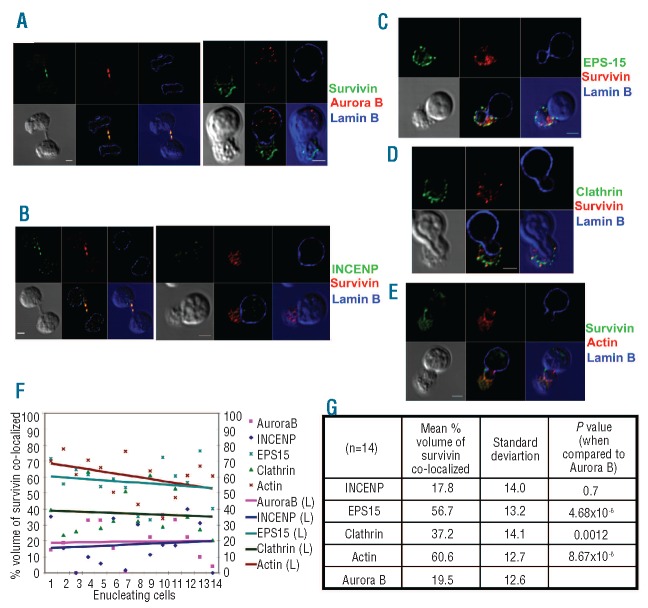
Figure 1.
Survivin co-localizes with EPS15, clathrin and actin in enucleating erythroblasts. (A, B) Cultured human primary erythroid cells were immunostained for survivin and lamin B and either aurora B kinase (A) or INCENP (B), and then imaged by confocal microscopy. A dividing cell (left) and an enucleating cell (right), from the same slide, are shown. (C, D) Erythroid cells were stained with anti-survivin and anti-lamin B, along with either anti-EPS15 or anticlathrin heavy chain antibodies, and then imaged by confocal microscopy. A phase contrast image was shown in gray in the bottom panels along with images showing merge of channels. (E) Images of primary human erythroid cells undergoing enucleation immunostained for survivin and lamin B and chemically stained for actin by rhodamine phalloidin. A representative z-section is shown for each cell. Bottom left panels depict phase contrast images, middle bottom panels show a merge of blue, red and green channels, and bottom right panels show a merge of red and green channels in the blue background of phase contrast image. Series of z sections for A-E are provided in Figure S2. Scale bar: 2 μm. (F) Confocal images of the immunofluorescence studies, including A-E, were analyzed for co-localization as explained in the Design and Methods section. This analysis revealed that the co-localization of survivin with EPS15, actin or clathrin is significantly higher than that of either aurora B or INCENP in enucleating erythroblasts. (G) The mean percentage volume of survivin co-localization with various proteins was calculated as described in the Design and Methods section. This analysis revealed that the co-localization of survivin with EPS15, clathrin or actin was significantly greater than that of survivin and aurora B (P=4.86E-6, P=0.0012 and P=8.67E-6, respectively).