Abstract
Background
Criteria for good candidate antigens for immunotherapy of acute myeloid leukemia are high expression on leukemic stem cells in the majority of patients with acute myeloid leukemia and low or no expression in vital tissues. It was shown in vaccination trials that Receptor for Hyaluronic Acid Mediated Motility (RHAMM/HMMR) generates cellular immune responses in patients with acute myeloid leukemia and that these responses correlate with clinical benefit. It is not clear however whether this response actually targets the leukemic stem cell, especially since it was reported that RHAMM is expressed maximally during the G2/M phase of the cell cycle. In addition, tumor specificity of RHAMM expression remains relatively unexplored.
Design and Methods
Blood, leukapheresis and bone marrow samples were collected from both acute myeloid leukemia patients and healthy controls. RHAMM expression was assessed at protein and mRNA levels on various sorted populations, either fresh or after manipulation.
Results
High levels of RHAMM were expressed by CD34+CD38+ and CD34- acute myeloid leukemia blasts. However, only baseline expression of RHAMM was measured in CD34+CD38- leukemic stem cells, and was not different from that in CD34+CD38- hematopoietic stem cells from healthy controls. RHAMM was significantly up-regulated in CD34+ cells from healthy donors during in vitro expansion and during in vivo engraftment. Finally, we demonstrated an explicit increase in the expression level of RHAMM after in vitro activation of T cells.
Conclusions
RHAMM does not fulfill the criteria of an ideal target antigen for immunotherapy of acute myeloid leukemia. RHAMM expression in leukemic stem cells does not differ significantly from the expression in hematopoietic stem cells from healthy controls. RHAMM expression in proliferating CD34+ cells of healthy donors and activated T cells further compromises RHAMM-specific T-cell-mediated immunotherapy.
Key words: leukemic stem cell, acute myeloid leukemia, cell therapy and immunotherapy, HMMR, RHAMM
Introduction
A priority-ranked list of cancer vaccine target antigens was published in 2009, ranking the cancer-associated antigens based on predefined and preweighted objective criteria determining the likelihood of their efficacy in cancer therapy.1 These criteria include therapeutic function in vaccine trials, immunogenicity, the number of patients with antigen-positive tumors, expression level, percentage of positive tumor cells and cellular location, as well as a role for the antigen in oncogenicity, a tumor-specific expression profile and expression in cancer stem cells. The last criterion, the evidence for expression on putative cancer stem cells, is likely to be very important in the context of acute myeloid leukemia (AML). Although 65 to 75% of patients under 60 years of age with AML reach a complete hematologic remission through the currently available standard therapy, the 5-year survival rate is less than 30%, because a high percentage of the patients relapse.2 Accumulating evidence supports the role of leukemic stem cells (LSC) in the high relapse rate of AML.2-4 These quiescent LSC, possessing biological properties rendering them resistant to chemotherapy and radiotherapy, are probably responsible for the minimal residual disease of AML and may eventually result in relapse. LSC share some properties with normal hematopoietic stem cells (HSC), such as a low division rate, self-renewal ability and expression of some surface markers including the CD34+CD38- phenotype. 3,4 It is generally believed that sensitive detection of minimal residual disease and targeted elimination of LSC can be a very efficient way to achieve more durable remissions or even a cure for AML.3,5 Immunotherapy is expected to be successful in this setting of minimal residual disease, complementary to prior standard treatment, by the elimination of the residual blasts, containing the LSC.
Receptor for Hyaluronic Acid Mediated Motility (RHAMM/HMMR/CD168), discovered by the SEREX (serological screening of cDNA expression libraries) method, has been described as a cancer-associated antigen and is involved in both tumorigenesis and progression or metastasis.6-11 Besides its expression in many solid tumors, RHAMM mRNA was detected in peripheral blood mononuclear cells of 60-70% of newly diagnosed AML patients.6,11 High expression of RHAMM has been correlated with a poor prognosis in patients with various types of solid tumors and hematologic malignancies such as B-cell chronic lymphocytic leukemia, multiple myeloma and AML.12-16 RHAMM-specific CD8+ T cells were detected in patients diagnosed with AML and chronic myeloid leukemia.17-19 Using a lymphoma mouse model, anti-tumor activity mediated by CD4+ T cells was observed after vaccination with RHAMM mRNA-transfected dendritic cells.20 A recently published report demonstrated prolonged survival of immune deficient mice injected with an AML cell line after adoptive transfer of RHAMM-specific T-cell receptor (TCR) transgenic lymphocytes.21 In addition, it was shown in clinical vaccination trials that RHAMM generates cellular immune responses and, importantly, clinical responses in some patients with AML, myelodysplastic syndrome and multiple myeloma. 22,23 RHAMM was, therefore, identified as one of the most promising leukemia-associated antigens in AML.
It is not clear however whether this RHAMM-specific response actually targets the true LSC. Expression of RHAMM in CD34+CD38- LSC has, to the best of our knowledge, never been specifically investigated. Promising results have been published concerning CD44, a hyaluronan receptor closely related to RHAMM, which has been described as a potential target to eliminate AML LSC.3 Another relatively unexplored criterion of leukemia-associated antigens concerning RHAMM is its tumor specificity. Greiner et al. showed that RHAMM is not expressed in peripheral blood mononuclear cells and CD34+ HSC from healthy volunteers.6,11 However, the specificity of RHAMM expression was shown to be not absolute, since testis, placenta and thymus showed significant RHAMM mRNA expression.6,24 Immunohistochemistry of spermatocytes, normal colonic mucosa and normal gastric mucosa revealed strong, weak and occasionally positive staining for RHAMM, respectively.12,16 RHAMM immunostaining was also demonstrated throughout all layers of the cornea and suprabasal layers of the limbus.25 Furthermore, it was reported that RHAMM is differentially expressed during the cell cycle, with maximal RHAMM mRNA expression in the G2/M phase.26 As a consequence, RHAMM expression might be up-regulated in actively dividing cells of physiological tissues.
In this study we investigated the expression pattern of RHAMM in various leukemic and non-leukemic hematopoietic cell populations that are relevant to immunotherapy of patients with AML.
Design and Methods
Samples from healthy volunteers and patients with acute myeloid leukemia
All samples were taken from patients with AML treated at Ghent University Hospital (Belgium) between 2009 and 2011. Samples were collected at the time of diagnosis or relapse as indicated in Table 1. AML samples (bone marrow, peripheral blood or leukapheresis) and cord blood, peripheral blood and leukapheresis samples (after HSC mobilization) from healthy donors were obtained and used following the guidelines of the Medical Ethical Committee of Ghent University Hospital, after informed consent had been obtained in accordance with the Declaration of Helsinki.
TABLE 1.
Clinical, immunophenotypic and genetic characteristics of the acute myeloid leukemia patients.
Cell lines
The K562 cell line, a chronic myeloid leukemia-blast crisisderived cell line, is often used as a positive control for RHAMM expression.6,11,17 OP9-GFP is a murine bone marrow stromal cell line constitutively expressing green fluorescent protein, which can be flow cytometrically analyzed in the fluorescein isothiocyanate (FITC) channel. Culture conditions are described in the Online Supplementary Design and Methods.
Isolation of subpopulations of acute myeloid leukemia and healthy donor cells
After thawing, CD34+ cells were first enriched by anti-CD34 magnetic activated cell sorting beads (MACS beads, Miltenyi) if the percentage of CD34+ cells in the sample was <70%. Subsequently, cells were sorted with the FACSAria II cell sorter (BD Biosciences) for the viable [assessed by lack of propidium iodide (Invitrogen) intake] CD34+CD38- and CD34+CD38+ cells to a purity of >99%, as determined by post-sorting analysis. If the percentage of CD34+ cells in the sample was >70%, samples were immediately sorted as described above. CD34- blasts were also isolated from five AML samples.
Isolation and in vitro culture of acute myeloid leukemia and cord blood samples
The procedures used for the isolation and in vitro culture of AML and cord blood samples were described by Van de Valle et al.27 and are detailed in the Online Supplementary Design and Methods.
T-cell activation
The T-cell activation is described in the Online Supplementary Design and Methods.
Flow cytometry and antibodies
Details on the flow cytometry equipment and techniques and antibodies used are given in the Online Supplementary Design and Methods.
Cell cycle analysis
Cell cycle analysis is described in the Online Supplementary Design and Methods.
In vivo transplantation experiment
Adult NOD.CB17-Prkdcscid/J (NOD/SCID) mice were given a sublethal dose of whole-body irradiation (3.5 Gy) and injected intraperitoneally with 200 μg of a rat monoclonal antibody against the murine IL2-Rβ chain.28Within 24 h after irradiation, mice were injected intravenously as described in the Online Supplementary Design and Methods.
Real-time quantitative polymerase chain reaction analysis
The techniques, apparatus, kits and primers used for the realtime quantitative polymerase chain reaction (RT-qPCR) analysis are described in the Online Supplementary Design and Methods. RHAMM primers were designed to amplify the four described isoforms of RHAMM indifferentially.15 Results were normalized to GAPDH expression and shown relative to the transcript level in K562 cells. If pre- and post-proliferation samples were compared, normalization was performed to the geometric mean of GAPDH, YWHAZ and R18S expression.29
Statistical analysis
Statistical analysis was performed in SPSS statistics version 19. The non-parametric related samples Wilcoxon's signed rank test and independent samples Mann-Whitney U test were used when indicated. P values less than 0.05 were considered statistically significant.
Results
RHAMM is not expressed in acute myeloid leukemic stem cells
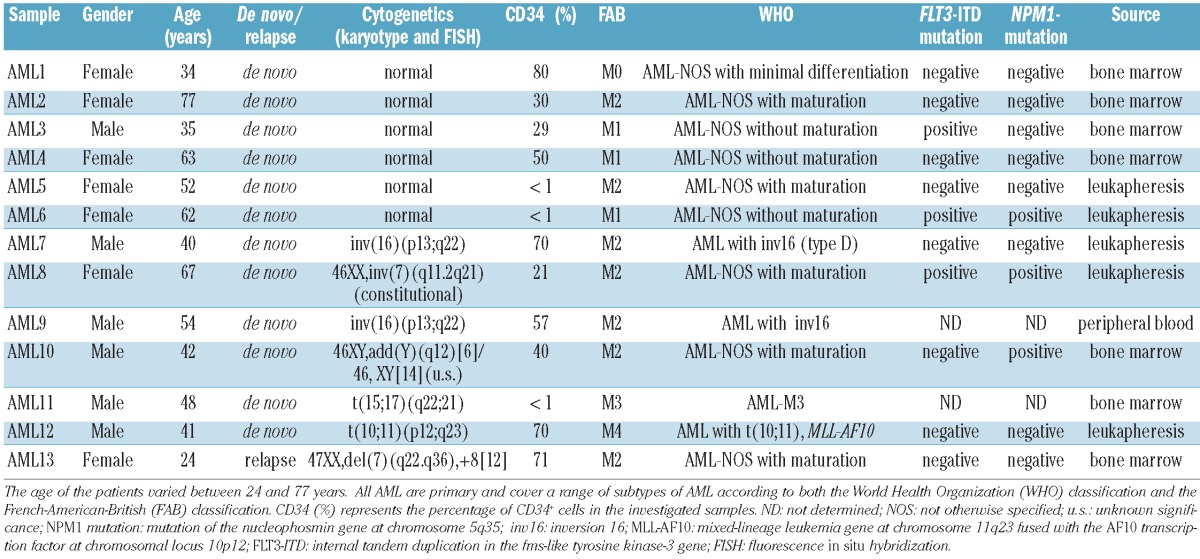
CD34+CD38+ AML blasts and CD34+CD38- LSC were isolated from bone marrow, peripheral blood or leukapheresis samples of 13 AML patients. The clinical, immunophenotypic and genetic features of the AML patients cover a range of subtypes of AML and are presented in Table 1. Samples were taken at diagnosis or relapse as indicated. Two cord blood samples and two leukapheresis samples from healthy donors were selected as controls. Figure 1A shows only background expression of RHAMM in CD34+CD38- HSC from healthy donors. This population is considered to be RHAMM-negative.6 No significant difference in RHAMM expression levels could be demonstrated between the CD34+CD38- and the CD34+CD38+ subpopulations from healthy donors (P=0.273). Expression of RHAMM in the CD34+CD38+ AML subpopulation was divergent, with relative expression levels varying from undetectable to 0.74, calculated relative to RHAMM expression in K562 cells. RHAMM expression in CD34- blasts was investigated in five patients and was not statistically different from the expression in the CD34+CD38+ blast population (P=0.893). Strikingly, only baseline RHAMM expression was observed in the CD34+CD38- LSC, which was not statistically different from the expression measured in healthy CD34+CD38- HSC (P=0.296). These RT-qPCR data were confirmed by flow cytometry. Figure 1B shows intracellular staining for RHAMM expression within the CD34+ AML cells for a representative AML sample (AML12): a subset of the CD34+CD38+ population clearly expressed RHAMM, whereas the CD34+CD38- cells were virtually all negative for RHAMM. This result provides evidence that, in addition to the differential RHAMM transcript level, a differential RHAMM protein expression profile can be seen in the LSC compared with the CD34+CD38+ AML control population.
Figure 1.
RHAMM expression profile in LSC versus HSC (A) From 13 AML samples and four samples from healthy donors two subpopulations were isolated by FACS sorting: CD34+CD38- LSC and the CD34+CD38+ control population from AML patients, and the CD34+CD38- HSC and CD34+CD38+ subpopulation from healthy donor samples. From five AML patients the CD34- blast population was isolated too. The box plot shows RHAMM expression levels measured by RT-qPCR on mRNA. Results were normalized to GAPDH expression and shown relative to the RHAMM transcript level in K562 cells. The o symbol represents an outlier, with a relative expression of RHAMM of 0.74. There is no significant difference in RHAMM expression level between the CD34+CD38- HSC samples and the CD34+CD38+ healthy subpopulation (P=0.273 calculated by Wilcoxon's test). Supplementary analysis showed no significant difference in RHAMM expression between the CD34- and the CD34+CD38+ AML blasts for five investigated samples (P=0.893 calculated by Wilcoxon's test). The CD34+CD38- LSC samples had a significantly lower RHAMM expression level compared with the CD34+CD38+ AML control population (P=0.001 calculated by Wilcoxon's test). No statistically significant difference could be shown between the LSC and the HSC (P=0.296 calculated by the Mann Whitney U test). (B) Intracellular staining for RHAMM on a representative AML sample (AML12). Plots are gated on the viable cells and show the further gating strategy. Gated on the CD34+ AML cells (left dot plot), the right dot plot shows that intracellular RHAMM expression can be mainly found in the CD34+CD38+ subpopulation. Gated on isotype control (not shown).
RHAMM expression in acute myeloid leukemia is cell cycle-dependent
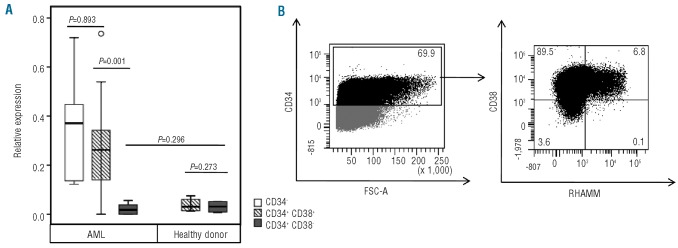
It was previously shown that RHAMM expression in human primary fibroblasts is differentially regulated during the cell cycle with maximal RHAMM mRNA expression in the G2/M phase.26 This has been linked to its important role in mitosis, when RHAMM binds to the mitotic spindle to regulate spindle integrity and stability.30 We, therefore, hypothesized that, also in AML, RHAMM expression may not be static during cell cycle progression. Figure 2 shows cell cycle analysis and RHAMM expression prior to and after in vitro expansion of AML samples. Freshly isolated AML cells were mainly in the G0/G1 phase of the cell cycle, while 5 days of in vitro culture allowed a subpopulation to be pushed towards S phase (20.1%) and G2/M phase (11.5%) of the cell cycle in this representative sample (Figure 2A). As hypothesized, in parallel with this shift in the distribution of the AML cells in the different phases of the cell cycle, an approximately 8-fold increase in RHAMM mRNA expression was observed after 5 days of in vitro culture of two representative AML samples (Figure 2B). These RT-qPCR results were confirmed by flow cytometry showing a clear upregulation of RHAMM protein expression after culture in the bulk of AML cells (Figure 2C). Interestingly, after 5 days of culture, although most cells had differentiated to CD34- cells, a small but distinct CD34+CD38- LSC subpopulation was sustained, in which RHAMM protein expression remained undetectable (Figure 2D).
Figure 2.
RHAMM expression before and after in vitro culture of AML cells. (A) Cell cycle analysis of a representative bulk AML sample (AML8) before (day 0) and after in vitro culture (day 5). Freshly isolated AML samples are mainly in the G0/G1 phase. Dean/Jett/Fox analysis after 5 days of in vitro culture shows 67% of cells in the G0/G1 phase, 20.1% in the S phase and 11.5% in the G2/M phase. (B) RT-qPCR analysis on mRNA is shown for the expression of RHAMM in freshly isolated bulk AML samples (day 0) and in AML samples after 5 days of in vitro culture (day 5). Results were normalized to the geometric mean of the expression of GAPDH, R18S and YWHAZ, and are shown relative to the RHAMM transcript level in K562 cells. Data shown are the mean values and standard deviations (SD) calculated from duplicate PCR of the same sample. Results are shown for two representative AML samples (AML8 and AML12). (C) Histogram demonstrating flow cytometric results of intracellular RHAMM expression on day 0 and day 5 after in vitro culture of a representative bulk AML sample (AML8). Compared with isotype staining (day 0 = day 5) (solid gray) and expression on day 0 (full line), clear up-regulation can be seen after 5 days of culture (dotted line). (D) Dot plots are gated on the viable cells and show the further gating strategy. Gated on the CD34+ AML cells (AML8; left dot plot), the right dot plot shows intracellular RHAMM expression after 5 days of in vitro culture. The expression in the CD34+CD38- subpopulation remains low, compared with that in the CD34+CD38+ population. Gated on isotype control (not shown).
In vitro expansion of cord blood-derived CD34+ cells causes up-regulation of RHAMM
Taking into account that the RHAMM protein and mRNA levels increased upon in vitro expansion in AML cells, we investigated whether this was also the case for non-malignant (both CD38+ and CD38-) CD34+ HSC. To explore the RHAMM expression profile of expanding HSC, human cord blood-derived CD34+ cells from five different donors were analyzed prior to and after in vitro culture. RT-qPCR showed a significant up-regulation of RHAMM mRNA in the expanded CD34+ cells compared with in freshly isolated CD34+ cells (Figure 3A). The expression levels in expanding healthy CD34+ cells were of comparable magnitude as those in AML samples under expansion conditions. These results suggest that not only malignant AML cells, but also CD34+ cells from healthy donors show cell cycle-dependent changes in the level of RHAMM mRNA. In addition to higher RHAMM mRNA expression, a strong up-regulation of cytoplasmic RHAMM protein levels was observed in the CD34+ cells after expansion (Figure 3B). This result could be linked again to the distribution of the cells in the different phases of the cell cycle prior to and after in vitro expansion, as shown in Figure 3C. Cell cycle analysis of a representative sample shows that freshly isolated CD34+ cells were virtually all in the G0/G1 phase, in contrast to the distribution of cells after expansion when a subpopulation shifted towards the S phase (29.3%) and G2/M phase (7.3%) of the cell cycle.
Figure 3.
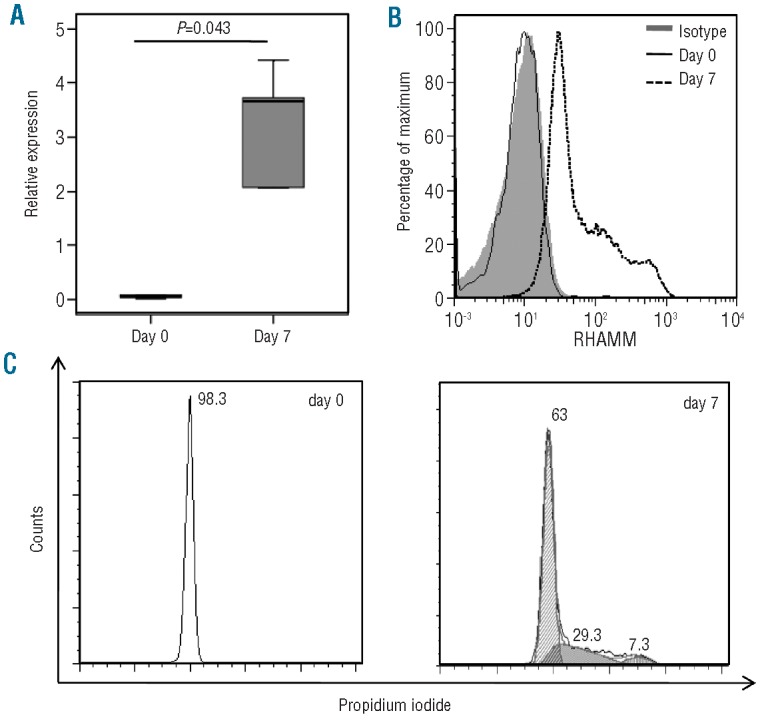
RHAMM expression in human cord bloodderived CD34+ cells before and after in vitro expansion (A) Box plot showing RHAMM mRNA expression in freshly isolated CD34+ cells (day 0) and in CD34+ cells after 7 days of in vitro culture (day 7) from five different cord blood donors. RHAMM expression was measured by RT-qPCR on mRNA and results were normalized to the geometric mean of the expression of GAPDH, R18S and YWHAZ and shown relative to the RHAMM transcript level in K562 cells. There is a significant difference in RHAMM expression level between day 0 and day 7 (P= 0.043 calculated by Wilcoxon's test). (B) Histogram showing flow cytometric results of intracellular RHAMM expression on day 0 and after 7 days of in vitro culture of CD34+ cells. Gated on the viable CD34+ GFP- cells. Compared with isotype staining (day 0 = day7) (solid gray) and with RHAMM expression on day 0 (full line), clear up-regulation can be seen after 7 days of culture (dotted line). Results shown are representative of three distinct cord blood donors analyzed. (C) Cell cycle analysis of a representative CD34+ cord blood sample before (day 0) and after in vitro culture (day 7). Freshly isolated CD34+ cells are virtually all in the G0/G1 phase. Dean/Jett/Fox analysis after 7 days of in vitro culture showed 63% of cells in the G0/G1 phase, 29.3% in the S phase and 7.3% in the G2/M phase.
Cord blood-derived CD34+ cells up-regulate RHAMM during engraftment in a NOD/SCID mouse model
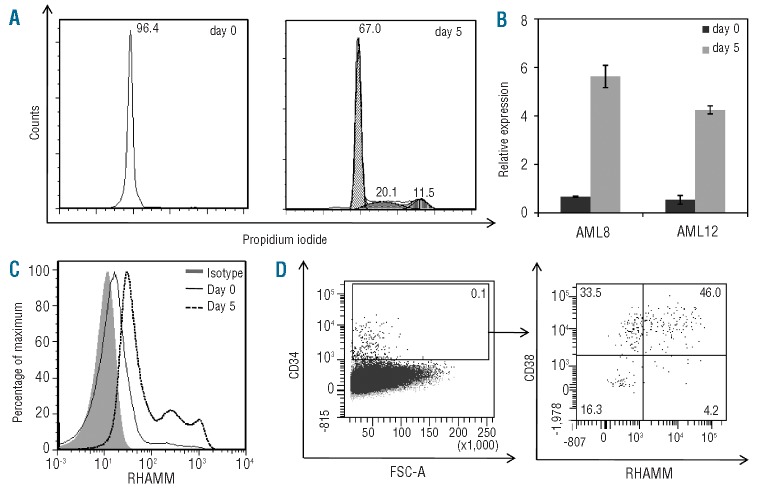
A NOD.CB17-Prkdcscid/J (NOD/SCID) mouse model was used to address the question whether the up-regulation of RHAMM observed in vitro during expansion of cord blood-derived CD34+ cells can be observed in vivo too and, therefore, whether it is clinically relevant. Two weeks after injection of cord blood-derived CD34+ cells, mice were sacrificed and bone marrow was collected. Engrafting hCD45+ hCD34+ mCD45- cells made up in average 1.88% (representative sample shown: 5.79%) of the total viable bone marrow cells and were sorted to high purity (Figure 4A). RHAMM expression in the CD34+ cells prior to injection and after 2 weeks of engraftment was determined by RT-qPCR. As shown earlier, RHAMM expression level was very low in freshly isolated healthy CD34+ cord blood samples (Figure 4B). Two weeks after transplantation, a significant up-regulation in RHAMM expression (P=0.012) was seen in the CD34+ human cord blood-derived cells: there was a mean 75.3-fold increase after 2 weeks. RHAMM primers were tested on whole mouse bone marrow of a non-injected mouse, and no RHAMM expression could be observed, indicating the specificity of the human RHAMM primers (data not shown). Because the monoclonal antibody against RHAMM is non-conjugated, it was technically not feasible to confirm these RT-qPCR results flow cytometrically, combining intracellular RHAMM staining with surface staining of all required markers. In conclusion, these data suggest that, also in vivo, RHAMM is clearly expressed on the engrafting non-malignant CD34+ cells in the bone marrow during the expansion phase after HSC transplantation.
Figure 4.
RHAMM expression before and after transplantation of human cord bloodderived CD34+ cells in NOD/SCID mice. Eight adult NOD/SCID mice were intravenously injected with 2.5×105 cord blood-derived CD34+ cells. (A) Plots are gated on the viable cells, and show the sorting strategy. Dot plots illustrate a representative mouse 2 weeks after transplantation of hCD34+ cord blood-derived cells. The percentage of hCD45+ mCD45- hCD34+ cells within the viable bone marrow cells is 5.79%. (B) Box plot showing the results of RT-qPCR analysis for RHAMM mRNA in freshly isolated CD34+ cells (pre-injection= pre) and in CD34+ cells isolated from the bone marrow 2 weeks after intravenous injection (post). Results were normalized to the geometric mean of the expression of GAPDH, R18S and YWHAZ, and are shown relative to the RHAMM transcript level in K562 cells. There is a significant difference in RHAMM expression level between the ‘pre’ and ‘post’ samples (P=0.012 calculated by Wilcoxon’s test), with firm up-regulation of RHAMM mRNA 2 weeks after transplantation.
In vitro activated T cells over-express RHAMM
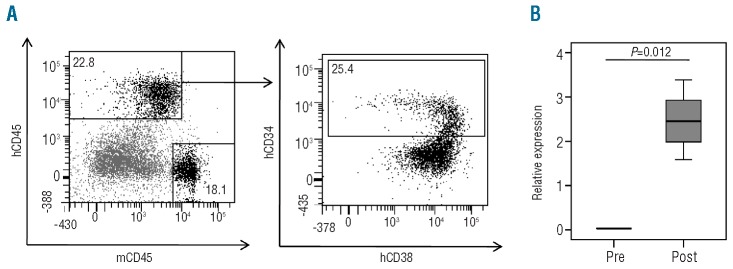
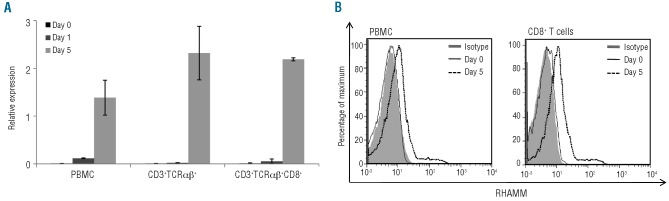
Immunotherapeutic strategies are based on two major principles. On the one hand, cytotoxic T cells can be isolated from the patient and selected or genetically engineered to express a TCR of interest to be subsequently expanded in vitro and re-transfused to the patient. On the other hand, cytotoxic T cells can be activated in vivo by vaccination with peptides, DNA or dendritic cells.22,23,31 In both cases activated cytotoxic T cells are indispensable in order to obtain immunological and clinical responses in the AML patient. As we observed up-regulation of RHAMM in cycling CD34+ cells from healthy donors, we also studied the change in RHAMM expression upon T-cell activation. No RHAMM mRNA expression could be detected in fresh peripheral blood mononuclear cells, freshly isolated TCRαβ+ T cells and freshly isolated CD8+ TCRαβ+ cytotoxic T cells (Figure 5A), in accordance with previous reports.6,24 Upon activation, a clear increase in RHAMM mRNA levels was observed. This up-regulation was confirmed by intracellular flow cytometric analysis (Figure 5B). Taken together, these data indicate that in vitro activation induces RHAMM expression in cytotoxic CD8+ T cells.
Figure 5.
RHAMM expression by in vitro activated T cells (A) RT-qPCR on mRNA from T-cell subpopulations (peripheral blood) from two different healthy donors. Bulk peripheral blood mononuclear cells (PBMC) were analyzed prior to and 1 and 5 days after in vitro activation by phytohaemagglutinin (PHA). From the same donors the CD3+TCRαβ+ and the CD3+TCRαβ+CD8+ fractions were sorted and analyzed unstimulated, on day 1 and on day 5 of CD3/CD28/CD49d activation. The results were normalized to the geometric mean of the expression of GAPDH, R18S and YWHAZ, and are shown relative to the expression level in K562 cells. Data shown are the mean values and SD calculated from two donors. (B) Histograms demonstrating flow cytometric results of intracellular RHAMM expression on day 0 and 5 days after in vitro activation by PHA of a representative bulk PBMC sample (left) and gated on the CD8+ T cell subpopulation (right). Compared with isotype staining (day 0 = day 5; solid gray) and expression on day 0 (full line), clear up-regulation can be seen after 5 days of stimulation (dotted line).
Discussion
Herein, we show that the expression of RHAMM, a cancer-associated antigen currently explored as a target for immunotherapy and especially for vaccination trials, is low to undetectable in the CD34+CD38- LSC of AML patients and not significantly different from that in the CD34+CD38- HSC of healthy donors. These results are in accordance with the putative role assigned earlier to RHAMM as a negative marker of the stem cell-containing population of human limbal epithelial cells. Ahmad et al. located RHAMM in all layers of corneal epithelium and suprabasal layers of the limbal epithelium. In contrast, RHAMM was completely absent in the basal layer of the limbus where the stem cells are located.25 In view of the fact that the beneficial effects of immunotherapeutic strategies for AML are expected to be mainly situated in targeting minimal residual disease after prior standard therapy, targeting the chemoresistant LSC is essential for a durable effect. However, the data presented here suggest that RHAMM-directed immunotherapeutic strategies will not target LSC but only the rapidly proliferating progeny. Such therapies may, therefore, offer little added value to standard therapy (e.g. poly-chemotherapy). Of course, we cannot exclude that the background level of RHAMM mRNA, detected in LSC, may be sufficient for recognition by high-avidity T cells. Still, the conclusion remains that, as RHAMM mRNA expression levels in HSC and LSC are not significantly different, both LSC and HSC may be attacked similarly by RHAMM-directed high-avidity T-cell-based therapies.
We observed that RHAMM expression in AML blasts is not static but cell cycle-dependent, in accordance with a previous study indicating that RHAMM is not constitutively expressed by all AML blasts, but only by a subpopulation. 13 These findings are in line with those of earlier studies demonstrating that RHAMM expression in human primary fibroblasts and the HeLa cell line varies during cell cycle, as low expression was measured in G0/G1 cells and a peak in mRNA level was observed during the G2/M phase.26,32 This expression pattern can be related to the intracellular function of RHAMM: it decorates the mitotic spindles and seems to be necessary for stable mitotic spindle formation and progression through the G2/M phase.7,26,30,33-35 In addition, other studies demonstrated a direct or indirect correlation between RHAMM and proliferation, both in malignant cells and in physiological nonhomeostatic settings such as wound healing and regeneration. 14,32,35-38 It is not, therefore, surprising that in a variety of hematologic and solid malignancies, RHAMM overexpression was described to be correlated with a poor prognosis.12-16 We strongly believe that this correlation is not only due to the function of RHAMM in cell motility and, consequently, the invasiveness of tumor cells,30 but can also be attributed to RHAMM being a genuine ‘proliferation marker’. The major drawback of the proliferationdependent expression of RHAMM is that non-malignant, actively dividing cells tend to express high levels of RHAMM too.
We confirm previous findings that freshly isolated CD34+ HSC from healthy donors do not express RHAMM mRNA.6,11 However, we observed a clear up-regulation of RHAMM after in vitro expansion of CD34+ HSC from healthy donors. These findings were confirmed in vivo during the process of CD34+ cell expansion in a mouse model of human stem cell transplantation. For ethical and practical reasons, we were unable to study CD34+ cells during the engraftment phase after HSC transplantation in patients. However, we and others have shown that human CD34+ cells transplanted into irradiated immunedeficient mice engraft in the bone marrow and expand dramatically during the first weeks after infusion.28 As this mouse model mimics in vivo engraftment of autologous (post-chemotherapy) or donor (allogeneic HSC transplantation) HSC, our data suggest a major limitation for immunotherapy targeting RHAMM: RHAMM-specific cytotoxic T cells might not be able to discriminate engrafting non-leukemic HSC from leukemic cells and might, therefore, hamper post-chemotherapy recovery or engraftment of allogeneic HSC in the context of allogeneic HSC transplantation. A recent study showed a reduction of colony-forming units of all lineages when freshly isolated HLA-A2+ CD34+ HSC from healthy donors were coincubated with TCR-transgenic lymphocytes specific for RHAMM in the context of HLA-A2, suggesting that freshly isolated CD34+ cells may express enough RHAMM to be killed by these T cells.21 However, it is more likely that CD34+ HSC up-regulated RHAMM expression in response to the growth factors added during the assay. Alternatively, since an allo-HLA-A2-restricted RHAMMspecific TCR was used, it is also possible that CD34+ cells were killed due to off-target promiscuity.39 Although, until today, no major toxicity has been reported in RHAMM vaccination trials,22,23 more potent immunotherapeutic strategies such as adoptive transfer of RHAMM-specific T cells might induce important hematologic and non-hematologic side effects.
We not only found RHAMM expression in HSC during hematologic recovery but also in cytotoxic T cells proliferating upon immune activation. Our results are consistent with those of Leisegang et al.,40 who showed that survivinspecific T cells underwent fratricide when activated, because of the target antigen, survivin, being also expressed in activated T cells. In a recently published paper by the same group on RHAMM-specific T cells, this issue was not addressed in depth as it was not reported whether HLA-A2 negative or positive effector T cells were used for treatment and whether differential T-cell toxicity was observed.21 Nevertheless, our data suggest that cellular therapy targeting RHAMM might be limited by in vivo fratricide of the RHAMM-specific T cells activated by the AML cells, and by collateral damage to neighboring activated T cells with unknown specificity. Similarly, vaccination strategies able to induce autologous RHAMM-specific T cells could be of limited efficacy because of apoptosis of the responding cytotoxic T cells.
Although RHAMM is a well-known and abundant cancer-associated antigen, over-expressed in several hematologic and solid malignancies, our data raise the question whether it should be used at all as a target for immunotherapy. We have not directly addressed whether certain splice variants may be uniquely associated with AML or malignancy in general. It is known that some truncated isoforms of the RHAMM protein have transforming properties when over-expressed in cell lines.33 In multiple myeloma, over-expression of mRNA of a splice variant without exon 4 was seen.15 If these truncated protein forms result in unique newly composed peptides specifically expressed in malignant cells, and one of those peptides binds with a high affinity to a major histocompatibility complex (MHC) class I allotype, new immunotherapeutic strategies could emerge that are more specific and, therefore, potentially safer and more efficient. However, our RT-qPCR results, using primers that were designed to amplify all four isoforms of RHAMM described until now, showed no expression in the LSC, which excludes expression of known alternatively spliced mRNA isoforms. The dual function of RHAMM, involving both an extracellular and intracellular localization, makes it more complicated to address the RHAMM protein expression level. Extracellular expression of RHAMM results from a redistribution of intracellular pools by nonconventional export to the extracellular compartment, which is not necessarily associated with increased synthesis or improved stability of RHAMM mRNA or protein.30 Surface staining of AML cells with an anti-RHAMM mono clonal antibody could not demonstrate clear extracellular RHAMM expression on AML blasts in our hands (data not shown), in accordance with the predominant intracellular localization of RHAMM in AML blasts described earlier in immunohistochemical stainings.13 Furthermore, it is expected that epitopes processed from intracellular RHAMM by the target cells and presented on MHC class I complexes will be by far the most important targets for recognition by cytotoxic T cells.
In conclusion, we have evaluated RHAMM as an immunotherapeutic target in the context of AML, guided by the internationally accepted criteria for prioritization of cancer-associated antigens.1 The expression in LSC is low to undetectable and RHAMM is not AML-specific, since it is also expressed in expanding healthy HSC and activated T cells, two clinically relevant populations in the context of a future integrated AML treatment, consisting of the standard induction chemotherapy followed by immunotherapy as a consolidation treatment or an allogeneic HSC transplant followed by AML-directed donor lymphocyte infusions. In brief, the two major benefits of immunotherapy over chemotherapy, the elimination of LSC and the reduction of side effects, are not likely to be achieved by RHAMM-directed immunotherapy.
Supplementary Material
Acknowledgments
The authors would like to thank Christiaan De Boever for performing art work. We would also like to thank Dr. Dominique De Bleser (Red Cross Flanders, Oost-Vlaanderen, Belgium) for providing cord blood samples, the medical staff of the Hematology Department of Ghent University Hospital for providing the AML samples, Dr. Tom Boterberg for the irradiation of the mice and Jet Robin and Eelke Vandenbergh for the animal care. Finally, we would like to thank Roos Colman from the Department of Biostatistics for help with the statistical analysis.
Funding: this work was supported by the Research Foundation – Flanders (Fonds voor Wetenschappelijk Onderzoek Vlaanderen, FWO) and its Odysseus research program, Stichting tegen Kanker, the geconcerteerde onderzoeksactiviteiten (GOA) of Ghent University, and the Interuniversity Attraction Poles Program (IUAP) supported by the Belgian Science Policy. YVC, SV and GV are supported by the Instituut voor de Aanmoediging van Innovatie door Wetenschap en Technologie in Vlaanderen (IWT). SS, TT and TK are supported by the Research Foundation – Flanders (Fonds voor Wetenschappelijk Onderzoek Vlaanderen, FWO).
Footnotes
TK and BV contributed equally to this manuscript as senior authors.
The online version of this article has a Suppplementary Appendix.
Authorship and Disclosures The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, et al. The prioritization of cancer antigens: a National Cancer Institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009; 15(17):5323–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006. 368(9550) 1894–907 [DOI] [PubMed] [Google Scholar]
- 3.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006; 12(10): 1167–74 [DOI] [PubMed] [Google Scholar]
- 4.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Med. 1997;3(7):730–7 [DOI] [PubMed] [Google Scholar]
- 5.Majeti R, Becker MW, Tian Q, Lee TL, Yan X, Liu R, et al. Dysregulated gene expression networks in human acute myelogenous leukemia stem cells. Proc Natl Acad Sci USA. 2009. 106(9):3396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greiner J, Ringhoffer M, Taniguchi M, Schmitt A, Kirchner D, Krahn G, et al. Receptor for hyaluronan acid-mediated motility (RHAMM) is a new immunogenic leukemia-associated antigen in acute and chronic myeloid leukemia. Exp Hematol. 2002. 30(9):1029–35 [DOI] [PubMed] [Google Scholar]
- 7.Telmer PG, Tolg C, McCarthy JB, Turley EA. How does a protein with dual mitotic spindle and extracellular matrix receptor functions affect tumor susceptibility and progression? Commun Integr Biol. 2011; 4(2):182–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greiner J, Bullinger L, Guinn BA, Dohner H, Schmitt M. Leukemia-associated antigens are critical for the proliferation of acute myeloid leukemia cells. Clin Cancer Res. 2008; 14(22):7161–6 [DOI] [PubMed] [Google Scholar]
- 9.Rein DT, Roehrig K, Schondorf T, Lazar A, Fleisch M, Niederacher D, et al. Expression of the hyaluronan receptor RHAMM in endometrial carcinomas suggests a role in tumour progression and metastasis. J Cancer Res Clin Oncol. 2003; 129(3):161–4 [DOI] [PubMed] [Google Scholar]
- 10.Wang C, Thor AD, Moore DH, 2nd, Zhao Y, Kerschmann R, Stern R, et al. The overexpression of RHAMM, a hyaluronan-binding protein that regulates ras signaling, correlates with overexpression of mitogen-activated protein kinase and is a significant parameter in breast cancer progression. Clin Cancer Res. 1998; 4(3):567–76 [PubMed] [Google Scholar]
- 11.Greiner J, Ringhoffer M, Taniguchi M, Li L, Schmitt A, Shiku H, et al. mRNA expression of leukemia-associated antigens in patients with acute myeloid leukemia for the development of specific immunotherapies. Int J Cancer. 2004; 108(5):704–11 [DOI] [PubMed] [Google Scholar]
- 12.Zlobec I, Terracciano L, Tornillo L, Gunthert U, Vuong T, Jass JR, et al. Role of RHAMM within the hierarchy of well-established prognostic factors in colorectal cancer. Gut. 2008; 57(10):1413–9 [DOI] [PubMed] [Google Scholar]
- 13.Tzankov A, Strasser U, Dirnhofer S, Menter T, Arber C, Jotterand M, et al. In situ RHAMM protein expression in acute myeloid leukemia blasts suggests poor overall survival. Ann Hematol. 2011; 90(8):901–9 [DOI] [PubMed] [Google Scholar]
- 14.Giannopoulos K, Mertens D, Buhler A, Barth TF, Idler I, Moller P, et al. The candidate immunotherapeutical target, the receptor for hyaluronic acid-mediated motility, is associated with proliferation and shows prognostic value in B-cell chronic lymphocytic leukemia. Leukemia. 2009; 23(3):519–27 [DOI] [PubMed] [Google Scholar]
- 15.Maxwell CA, Rasmussen E, Zhan F, Keats JJ, Adamia S, Strachan E, et al. RHAMM expression and isoform balance predict aggressive disease and poor survival in multiple myeloma. Blood. 2004; 104(4):1151–8 [DOI] [PubMed] [Google Scholar]
- 16.Ishigami S, Ueno S, Nishizono Y, Matsumoto M, Kurahara H, Arigami T, et al. Prognostic impact of CD168 expression in gastric cancer. BMC Cancer. 2011; 11:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greiner J, Li L, Ringhoffer M, Barth TF, Giannopoulos K, Guillaume P, et al. Identification and characterization of epitopes of the receptor for hyaluronic acidmediated motility (RHAMM/CD168) recognized by CD8+ T cells of HLA-A2-positive patients with acute myeloid leukemia. Blood 2005; 106(3):938–45 [DOI] [PubMed] [Google Scholar]
- 18.Schmitt M, Li L, Giannopoulos K, Chen J, Brunner C, Barth T, et al. Chronic myeloid leukemia cells express tumor-associated antigens eliciting specific CD8+ T-cell responses and are lacking costimulatory molecules. Exp Hematol. 2006; 34(12):1709–19 [DOI] [PubMed] [Google Scholar]
- 19.Greiner J, Schmitt M, Li L, Giannopoulos K, Bosch K, Schmitt A, et al. Expression of tumor-associated antigens in acute myeloid leukemia: Implications for specific immunotherapeutic approaches. Blood. 2006; 108(13):4109–17 [DOI] [PubMed] [Google Scholar]
- 20.Fukui M, Ueno K, Suehiro Y, Hamanaka Y, Imai K, Hinoda Y. Anti-tumor activity of dendritic cells transfected with mRNA for receptor for hyaluronan-mediated motility is mediated by CD4+ T cells. Cancer Immunol Immunother. 2006; 55(5):538–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spranger S, Jeremias I, Wilde S, Leisegang M, Stärck L, Mosetter B, et al. T-cell receptortransgenic lymphocytes specific for HMMR/Rhamm limit tumor outgrowth in vivo. Blood. 2012; 119(15):3440–9 [DOI] [PubMed] [Google Scholar]
- 22.Schmitt M, Schmitt A, Rojewski MT, Chen J, Giannopoulos K, Fei F, et al. RHAMM-R3 peptide vaccination in patients with acute myeloid leukemia, myelodysplastic syndrome, and multiple myeloma elicits immunologic and clinical responses. Blood. 2008; 111(3):1357–65 [DOI] [PubMed] [Google Scholar]
- 23.Greiner J, Schmitt A, Giannopoulos K, Rojewski MT, Gotz M, Funk I, et al. Highdose RHAMM-R3 peptide vaccination for patients with acute myeloid leukemia, myelodysplastic syndrome and multiple myeloma. Haematologica. 2010; 95(7):1191–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pilarski LM, Miszta H, Turley EA. Regulated expression of a receptor for hyaluronanmediated motility on human thymocytes and T cells. J Immunol. 1993; 150(10):4292–302 [PubMed] [Google Scholar]
- 25.Ahmad S, Kolli S, Li DQ, de Paiva CS, Pryzborski S, Dimmick I, et al. A putative role for RHAMM/HMMR as a negative marker of stem cell-containing population of human limbal epithelial cells. Stem Cells. 2008; 26(6):1609–19 [DOI] [PubMed] [Google Scholar]
- 26.Sohr S, Engeland K. RHAMM is differentially expressed in the cell cycle and downregulated by the tumor suppressor p53. Cell Cycle. 2008; 7(21):3448–60 [DOI] [PubMed] [Google Scholar]
- 27.Van de Walle I, De Smet G, Gartner M, De Smedt M, Waegemans E, Vandekerckhove B, et al. Jagged2 acts as a Delta-like Notch ligand during early hematopoietic cell fate decisions. Blood. 2011; 117(17):4449–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerre TC, De Smet G, De Smedt M, Offner F, De Bosscher J, Plum J, et al. Both CD34+38+ and CD34+38- cells home specifically to the bone marrow of NOD/LtSZ scid/scid mice but show different kinetics in expansion. J Immunol. 2001; 167(7):3692–8 [DOI] [PubMed] [Google Scholar]
- 29.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome biology. 2002; 3(7):RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maxwell CA, McCarthy J, Turley E. Cellsurface and mitotic-spindle RHAMM: moonlighting or dual oncogenic functions?. J Cell Sci. 2008; 121(Pt 7):925–32 [DOI] [PubMed] [Google Scholar]
- 31.Amano T, Kajiwara K, Yoshikawa K, Morioka J, Nomura S, Fujisawa H, et al. Antitumor effects of vaccination with dendritic cells transfected with modified receptor for hyaluronan-mediated motility mRNA in a mouse glioma model. J Neurosurg. 2007; 106(4):638–45 [DOI] [PubMed] [Google Scholar]
- 32.Yang CW, Su JY, Tsou AP, Chau GY, Liu HL, Chen CH, et al. Integrative genomics based identification of potential human hepatocarcinogenesis-associated cell cycle regulators: RHAMM as an example. Biochem Biophys Res Commun. 2005; 330(2):489–97 [DOI] [PubMed] [Google Scholar]
- 33.Tolg C, Hamilton SR, Morningstar L, Zhang J, Zhang S, Esguerra KV, et al. RHAMM promotes interphase microtubule instability and mitotic spindle integrity through MEK1/ERK1/2 activity. J Biol Chem. 2010; 285(34):26461–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maxwell CA, Keats JJ, Belch AR, Pilarski LM, Reiman T. Receptor for hyaluronanmediated motility correlates with centrosome abnormalities in multiple myeloma and maintains mitotic integrity. Cancer Res. 2005; 65(3):850–60 [PubMed] [Google Scholar]
- 35.Tolg C, Hamilton SR, Nakrieko KA, Kooshesh F, Walton P, McCarthy JB, et al. Rhamm-/-fibroblasts are defective in CD44-mediated ERK1,2 motogenic signaling, leading to defective skin wound repair. J Cell Biol. 2006; 175(6):1017–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pilarski LM, Masellis-Smith A, Belch AR, Yang B, Savani RC, Turley EA. RHAMM, a receptor for hyaluronan-mediated motility, on normal human lymphocytes, thymocytes and malignant B cells: a mediator in B cell malignancy? Leuk Lymphoma. 1994; 14(5-6):363–74 [DOI] [PubMed] [Google Scholar]
- 37.Hatano H, Shigeishi H, Kudo Y, Higashikawa K, Tobiume K, Takata T, et al. Overexpression of receptor for hyaluronanmediated motility (RHAMM) in MC3T3-E1 cells induces proliferation and differentiation through phosphorylation of ERK1/2. J Bone Miner Metab. 2012; 30(3):293–303 [DOI] [PubMed] [Google Scholar]
- 38.Zaman A, Cui Z, Foley JP, Zhao H, Grimm PC, Delisser HM, et al. Expression and role of the hyaluronan receptor RHAMM in inflammation after bleomycin injury. Am J Respir Cell Mol Biol. 2005; 33(5):447–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Falkenburg WJ, Melenhorst JJ, van de Meent M, Kester MG, Hombrink P, Heemskerk MH, et al. Allogeneic HLA-A*02-restricted WT1-specific T cells from mismatched donors are highly reactive but show off-target promiscuity. J Immunol. 2011; 187(5):2824–33 [DOI] [PubMed] [Google Scholar]
- 40.Leisegang M, Wilde S, Spranger S, Milosevic S, Frankenberger B, Uckert W, et al. MHCrestricted fratricide of human lymphocytes expressing survivin-specific transgenic T cell receptors. J Clin Invest. 2010; 120(11):3869–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.