocal microscopy. In order to obtain an unbiased calculation of the percentage of co-localization between proteins, we used a software pluggin for ImageJ called Co-localisation Threshold pluggin (Figure 1F and G and Online Supplementary Appendix). The method used for threshold identification of channel intensity and quantification, and the validity, effectiveness and statistical significance of this software has been reported by Costes et al.16 As expected, survivin co-localized with INCENP and aurora-B in dividing cells within the midbody region (Figure 1A and B). In stark contrast, survivin failed to co-localize with either aurora B (19.5%) or INCENP (17.8%) in erythroblasts undergoing enucleation (Figure 1A, B, F and G and Online Supplementary Figure S3A and B). Within enucleating cells, survivin was largely restricted to the cytoplasm where it co-localized with EPS15 (56.7%) and actin (60.6%) (Figure 1C, E-G and Online Supplementary Figure S3C and E). In addition, we also saw a partial overlap between the localization of survivin and clathrin (37.2%). On the other hand, aurora B and INCENP were localized within the nucleus (enclosed within lamin B staining), or the nucleus and cytoplasm, respectively, in regions that did not overlap with survivin (Figure 1A and B and Online Supplementary Figure S3A and B). These findings demonstrate that aurora B and INCENP do not exist within the supernumerary protein complex containing survivin, EPS15 and clathrin in enucleating erythroblasts.
Figure 1.
Survivin co-localizes with EPS15, clathrin and actin in enucleating erythroblasts. (A, B) Cultured human primary erythroid cells were immunostained for survivin and lamin B and either aurora B kinase (A) or INCENP (B), and then imaged by confocal microscopy. A dividing cell (left) and an enucleating cell (right), from the same slide, are shown. (C, D) Erythroid cells were stained with anti-survivin and anti-lamin B, along with either anti-EPS15 or anticlathrin heavy chain antibodies, and then imaged by confocal microscopy. A phase contrast image was shown in gray in the bottom panels along with images showing merge of channels. (E) Images of primary human erythroid cells undergoing enucleation immunostained for survivin and lamin B and chemically stained for actin by rhodamine phalloidin. A representative z-section is shown for each cell. Bottom left panels depict phase contrast images, middle bottom panels show a merge of blue, red and green channels, and bottom right panels show a merge of red and green channels in the blue background of phase contrast image. Series of z sections for A-E are provided in Figure S2. Scale bar: 2 μm. (F) Confocal images of the immunofluorescence studies, including A-E, were analyzed for co-localization as explained in the Design and Methods section. This analysis revealed that the co-localization of survivin with EPS15, actin or clathrin is significantly higher than that of either aurora B or INCENP in enucleating erythroblasts. (G) The mean percentage volume of survivin co-localization with various proteins was calculated as described in the Design and Methods section. This analysis revealed that the co-localization of survivin with EPS15, clathrin or actin was significantly greater than that of survivin and aurora B (P=4.86E-6, P=0.0012 and P=8.67E-6, respectively).
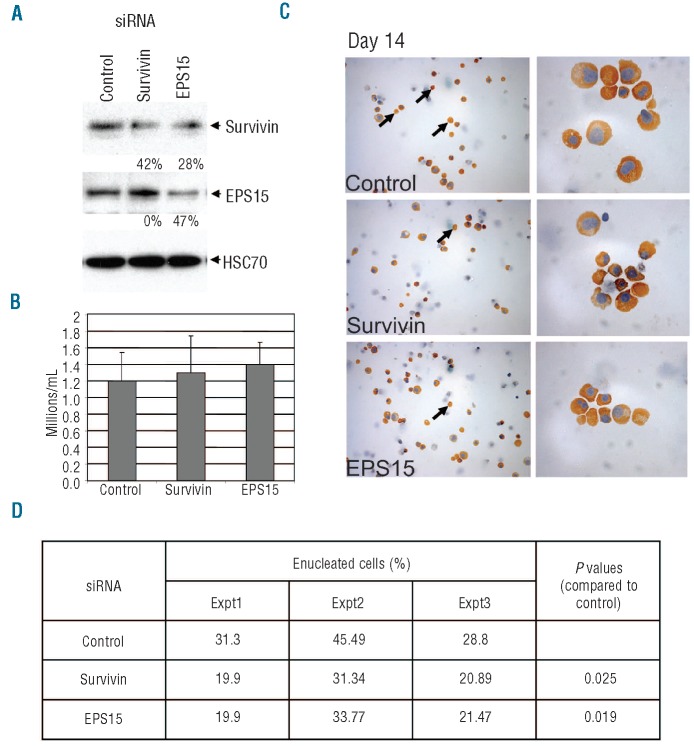
Survivin and EPS-15 are required for enucleation
siRNA mediated knockdown of clathrin inhibits enucleation of primary human erythroblasts, supporting our model that endocytic vesicle trafficking plays an important role in this process.7 To evaluate the contributions of survivin and EPS15 to enucleation, we knocked each gene down in primary human erythroblasts derived from CD34+ cells.15 In this system, the majority of cells have reached the orthochromatic stage and exited the cell cycle by Day 12; by Day 14 approximately 30-40% of the cells have enucleated. In the design and during this experiment, we were careful not to expose proliferating erythroblasts to survivin and EPS15 knockdown. We transfected erythroblasts on Days 10 and 11 of culture with siRNAs against survivin, EPS15, or a control siRNA, measured the extent of protein knock-down on Day 13, and quantified the numbers of enucleated cells on Day 14 (Figure 4). Since we transfect the siRNA on Days 10 and 11, we expect a decrease in protein levels on Day 12, by which time more than 95% of the erythroblasts have exited the cell cycle and have reached the orthochromatic stage. We discovered that knockdown of survivin or EPS15 significantly inhibited enucleation without affecting the survival or differentiation of erythroblasts (Figure 4). From these data, we conclude that survivin and EPS15 are each required for optimal human erythroblast enucleation.
Figure 4.
Knockdown of survivin or EPS15 block enucleation of primary erythroblasts. (A) Human primary erythroid cells derived from CD34+ cells were transfected with siRNAs against survivin, EPS15 or a control siRNA on Days 10 and 11. The knockdown efficiencies were determined by Western blot analysis on Day 13. Using Image J software, we determined that survivin and EPS15 knockdown conditions showed a reduction of 42 and 47% of protein levels, respectively. (B) The total number of live cells in various siRNA treated conditions were counted by trypan blue exclusion on Day 13 and the mean ± standard deviation of 3 independent experiments were plotted. (C) Benzidine stained cytospin images of 14 days differentiated human erythroid cells after treatment with various siRNAs are shown (magnification: left 400X; right 1000X oil immersion objective). Cells were collected on Day 14, washed, cytospun onto poly (L) lysine coated slides and stained with benzidine and hematoxylin. Hemoglobinized erythroblasts and reticulocytes stain positive for benzidine (golden brown) and hematoxylin stains the nuclei into blue color. Arrows denote enucleated reticulocytes. (D) Knockdown of survivin and EPS15, each lead to a significant reduction in enucleation of primary human erythroblasts. The percentages of enucleated cells relative to the total number of benzidine positive cells are shown for 3 independent experiments.
Loss of survivin inhibits enucleation by disrupting formation of cytoplasmic vacuoles
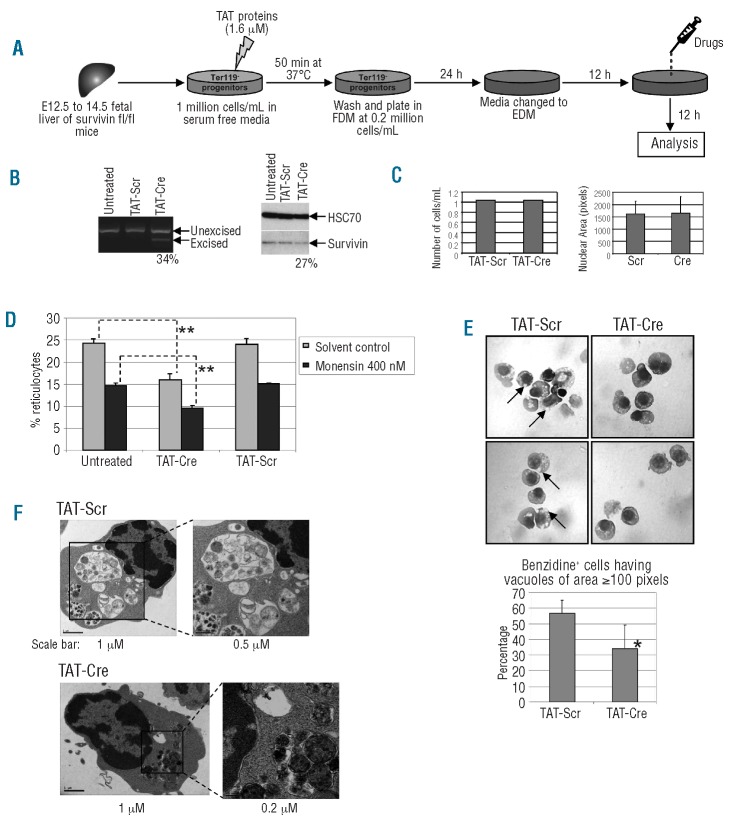
To further demonstrate the requirement for survivin in enucleation, we deleted survivin from primary murine fetal liver erythroblasts and monitored enucleation. Fetal liver erythroblasts were harvested from E14.5 survivin-floxed (Surfl/fl) embryos and incubated with TAT-Cre fusion protein, an unrelated TAT-scrambled protein, or media alone (Figure 5A). After 36 h of culture, we quantified the extent of survivin gene excision and the level of survivin protein in the different populations. We observed a 27% reduction in survivin protein in the TAT-Cre treated cells, an effect that was consistent with the 34% excision of the survivin gene as determined by PCR analysis (Figure 5B). Neither the scrambled TAT, nor the media control sample led to excision of the survivin gene. Next, after 48 h of culture, we determined the proportion of erythroblasts that underwent enucleation by flow cytometry after staining with Syto 64 and Ter119. We found that deletion of survivin caused a significant decrease in the enucleation of fetal liver erythroblasts, but did not affect cell number, differentiation or nuclear condensation (Figure 5C and D and Online Supplementary Figure S4). Furthermore, the addition of 400 nM monensin, a lipid soluble Na+ ionophore that impairs vesicle trafficking through multiple pathways,7,17 led to a further 30% reduction in the extent of enucleation of Surfl/fl progenitors. These results confirm that survivin is required for enucleation of primary murine erythroblasts.
Figure 5.
Loss of survivin inhibits enucleation by suppressing vacuole formation. (A) Schematic depiction of the experimental approach. (B) The extent of survivin gene excision was evaluated by PCR (left) and the level of survivin protein in cultured cells was measured by Western blot analysis (right) 36 h after treatment. The percentages of excised allele and percent reduction of survivin protein, determined by ImageJ software, are shown below the blots. HSC-70 was used as a loading control. (C) The numbers of live cells at 36 h, enumerated by trypan blue staining, are shown for TAT-Cre and TAT-Scr conditions (left). Nuclear size of benzidine positive cells after 48 h of culture were determined by ImageJ software and the mean ± standard deviation of 3 independent experiments are shown (right). D) Effect of TAT-Cre or TAT-Scrambled (Scr) peptide treatment, or no treatment, on enucleation of Ter119- Surfl/fl erythroid progenitors. Dark bars correspond to cultures that were also treated with 400 nM monensin after 36 h of incubation. Data are shown as mean ± standard deviation for 3 independent experiments. **P<0.01. (E) Top: benzidine and hematoxylin stained cytospins of TAT-Scr and TAT-Cre treated Surfl/fl erythroid progenitors after 48 h of culture. Cells were viewed using a light microscope with 100X oil immersion objective. Representative images for each condition are shown. (Top) arrows point to large vacuoles in control cells. (Bottom) the number of benzidine positive cells that harbored vacuoles with an area larger than 100 pixels were enumerated and the means ± standard deviations of 5 independent experiments are shown (right). *P<0.05. (F) Transmission electron microscopic images of Surfl/fl fetal liver erythroblasts cultured with TAT-Scr, TAT-Cre, or 1 μM monensin along with control are shown.
To investigate the mechanism by which loss of survivin inhibits enucleation, we analyzed the morphology of benzidine/hematoxylin stained cells collected from Surfl/fl fetal liver cultures treated with TAT-Cre and TAT-Scr. After 48 h of culture, we observed that the majority of erythroid cells treated with TAT-Scr (control) harbored relatively large vacuoles in their cytoplasm, whereas the preponderance of cells treated with TAT-Cre did not show similarly sized vacuoles (Figure 5E). To assess the difference in vacuole size between the two groups, we used ImageJ software and measured the area of the largest vacuoles in 100 benzidine positive cells from each condition. Using the benchmark of 100 pixels as the size of a large vacuole, we found that the TAT-Cre culture had 40% fewer cells with 100 or more pixels vacuole when compared to control (n=5, P=0.015). In addition, the average size of the vacuoles within cells was smaller after TAT-Cre than control TAT protein. Loss of survivin, however, did not affect the average number of vacuoles per cell (Online Supplementary Figure S5).
Electron microscopy confirmed that there were marked differences in the composition of erythroid cells cultured with TAT-Cre versus TAT-Scr (Figure 5F). The majority of erythroid cells in the TAT-Scr conditions (∽90%) harbored multiple, large vacuoles in the cytoplasm. In contrast, only half of the erythroid cells within the TAT-Cre cultures harbored vacuoles with a similar appearance. Furthermore, approximately 40% of erythroid cells treated with TAT-Cre harbored vesicles that were filled with undegraded organelles resembling autophagosomes (Figure 5F). These results demonstrate that the enucleation defect caused by survivin knockout is associated with aberrant vacuole formation and/or fusion.
Survivin knockout-induced enucleation defect is reversed by vacuolin-1
We then asked whether the enucleation block observed in survivin deficient cells is genuinely due to its effect on enucleation per se or to cell death and cell cycle arrest. We previously reported that vacuolin-1, a chemical that induces vacuole formation by homotypic fusion of endosomes and lysosomes,18,19 when added to the fetal liver erythroblast culture at 36 h after plating, increases enucleation of erythroblasts without affecting the cell cycle, apoptosis or differentiation.7 If survivin knockout caused cell death in erythroblasts, then adding vacuolin-1 at 36 h would not rescue the phenotype. We found that vacuolin- 1 rescued the enucleation defect of Surfl/fl fetal liver erythroblasts deleted for survivin with TAT-Cre (Figure 6A). Also, if vacuolin-1 can drive the erythroblasts towards enucleation while bypassing a cell cycle block, it should rescue enucleation efficiency in erythroblasts that are blocked in cell cycle, for example blebbistatin, which arrests dividing cells in cytokinesis.20 Indeed, vacuolin-1 failed to rescue the enucleation block caused by addition of blebbistatin (Figure 6B). The fact that vacuolin-1 rescues the enucleation block caused by survivin deletion, but not the cytokinetic block induced by earlier addition of blebbistatin, serves as further evidence that survivin participates in enucleation in a manner that is distinct from its cell cycle function.
Figure 6.
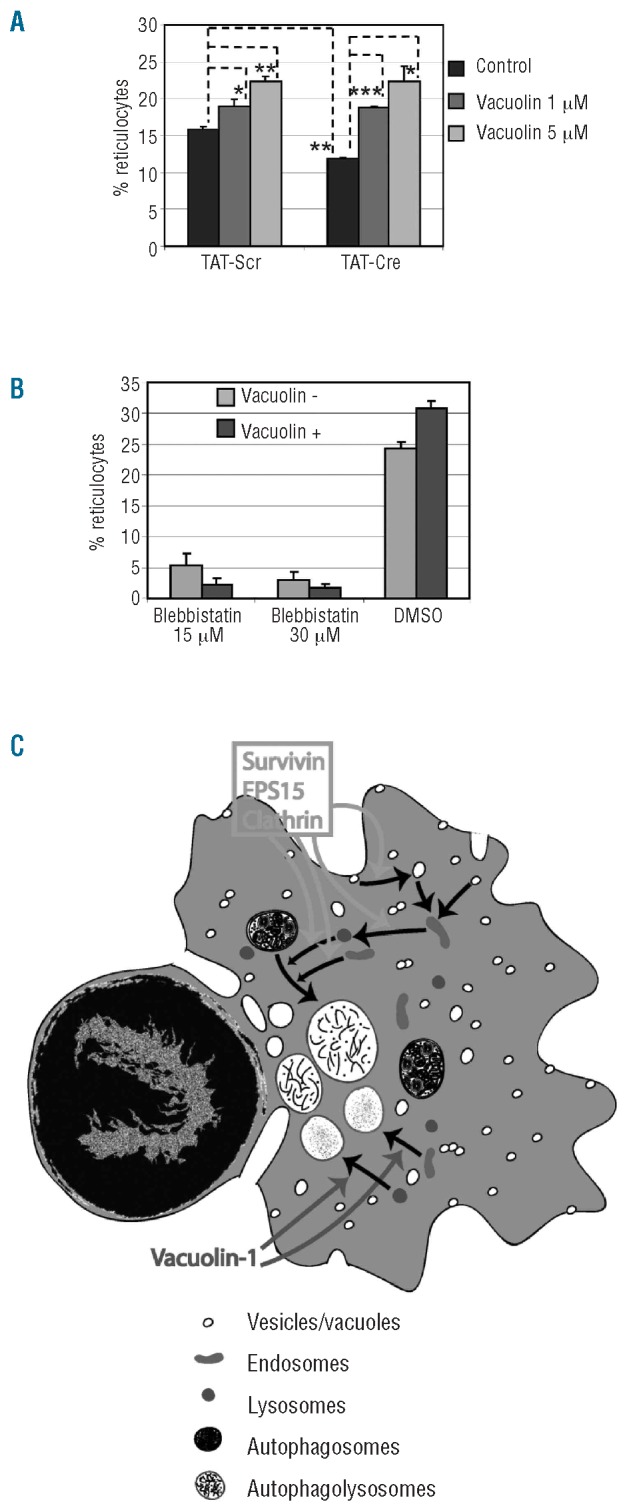
Vacuolin-1 rescues the enucleation defect in survivin deficient cells. (A) Effect of vacuolin-1 on enucleation of Surfl/fl fetal liver erythroblasts cultured with TAT-Scr and TAT-Cre. The means ± standard deviations for 3 independent experiments are shown. The dotted lines indicate the data sets that were evaluated for statistical differences. *P<0.05, **P<0.01 and ***P<0.001. (B) Effect of vacuolin- 1 on enucleation of WT mouse fetal liver erythroblasts in the absence or presence of blebbistatin. Blebbistatin and vacuolin-1 were added at 24 and 36 h time points, respectively, and their effect on enucleation was analyzed at 48 h time point by flow cytometry. Data are depicted as mean ± standard deviation for 3 independent experiments. (C) Model of enucleation. Survivin, EPS15, and clathrin are predicted to cooperate in formation, movement and/or fusion of vesicles, endosomes or lysosomes while vacuolin-1 induces vacuoles by homotypic fusion of endosomes and lysosomes.
Discussion
Enucleation is the final step in the derivation of reticulocytes from mammalian erythroblasts. We recently showed that asymmetric cytokinesis, one possible mechanism of enucleation, is not playing a major part in this process.7 Rather we discovered that vesicle trafficking and fusion of vesicles/vacuoles in the region between the nuclei and incipient reticulocyte contributes to completing enucleation. Indeed, when comparing the processes of cytokinesis with enucleation,21 except for the completion step of cytokinesis, in which vesicle trafficking is important, all other stages are significantly different. Although earlier components of cytokinesis are not involved in the mechanism of enucleation, here we reveal that survivin, an essential component of the CPC that has many different functions,4 is required for enucleation. Since neither nocodazole nor colchicine affected enucleation,7 it is unlikely that survivin promotes enucleation by affecting microtubule dynamics.22,23 Rather, our identification of an interaction among survivin, EPS15 and clathrin supports our model that survivin has a novel function in vesicle trafficking and enucleation.
In analyzing the role of survivin in enucleation, we discovered that deletion of survivin was associated with multiple erythroblast defects. Most prominent among these defects was a reduction in the number and size of cytoplasmic vacuoles. In addition, electron micrographs suggest that the composition of the vacuoles in control and survivin-deleted cultures differ. Vacuoles within the vast majority of TAT-Scr cultures resemble autophago-lysosomes, which participate in degradation of cellular organelles such as mitochondria. In contrast, fewer than half of the erythroblasts within the TAT-Cre culture harbored vacuoles with a similar autophago-lysosome appearance. Instead, many TAT-Cre treated cells harbored smaller vesicles that resemble autophagosomes, which contain undegraded organelles. Although autophagosomes play an essential role in removing organelles from nascent reticulocytes, recent reports suggest that autophagy is not required for enucleation.24,25 We, therefore, conclude that a general defect in the endosome/lysosomal trafficking pathway is manifested as an increase in autophagosomes in the absence of survivin. Our study provides evidence for a function of late endosomes, lysosomes and autophagolysosomes, and for coordination between enucleation and organelle degradation in enucleating cells (Figure 6C). Studies to determine the role of survivin in specific components of vesicle trafficking, and the extent to which survivin contributes to membrane reorganization in normal cytokinesis, are warranted.
EPS15 is a well conserved protein that has three N-terminal EH (EPS15-homology)-domains, a coiled coil middle region determined by heptad repeats and a C-terminal region containing DPR repeats and a proline rich region.26 Even though there is speculation about its possible non-vesicle trafficking roles, such as the neoplastic transformation of cells,8 the role of EPS15 in clathrin-mediated endocytosis is widely accepted.11,27 EPS15 is bound to clathrin adapters AP-2 (plasma membrane to endosome traffic) and AP-1 (trans-golgi network to endosome traffic), and participates in clathrin mediated vesicle trafficking (Figure 6C). Also, it has been shown that AP-2 containing clathrin coats are assembled as patches around lysosomes and induce formation of vesicle buds from them under physiological conditions in vitro.28 Here we show that survivin interacts with EPS15 and clathrin, and that all three genes are required for optimal enucleation. It is important to note here that these proteins are in a supernumerary complex of more than 600 kDa molecular weight and we only know three proteins in that complex. Furthermore, it is not yet known whether survivin and EPS15 bind to each other directly or indirectly. Since around 60% of actin localization pattern coincides with survivin (Figure 1E-G), and since actin has known roles in vesicle trafficking,7,29-31 we can assume a collaborative role for survivin, EPS15 and actin in enucleating erythroblasts.
How does survivin function in vesicle trafficking and enucleation? Survivin may be involved in the movement of vesicles, endosomes or lysosomes, or could also play a role in vesicle fusion along with EPS15. Alternatively, instead of having a general role in vesicle formation, similar to clathrin and EPS15, survivin might function as a selective trafficking agent for a subset of proteins or organelles. Further studies are needed to address these issues.
Supplementary Material
Acknowledgments
the authors thank Jonathan Licht, Gerd Blobel and Mitchell Weiss for discussions and critical reading of the manuscript. We also thank Wei-Jen Tang and Yuequan Shen for their assistance with protein purification, Don Wolfgeher for the mass spectrometry analysis, Ed Conway and Astar Winoto for the survivin floxed mice, and Vitas Bindokas, William Russin and Teng Leong Chew for their assistance in microscopy.
Funding: this work was supported by grants from NIDDK (DK074693 to JDC), the Samuel Waxman Cancer Research Foundation (to JDC), NCI (CA98550 to AW) and the Giving Tree Foundation (to AW), and by the Katten Muchin Rosenman Travel Scholarship Award from the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. Imaging work was performed at the Northwestern University Cell Imaging Facility supported by NCI CCSG P30 CA060553 awarded to the Robert H Lurie Comprehensive Cancer Center. We acknowledge use of the Leica EM-PACT2 highpressure freezer and AFS2 freezesu-bstitution instruments in the Biological Imaging Facility (Northwestern University) funded by the NIH Shared Instrumentation Grant (SRR022494A)
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures: The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3(8):917-21 [DOI] [PubMed] [Google Scholar]
- 2.Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol. 2007;8(10):798-812 [DOI] [PubMed] [Google Scholar]
- 3.Okada H, Bakal C, Shahinian A, Elia A, Wakeham A, Suh WK, et al. Survivin loss in thymocytes triggers p53-mediated growth arrest and p53-independent cell death. J Exp Med. 2004;199(3):399-410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8(1):61-70 [DOI] [PubMed] [Google Scholar]
- 5.Gurbuxani S, Xu Y, Keerthivasan G, Wickrema A, Crispino JD. Differential requirements for survivin in hematopoietic cell development. Proc Natl Acad Sci USA. 2005;102(32):11480-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung CG, Xu Y, Mularski B, Liu H, Gurbuxani S, Crispino JD. Requirements for survivin in terminal differentiation of erythroid cells and maintenance of hematopoietic stem and progenitor cells. J Exp Med. 2007;204(7):1603-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keerthivasan G, Small S, Liu H, Wickrema A, Crispino JD. Vesicle trafficking plays a novel role in erythroblast enucleation. Blood. 2010;116(17):3331-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fazioli F, Minichiello L, Matoskova B, Wong WT, Di Fiore PP. eps15, a novel tyrosine kinase substrate, exhibits transforming activity. Mol Cell Biol. 1993;13(9):5814-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tebar F, Sorkina T, Sorkin A, Ericsson M, Kirchhausen T. Eps15 is a component of clathrin-coated pits and vesicles and is located at the rim of coated pits. J Biol Chem. 1996;271(46):28727-30 [DOI] [PubMed] [Google Scholar]
- 10.Carbone R, Fre S, Iannolo G, Belleudi F, Mancini P, Pelicci PG, et al. eps15 and eps15R are essential components of the endocytic pathway. Cancer Res. 1997;57(24):5498-504 [PubMed] [Google Scholar]
- 11.Chi S, Cao H, Chen J, McNiven MA. Eps15 mediates vesicle trafficking from the trans-Golgi network via an interaction with the clathrin adaptor AP-1. Mol Biol Cell. 2008;19(8):3564-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slepnev VI, Ochoa GC, Butler MH, Grabs D, De Camilli P. Role of phosphorylation in regulation of the assembly of endocytic coat complexes. Science. 1998;281(5378):821-4 [DOI] [PubMed] [Google Scholar]
- 13.Eggert US, Mitchison TJ, Field CM. Animal cytokinesis: from parts list to mechanisms. Annu Rev Biochem. 2006;75:543-66 [DOI] [PubMed] [Google Scholar]
- 14.Albertson R, Riggs B, Sullivan W. Membrane traffic: a driving force in cytokinesis. Trends Cell Biol. 2005;15(2):92-101 [DOI] [PubMed] [Google Scholar]
- 15.Kang JA, Zhou Y, Weis TL, Liu H, Ulaszek J, Satgurunathan N, et al. Osteopontin regulates actin cytoskeleton and contributes to cell proliferation in primary erythroblasts. J Biol Chem. 2008;283(11):6997-7006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costes SV, Daelemans D, Cho EH, Dobbin Z, Pavlakis G, Lockett S. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J. 2004;86(6):3993-4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mollenhauer HH, Morre DJ, Rowe LD. Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity. Biochim Biophys Acta. 1990;1031(2):225-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cerny J, Feng Y, Yu A, Miyake K, Borgonovo B, Klumperman J, et al. The small chemical vacuolin-1 inhibits Ca(2+)- dependent lysosomal exocytosis but not cell resealing. EMBO Rep. 2004;5(9):883-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huynh C, Andrews NW. The small chemical vacuolin-1 alters the morphology of lysosomes without inhibiting Ca2+-regulated exocytosis. EMBO Rep. 2005;6(9):843-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng MM, Chang F, Burgess DR. Movement of membrane domains and requirement of membrane signaling molecules for cytokinesis. Dev Cell. 2005;9(6):781-90 [DOI] [PubMed] [Google Scholar]
- 21.Keerthivasan G, Wickrema A, Crispino JD. Erythroblast enucleation. Stem Cells Int. 2011;2011:139851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemke C, Linss W. Remarks on the role of microtubules in enucleating normoblasts. Anat Anz. 1984;156(5):427-31 [PubMed] [Google Scholar]
- 23.Koury ST, Koury MJ, Bondurant MC. Cytoskeletal distribution and function during the maturation and enucleation of mammalian erythroblasts. J Cell Biol. 1989;109(6 Pt 1):3005-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kundu M, Lindsten T, Yang CY, Wu J, Zhao F, Zhang J, et al. Ulk1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood. 2008;112(4):1493-502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, Chen M, et al. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454(7201):232-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salcini AE, Chen H, Iannolo G, De Camilli P, Di Fiore PP. Epidermal growth factor pathway substrate 15, Eps15. Int J Biochem Cell Biol. 1999;31(8):805-9 [DOI] [PubMed] [Google Scholar]
- 27.Polo S, Confalonieri S, Salcini AE, Di Fiore PP. EH and UIM: endocytosis and more. Sci STKE. 2003;2003(213):re17. [DOI] [PubMed] [Google Scholar]
- 28.Traub LM, Bannykh SI, Rodel JE, Aridor M, Balch WE, Kornfeld S. AP-2-containing clathrin coats assemble on mature lysosomes. J Cell Biol. 1996;135(6 Pt 2):1801-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merrifield CJ, Perrais D, Zenisek D. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell. 2005;121(4):593-606 [DOI] [PubMed] [Google Scholar]
- 30.Le Clainche C, Pauly BS, Zhang CX, Engqvist-Goldstein AE, Cunningham K, Drubin DG. A Hip1R-cortactin complex negatively regulates actin assembly associated with endocytosis. EMBO J. 2007;26(5):1199-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soldati T, Schliwa M. Powering membrane traffic in endocytosis and recycling. Nat Rev Mol Cell Biol. 2006;7(12):897-908 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.