Abstract
Background
Previous data suggest that the response of chronic myeloid leukemia cells to imatinib is dose-dependent. The potential benefit of initial dose intensification of imatinib in pre-treated patients with chronic phase chronic myeloid leukemia remains unknown.
Design and Methods
Two hundred and twenty-seven pre-treated patients with chronic myeloid leukemia in chronic phase were randomly assigned to continuous treatment with a standard dose of imatinib (400 mg/day; n=113) or to 6 months of high-dose induction with imatinib (800 mg/day) followed by a standard dose of imatinib as maintenance therapy (n=114).
Results
The rates of major and complete cytogenetic responses were significantly higher in the high-dose arm than in the standard-dose arm at both 3 and 6 months (major cytogenetic responses: 36.8% versus 21.2%, P=0.01 and 50.0% versus 34.5%, P=0.018; complete cytogenetic responses: 22.8% versus 6.2%, P<0.001 and 40.4% versus 16.8%, P<0.001) on the basis of an intention-to-treat analysis. At 12 months, the difference between treatment arms remained statistically significant for complete cytogenetic responses (40.4% versus 24.8%, P=0.012) but not for major cytogenetic responses (49.1% versus 44.2%, P=0.462). The rate of major molecular responses was also significantly better at 3 and 6 months in the high-dose arm (month 3: 14.9% versus 3.5%, P=0.003; month 6: 32.5% versus 8.8%, P<0.001). Overall and progression-free survival rates were comparable between arms, but event-free survival was significantly worse in the high-dose arm (P=0.014).
Conclusions
Standard-dose imatinib remains the standard of care for pre-treated patients with chronic phase chronic myeloid leukemia (Clinicaltrials.gov identifier: NCT00327262).
Key words: CML, chronic phase, pre-treated, imatinib, high-dose, phase III study
Introduction
From a global perspective, optimized dosing schedules for imatinib treatment in a second-line context are of great interest because imatinib has not been approved for first-line treatment in many countries of the world so far. Patients are currently still often receiving drugs from the pre-imatinib era, such as interferon-α, hydroxyurea and busulfan. The impressive efficacy and safety results of the IRIS trial comparing imatinib 400 mg/day with interferon-α in combination with low-dose cytarabine in patients with newly diagnosed chronic phase (CP) chronic myeloid leukemia (CML)1,2 established imatinib 400 mg once daily as the first-line standard treatment in these patients. Notably, the initial phase I study was performed in pre-treated patients and revealed a clear dose-response relationship without the maximum tolerated dose being reached.3 Higher doses of imatinib have been shown to improve or restore remissions in non-randomized clinical trials including patients not responding sufficiently to a dose of 400 mg.4-6 Considering the importance of drug transporters (e.g. Oct-1 and PgP)7,8 in regulating the import and export of imatinib from the cytoplasm, optimized dosing schedules of the drug are needed to achieve higher intracellular drug levels with, consequently, more effective inhibition of the target kinase. Data from Hughes et al. supported this concept by showing that at least a proportion of CML patients achieving only a suboptimal response to standard-dose (SD) imatinib have low OCT-1 activity, a problem that can be overcome by imatinib dose-intensification.9 Moreover, previously conducted non-randomized phase II as well as randomized phase III studies performed both in CP-CML patients receiving second-line treatment after the failure of interferon-α5 and in patients with newly diagnosed early CP-CML6,10-14 suggest that a more aggressive dosing schedule (800 mg/day) induces faster responses and higher cytogenetic and molecular response rates, although these did not translate into improved survival rates. Furthermore, deeper responses achieved earlier have been demonstrated to be linked to a better long-term progression-free survival (PFS),9,15-17 supporting the use of more dose-intense imatinib induction therapy. With regards to safety, higher imatinib doses were generally well tolerated in pre-treated CP-CML patients with the exception of an increased rate of myelosuppression causing dose reductions in a substantial portion of patients treated with 800 mg/day5 The very recently presented results of the German CML IV study also support the potential of a tolerability-adapted high-dose (HD) imatinib schedule in newly diagnosed CML patients13 as this schedule induced significantly higher major molecular response rates than did either SD imatinib or SD imatinib in combination with interferon-α.
We chose to investigate an alternative approach to continuous HD imatinib and initiated a prospective international, multicenter randomized phase III study in which we limited HD imatinib (800 mg/day) to the first 6 months as induction, which was followed by 400 mg/day imatinib as “maintenance” therapy in the experimental arm. This dosing strategy was compared to continuous SD imatinib (i.e. 400 mg/day). The study was performed in a cohort of patients at high risk of disease acceleration, i.e. pre-treated, but imatinib-naïve CML patients in late CP, who had not achieved a major cytogenetic remission (MCyR) in response to their prior treatment at the time of enrollment into this study. The data presented here are the final results of the study after a median observation period of 24 months.
Design and Methods
Study design
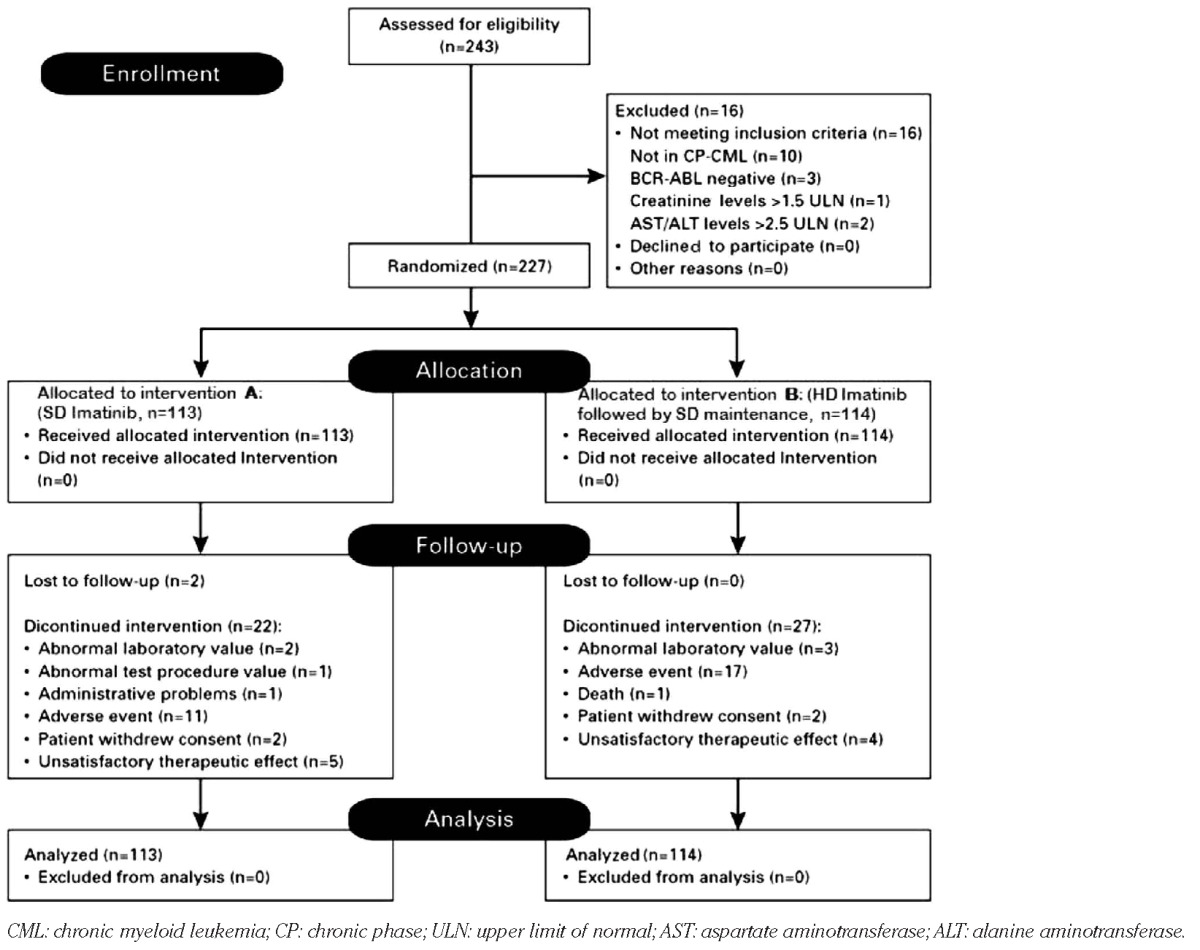
This multicenter, randomized, open-label, phase III study was performed in 13 centers in seven different countries: Austria, Bulgaria, Latvia, Lithuania, Macedonia, Serbia and Ukraine. The eligibility criteria have been described in detail elsewhere.18 In brief, CML patients in CP aged over 18 years had to have been pre-treated with drugs other than a bcr-abl-specific tyrosine kinase inhibitor for at least 12 months and should not have achieved a MCyR or anything better by study entry. All patients provided written informed consent to their participation in the study in accordance with the Declaration of Helsinki. The trial was reviewed and ethically approved at all participating centers and was registered at ClinicalTrials.gov, a service of the National Institutes of Health, with the identifying number NCT00327262. The trial was operated by the Central European Leukemia Study Group (CELSG), and data were collected and processed by the CELSG trial center at the Medical University of Innsbruck. Two hundred and forty-three patients with CML previously treated with drugs other than tyrosine kinase inhibitors were screened: 16 patients were not eligible for various reasons as outlined in the consort diagram (Table 1) and did not, therefore, receive any study drug. The remaining 227 patients who were all in CP at the time of randomization were considered the intent-to-treat population. The study was named “ISTAHIT” which stands for imatinib standard dose versus high dose induction trial.
TABLE 1.
The Consort flow diagram for the phase III CELSG CML 11 “ISTAHIT” study.
Treatment and dose modifications
Patients were randomized in a 1:1 ratio to either SD treatment (400 mg QD; 24 months) (arm A) or to experimental HD therapy (arm B). In arm B imatinib was administered for 6 months at 800 mg/day (400 mg BID) and then for 18 months at a dose of 400 mg QD. If patients experienced grade 2 non-hematologic toxicity, the study drug had to be withheld until the toxicity had resolved to grade 1 or less and was then resumed at the same dose. If the grade 2 toxicity recurred, imatinib had to be withheld until the toxicity had resolved to grade 1 or less and then re-introduced at a lower dose (300 mg QD in arm A or 300 mg BID in arm B or further decreased to 400 mg QD and further to 300 mg QD when grade 2 toxicity recurred at the previous dose level). In the case of recurring grade 2 toxicity with 300 mg QD the patient went off study. The same stepwise dosing reduction was performed in the case of a grade 3/4 non-hematologic toxicity.
If a patient experienced grade 3/4 hematologic toxicity according to the CTC3.0 toxicity grading, imatinib was withheld until the toxicity had resolved to grade 2 or less. If the toxicity resolved within 2 weeks, imatinib was resumed at the same dose. If the grade 3/4 toxicity recurred or persisted for more than 2 weeks, imatinib was withheld and then restarted at 300 mg QD in arm A and at 300 mg BID in arm B. If grade 3/4 toxicity recurred, the dose was reduced further (300 mg QD in arm A and 400 mg QD in arm B). If grade 3/4 toxicity recurred in patients taking 300 mg QD, the patient went off study. No dose reductions were performed for grade 3/4 anemia. Red blood transfusions or recombinant human erythropoietin and granulocyte colony-stimulating factor were allowed at the discretion of the investigator to treat anemia or leukocytopenia, respectively. Patients who progressed to accelerated phase or blast crisis went off study.
End-points
The primary end-point for evaluation in the study was the proportion of patients who achieved a MCyR after 12 months of therapy. This end-point was chosen because at the time the study was designed, in the pre-imatinib era, the achievement of a MCyR, in particular at 12 months, was associated with a significantly superior survival.19 Secondary end-points were the achievement of CCyR and major molecular remission (according to the international scale), tolerability of standard versus high-dose imatinib, event-free survival (EFS), PFS and overall survival (OS).
Definition of response and response monitoring
Blood counts, biochemistry and clinical evaluations were performed at baseline and at 1.5, 3, 6, 12, 18 and 24 months. All response criteria were assessed strictly according to the European LeukemiaNet recommendations.20 Bone marrow morphology, cytogenetic analyses and quantitative real-time polymerase chain reaction analyses from peripheral blood were performed at baseline, 1.5, 3, 6, 12 and 24 months. Cytogenetic responses were assessed locally, whereas molecular monitoring was done centrally at the European LeukemiaNet-certified reference laboratory of the Children's Cancer Research Institute (CCRI)/LabDia Labordiagnostik in Vienna, Austria. For molecular monitoring, peripheral blood was collected into four Paxgene RNA-stabilization tubes (PreAnalytiX, Hombrechtikon, Switzerland), each containing 2.5 mL blood. The tubes were stored locally at -20°C until shipment on dry ice every 3-6 months to the CCRI/LabDia. Total RNA was extracted using the PAXgene Blood RNA kit (PreAnalytiX, Hombrechtikon, Switzerland) strictly according to the manufacturer's instructions. Reverse transcription and real-time polymerase chain reaction analyses were carried out as described elsewhere.21 Quantitative analyses of BCR-ABL expression were performed in relation to the ABL gene. The molecular response was assessed using the international scale (IS).21-23
Statistical analyses
This report presents the final analysis of a multicenter, randomized, open-label, two-arm parallel group, phase III clinical trial comparing SD imatinib with HD imatinib in pre-treated CML patients. Demographic characteristics, diagnosis, extent of cancer, disease history, toxicity, adverse events and medication administered were compared applying Fisher's exact test, the χ2 test, t-test or Mann-Whitney U-test, as appropriate. Response rates at different time-points were summarized using contingency table analyses and compared between treatment groups using χ2 tests. EFS, PFS and OS were compared applying the Kaplan-Meier estimator together with the log-rank test. Events were defined as death from any cause during treatment, progression to accelerated phase or blast crisis, loss of a complete hematologic response, or loss of a MCyR. According to the protocol and due to an interim analysis,18 the significance level for the final analysis was set at 0.048. All tests were two-sided. The study was designed with a power of 90% to detect a 20% difference in the rate of MCyR within 12 months after randomization. The reported cytogenetic and molecular responses as well as EFS, PFS and OS were analyzed according to the intention-to-treat principle.
Results
Patients' characteristics
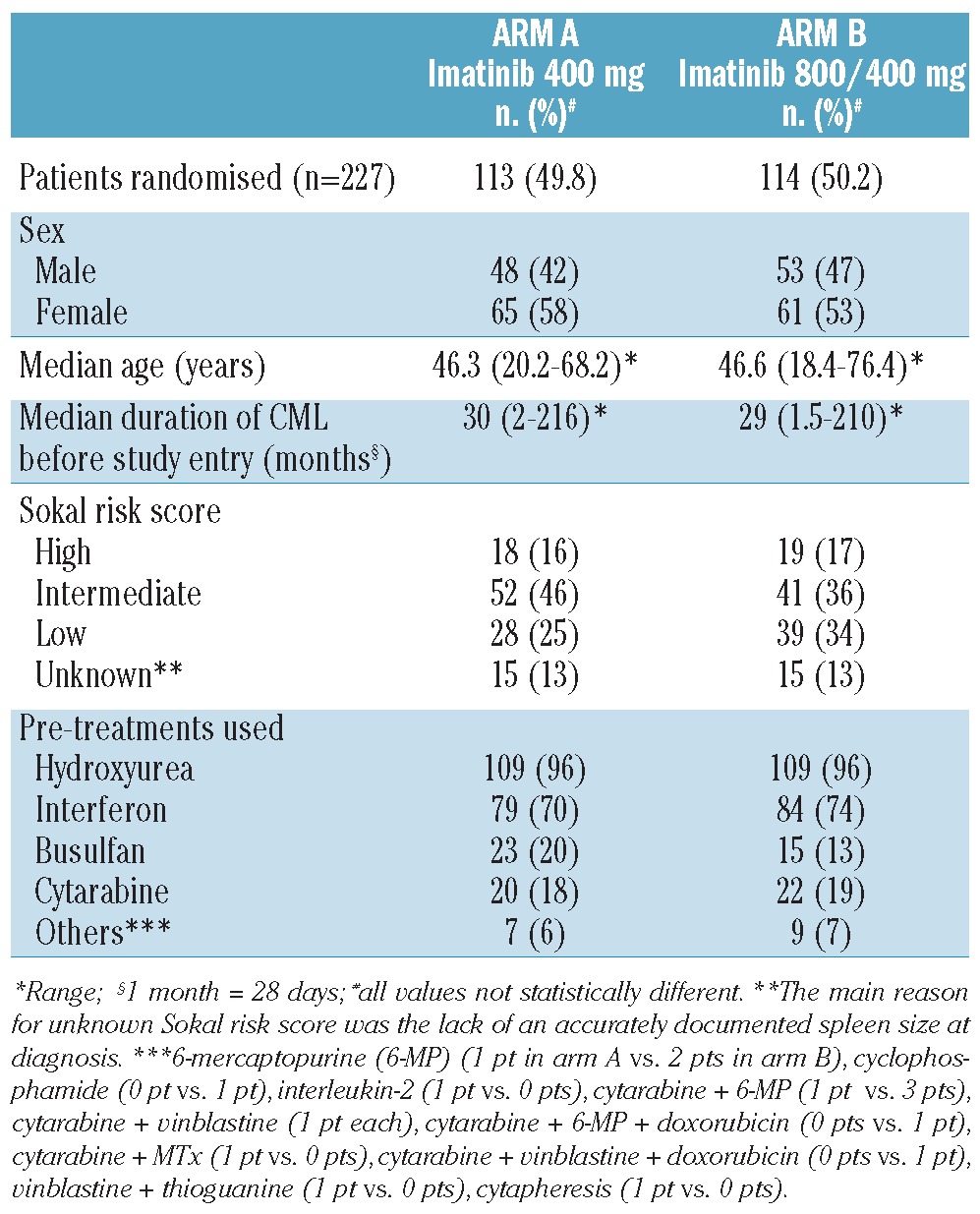
Two hundred and forty-three patients with pre-treated CML were screened but 16 patients were not eligible for various reasons, as stated in the Consort diagram (Table 1). Of the remaining 227 patients, 113 patients were randomized into the SD arm A and 114 patients into the experimental HD arm B. We did not detect any statistically significant differences with regards to patients' sex, age, Sokal scores at diagnosis or type of previous treatment which included hydroxyurea, interferons, busulfan, cytarabine and “others” (Table 2). The median number of previous treatments was two.
TABLE 2.
Patients' demographics. The median age, duration of CML, Sokal scores and pre-treatments are presented.
Hematologic, cytogenetic and molecular responses
Hematologic responses were evaluated according to the European LeukemiaNet criteria and were not significantly different between the study arms throughout the whole observation period (data not shown). In contrast, cytogenetic responses, analyzed on an intention-to-treat basis, were generally higher in the experimental HD arm, with statistically significant differences in the rates of MCyR at 3 and 6 months (month 3: 21.2% arm A, 36.8% arm B, P=0.01; month 6: 34.5% arm A, 50.0% arm B, P=0.018; Figure 1A). Notably, the effect of HD imatinib induction therapy resulted in a sustained improvement of CCyR rates also detectable 12 months after randomization, when patients had already received SD imatinib in both arms (month 3: 6.2% arm A, 22.8% arm B, P<0.001; month 6: 16.8% arm A, 40.4% arm B, P<0.001; month 12: 24.8% arm A, 40.4% arm B, P=0.012; Figure 1B). Interestingly, the rates of MCyR at 12 months (the primary end-point of the study) were not significantly different (44.2% arm A, 49.1% arm B). In line with the improved cytogenetic response rates, major molecular responses (using the IS) were also significantly superior at 3 and 6 months in the HD arm (month 3:3.5% arm A, 14.9% arm B, P=0.003; month 6: 8.8% arm A, 32.5% arm B, P<0.001; Figure 1C). Of note, the highest cumulative rates of MCyR and CCyR were achieved in the subgroup of patients with low and intermediate Sokal risk scores treated with HD imatinib (cumulative MCyR at 24 months 65.0% arm A and 77.5% arm B, P=0.01; cumulative CCyR at 24 months 48.8% arm A and 62.5% arm B, P=0.003). The cumulative MCyR rate in Sokal low/intermediate-risk patients in the HD arm was significantly better than that of Sokal high-risk patients treated with HD imatinib (MCyR: P=0.031) and also better than that of Sokal low/intermediate-risk patients treated with SD imatinib (P=0.013). Likewise, the cumulative CCyR rate in patients with Sokal low/intermediate risk was significantly better in the HD arm than in the imatinib SD arm A (P=0.003, Online Supplementary Figure S1).
Figure 1.
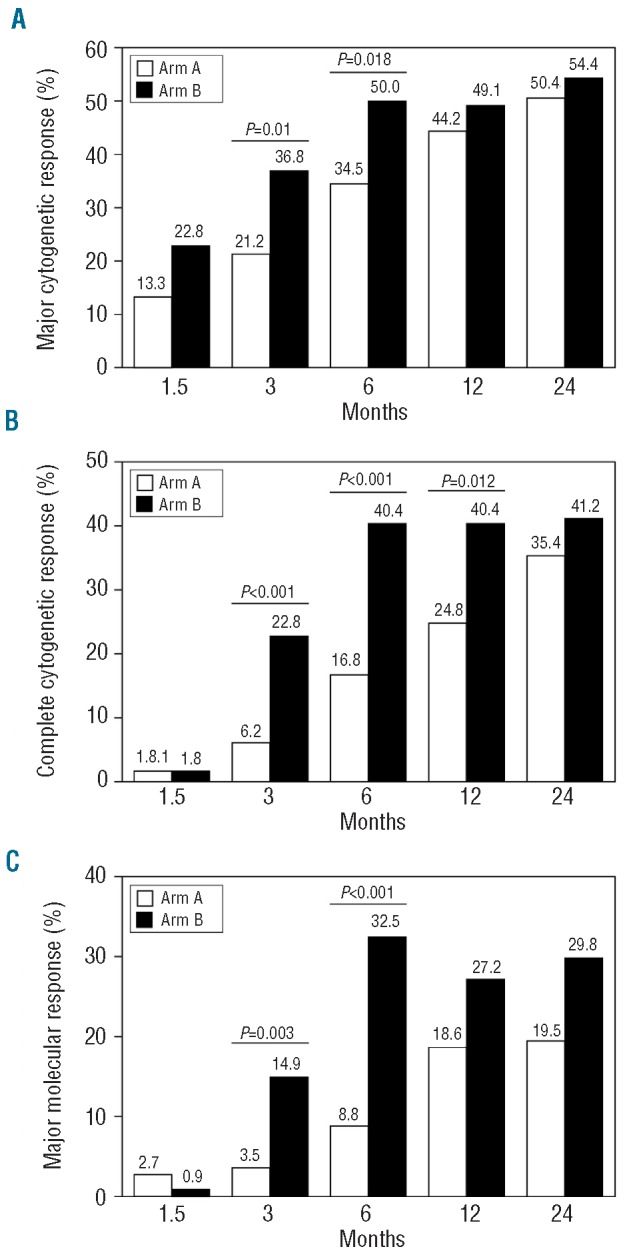
Cytogenetic and molecular responses at different time points by intention-to-treat analysis. Major cytogenetic responses (A), complete cytogenetic responses (B) and major molecular responsesIS (C) are shown as response rates at 1.5, 3, 6, 12 and 24 months for the SD arm (arm A; white bars) and the experimental HD arm (arm B; black bars).
Progression-free, event-free and overall survival
In spite of significantly higher and earlier complete cytogenetic and major molecular response rates in the HD imatinib induction arm, no improvement in PFS and OS was achieved by the dose intensification (Figures 2A-C). Unexpectedly, however, EFS was even significantly inferior in the experimental HD arm than in the SD imatinib arm (Figure 2C). Events in the HD arm comprised progression to accelerated phase (n=4), blast crisis (n=6), death (n=7), loss of complete hematologic response (n=9) and loss of MCyR (n=12), whereas in the SD arm only five patients progressed to blast crisis, two to accelerated phase, three died within 24 months after treatment initiation, nine lost a complete hematologic response and four lost a MCyR. Subsequent analyses revealed that patients with events in the HD arm had more often had dose reductions during the first 6 months of therapy (P=0.098), had larger spleens (P=0.087) and had had CML for longer before inclusion into the trial (P=0.064) when compared to patients who did not have events. In contrast, no difference in biological characteristics, such as clonal evolution prior to study inclusion, age, Sokal score, gender or type of prior therapy could be identified to be associated with the appearance of an event in the HD arm. Furthermore, we performed landmark analyses to categorize patients in both arms into complete and non-complete cytogenetic responders after the HD phase (i.e. 6 months of therapy) and subsequently analyzed EFS. This analysis revealed that patients in the HD arm who had not achieved a CCyR at the time of dose reduction (i.e. at 6 months) had significantly lower EFS and PFS rates when compared to patients in the HD arm who had achieved a CCyR by 6 months as well as when compared to patients receiving SD imatinib in arm A irrespective of the achievement of a CCyR (Figures 3A-B). It is noteworthy that the achievement of a CCyR in the high-dose arm was clearly associated with dose intensity during the first 6 months of therapy. After 6 months 68% of patients receiving continuous HD imatinib achieved a CCyR, whereas the proportion was only 30% in individuals who needed a dose reduction or treatment discontinuation due to toxicity (P<0.001). Moreover, dose interruptions lasted longer in patients who did not achieve a CCyR by 6 months than in patients who did achieve a CCyR in both treatment arms (mean/median: arm A 23/25 versus 13/13.5 days, P=0.11; arm B 30/25.5 versus 14/13 days, P<0.001). Accordingly, in the HD arm the mean dose was 752.6 mg (min/max: 561.9 – 800 mg/day) in patients with CCyR after 6 months as opposed to 642.8 mg (min/max: 334.5 – 800 mg/day) in patients who did not have a CCyR (median doses: 795.2 versus 635.7 mg/day, respectively; P<0.001).
Figure 2.
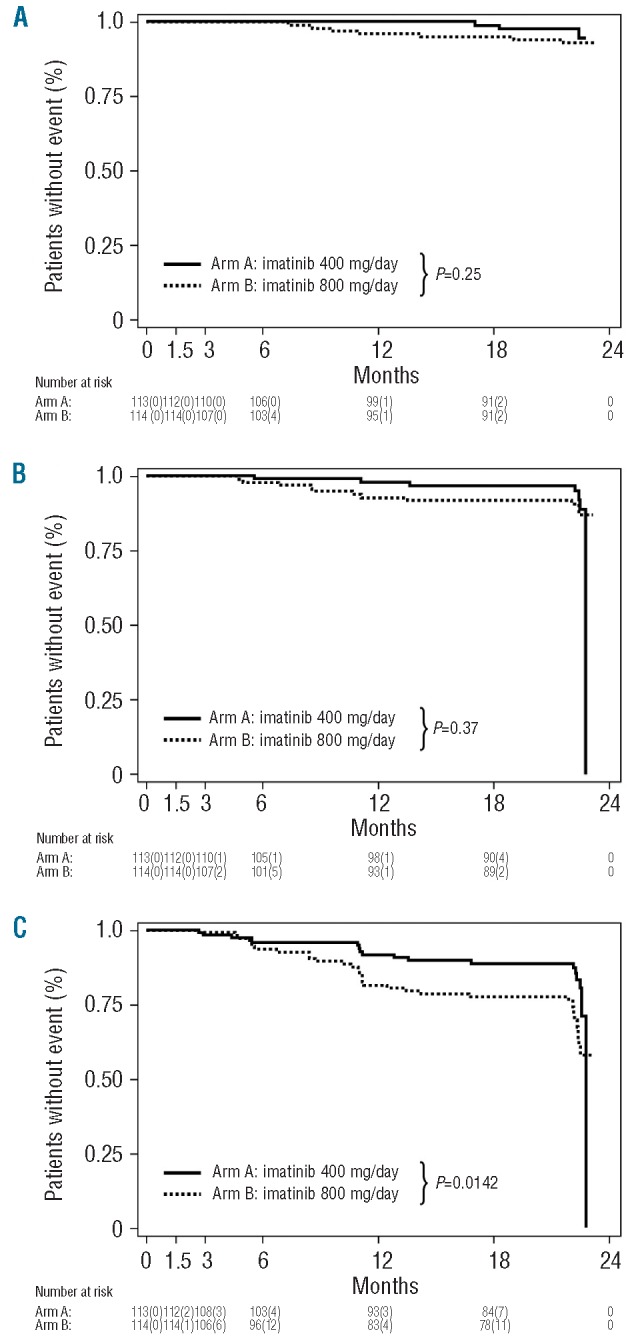
Kaplan-Meier survival curves for pre-treated CP CML patients who received SD imatinib (400 mg QD, arm A) or HD imatinib induction (800 mg for 6 months, followed by imatinib 400 mg QD thereafter; arm B). Overall survival (A), progression-free survival (B), event-free survival (C). Events were defined as follows: death from any course during treatment, progression to accelerated phase or blast crisis, loss of a complete haematologic response, loss of a MCyR.
Figure 3.
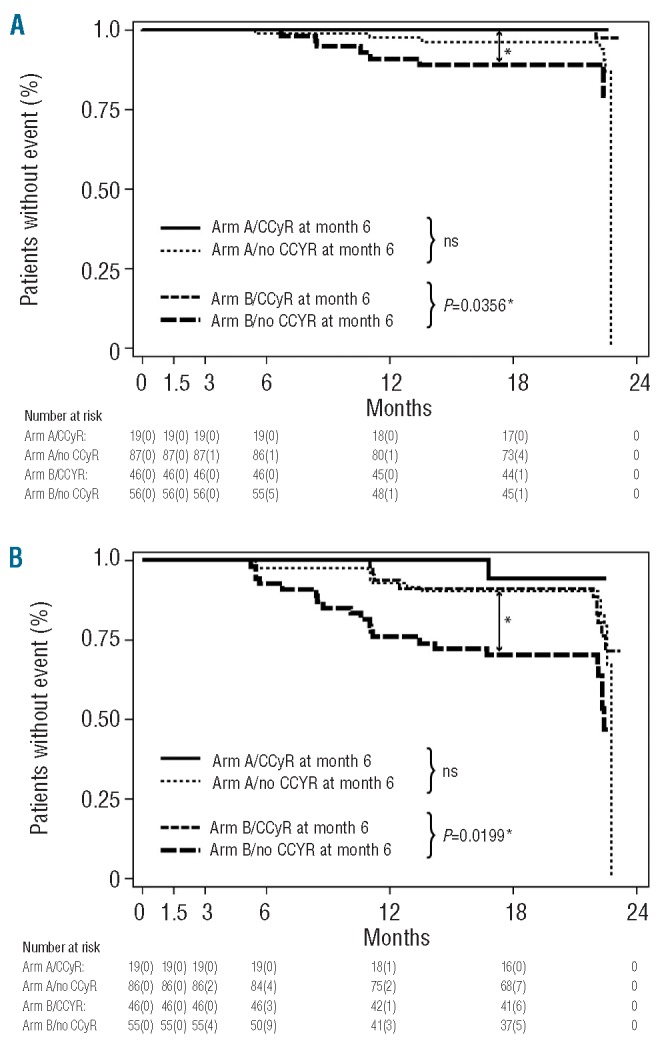
Landmark analyses of PFS and EFS according to CCyR at 6 months. A total of 199 patients (103 patients in arm A and 96 patients in arm B who were still on study treatment and could, therefore, be analyzed by conventional cytogenetics at month 6 after treatment initiation were categorized according to CCyR. The following end-points were analyzed: progression into accelerated phase and blast crisis (A) and EFS (B). Events were defined as follows: death from any cause during treatment, progression to accelerated phase and blast crisis, loss of a complete hematologic response, loss of a MCyR.
Toxicity
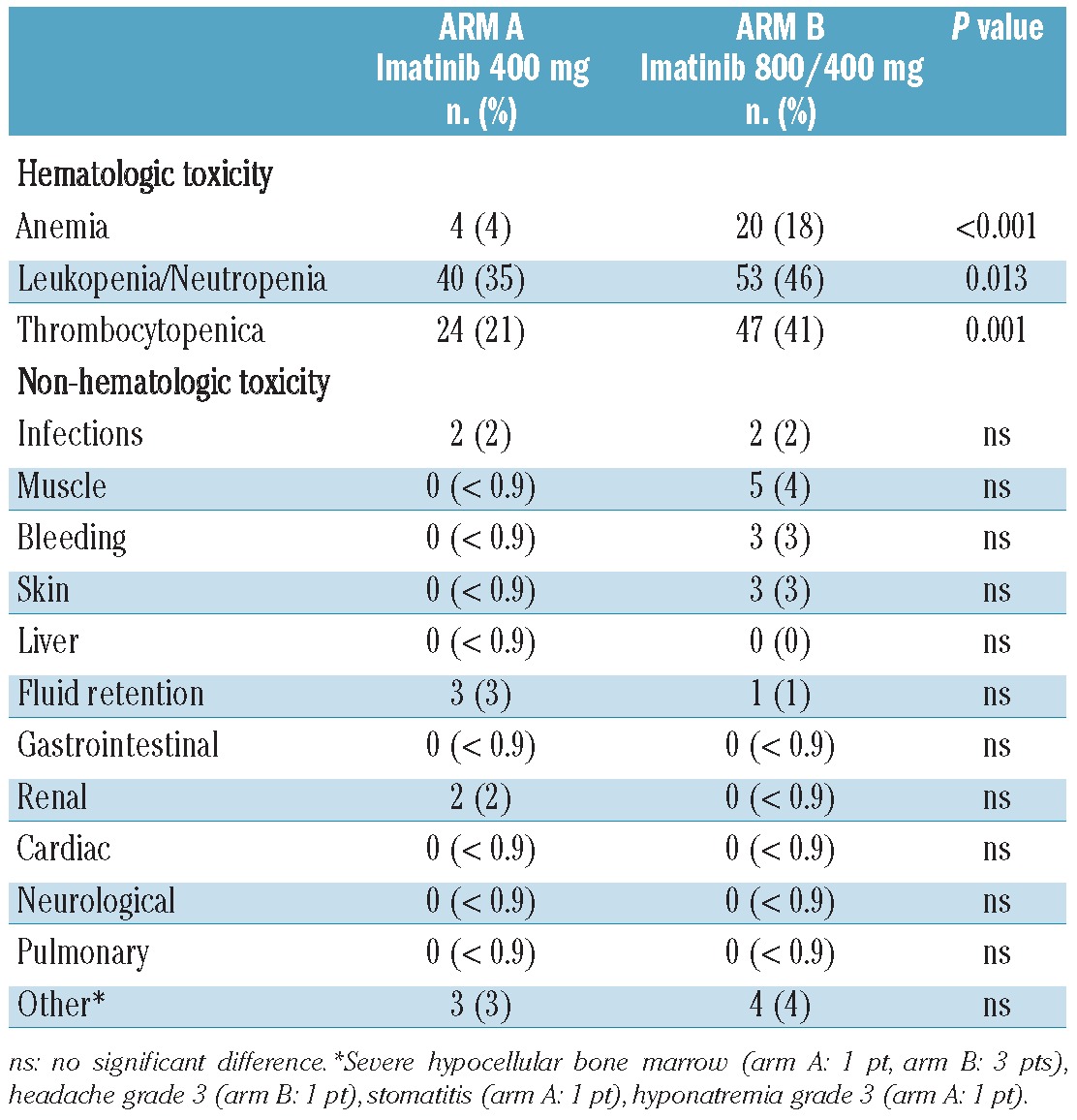
In terms of tolerability WHO grade 3 and 4 non-hematologic toxicities were not increased by dose intensification of imatinib during the first 6 months of therapy. In contrast, but not unexpectedly, WHO grade 3 and 4 hematologic toxicities were significantly more frequent in the experimental HD arm (Table 3). Notably, leukopenia did not translate into higher rates of grade 3 and 4 infectious complications, and thrombocytopenia was not associated with major bleeding events in the HD arm. The more frequent hematologic toxicities explain the higher rate of dose interruptions in the HD arm (65 cases) than in the SD arm A (47 cases) (P=0.024). As a consequence, the median cumulative dose of imatinib during the first 6 months was 718 mg in arm B compared to 400 mg in arm A.
TABLE 3.
Grade 3 and 4 hematologic and non-hematologic toxicities.
Discussion
Imatinib 400 mg once daily is the standard of care for CP CML as it induces high rates of cytogenetic and molecular responses not only in patients with de novo disease, but also in pre-treated patients.1,2,25-27 Nevertheless, for a substantial number of patients, this treatment is still suboptimal and needs further improvement. We, therefore, investigated whether a higher starting dose of imatinib (i.e. 800 mg/day) could improve cytogenetic and molecular remission rates and consequently affect EFS, PFS and OS in pre-treated patients with late CP CML, who are known to be at higher risk of disease progression.28 From a global perspective the data obtained from this study are of particular interest as a considerable proportion of CML patients in developing countries are still pre-treated with drugs other than bcr-abl-specific tyrosine kinase inhibitors. The patent protection of imatinib will expire in 2015/2016 and imatinib will then probably become the standard of care for CP CML patients also in these countries because of lower prices for the patent-free drug. The reason for limiting the time of HD imatinib to a 6 months “induction phase” was based on findings from studies in pre-treated CP CML patients showing that a significant proportion of patients treated with imatinib 800 mg/day had to have dose reductions because of toxicity at month 6.5 The design of the study presented here is, therefore, unique in comparison to other randomized phase III HD imatinib trials11-14 as it was performed in pre-treated CP CML patients and HD imatinib was not given throughout the whole study period, but limited to the first 6 months and followed by a SD “maintenance phase”. The data we evaluated in our SD arm are comparable to those from a phase II study of continuous SD imatinib in a similar cohort of CML patients in late CP.29 The final results of our study clearly show that cytogenetic and molecular responses to HD imatinib occur more rapidly and are more marked than those achieved in response to treatment with SD imatinib. This is consistent with findings from previous randomized11-14 as well as non-randomized clinical trials.5,6,10 The more rapid decline in tumor burden in the entire subpopulation of patients did not, however, translate into either a superior OS or an improved PFS. Interestingly, the EFS of the patients in our study was significantly worse in those patients who received HD followed by SD imatinib than in those who received continuous SD imatinib. This is surprising as superior cytogenetic and molecular responses have been clearly associated with superior survival rates so far.9,15-17,25,30-32 We, therefore, performed various sub-analyses that included also EFS analyses in patients achieving or not achieving a CCyR during the 6 months of HD imatinib (the end of the HD “induction” period). These studies revealed that the vast majority of events occurred in the subpopulation of patients in the HD imatinib arm who had not achieved a CCyR by 6 months. Moreover, we identified dose reduction in the HD cohort as an important variable for the achievement of CCyR at 6 months, which was reflected by significantly lower mean and median doses in patients not achieving CCyR. These patients are at a particularly high risk of having an event. Continuous administration of the higher doses does, therefore, appear to play an important role in the achievement of an optimal outcome. These data might also suggest that dose reduction from 800 mg to 400 mg imatinib once daily may be particularly deleterious for pre-treated CP CML patients who have not achieved a CCyR with 6 months of HD imatinib therapy. Our data are, therefore, in line with recently published data suggesting a superior outcome in the case of the achievement of a CCyR.15,16,25,30-33 It does, however, remain to be determined in future studies whether ongoing HD treatment of patients not achieving a CCyR at 6 months may compensate for the negative impact of dose reduction in this particular subgroup of patients. Moreover, the observation that HD imatinib induction followed by SD maintenance did not significantly affect PFS or OS is in line with results of previous randomized phase III studies testing a durable HD concept in comparison to SD imatinib in de novo CP CML patients [i.e. the tyrosine kinase inhibitor optimization and selectivity (TOPS) study,11 the prospective trial of the European LeukemiaNet,12 the French Spirit trial14 and the German CML study IV13 that compared imatinib 400 mg with a tolerability-adapted imatinib 800 mg dose per day]. In the light of our data the approach of the German CML IV trial to adapt the higher imatinib dose according to tolerability instead of reducing HD imatinib at a predefined definite time-point might be a better strategy for optimizing treatment outcome. However, this approach also has not been demonstrated to have any OS benefit so far. From the safety aspect, as expected and in line with previous data from HD imatinib trials,5,6,10-12 treatment for 6 months with dose-intense imatinib was paralleled by higher hematologic toxicity rates, although these did not translate into higher rates of severe infections or major bleeding events. The frequencies of non-hematologic grade 3 and 4 adverse events within the first 6 months were comparable between the two treatment arms. Thus, HD induction treatment followed by SD maintenance therapy was generally well tolerated and no additional safety concerns were observed. However, the hematologic toxicity of HD imatinib is clearly higher than that of SD treatment and results in frequent dose reduction and/or treatment discontinuation. As a substantial proportion of the patients had received interferon before inclusion into the study (70% in arm A and 74% in arm B) we also analyzed whether prior exposure to interferon had an impact on toxicities or affected the levels of response to imatinib. Interestingly, treatment with interferon prior to imatinib did not affect either response rates or toxicities (data not shown). Overall, we conclude that the strategy of HD induction treatment with 800 mg/day followed by SD maintenance induces higher CCyR and major molecular response rates without improving OS or PFS. Patients receiving HD imatinib more frequently have hematologic toxicities requiring dose modification, which negatively affects the achievement of CCyR. Patients not achieving a CCyR at 6 months in the HD arm were at a particularly high risk of developing an event after dose reduction to 400 mg once daily, which is mirrored by a markedly worse EFS in this cohort of patient. Thus, as we could not demonstrate an improved OS, PFS or EFS in the experimental HD imatinib treatment arm, imatinib 400 mg/day remains the standard of care for pre-treated patients with CP CML. However, a longer follow-up of the patients treated in this trial may be necessary to clarify whether an early improved major molecular response might possibly add a beneficial effect, at least in a selected group of patients. An increase in the dose of imatinib does, however, remain an option in the case of a suboptimal response according to defined European LeukemiaNet criteria if no imatinib-resistant mutation is detectable and no toxic side effects occur under imatinib 400 mg once daily dosing.30 In the case of treatment failure during any dosing schedule, a switch from imatinib to a second generation tyrosine kinase inhibitor appears to be the most appropriate treatment decision.
Supplementary Material
Acknowledgments
the excellent work of Roswitha Flener and colleagues from Result (CRO), Anton Klingler (who generated and provided the electronic CRF) and Wenzel Dennig who organized the drug supply to the centers are gratefully acknowledged. We thank Christiane Pfleger for typing and Birgit Petzer for proof-reading the manuscript.
Funding: this study was supported by an unrestricted research grant form Novartis to the CELSG.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003; 348(11):994–04 [DOI] [PubMed] [Google Scholar]
- 2.Hochhaus A, Druker B, Sawyers C, Guilhot F, Schiffer CA, Cortes J, et al. Favorable long-term follow-up results over 6 years for response, survival, and safety with imatinib mesylate therapy in chronic-phase chronic myeloid leukemia after failure of interferon-alpha treatment. Blood.2008; 111(3):1039–43 [DOI] [PubMed] [Google Scholar]
- 3.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001; 344(14):1031–7 [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian HM, Talpaz M, O'Brien S, Giles F, Carcia-Manero G, Faderl S, et al. Dose escalation of imatinib mesylate can overcome resistance to standard-dose therapy in patients with chronic myelogenous leukemia. Blood. 2003; 101(2):473–5 [DOI] [PubMed] [Google Scholar]
- 5.Cortes J, Giles F, O'Brien S, Thomas D, Garcia-Manero G, Rios MB, et al. Result of high-dose imatinib mesylate in patients with Philadelphia chromosome-positive chronic myeloid leukemia after failure of interferon-alpha. Blood. 2003; 102(l):83–6 [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian H, Talpaz M, O'Brien S, Garcia-Manero G, Verstovsek S, Giles F, et al. High-dose imatinib mesylate therapy in newly diagnosed Philadelphia chromosome-positive chronic phase chronic myeloid leukemia. Blood. 2004; 103(8):2873–8 [DOI] [PubMed] [Google Scholar]
- 7.White DL, Saunders VA, Dang P, Engler J, Venables A, Zrim S, et al. Most CML patients who have a suboptimal response to imatinib have low OCT-1 activity: higher doses of imatinib may overcome the negative impact of low OCT-1 activity. Blood. 2007; 110(12):4064–72 [DOI] [PubMed] [Google Scholar]
- 8.Rumpold H, Wolf AM, Gruenewald K, Gastl G, Gunsilius E, Wolf D. RNAi-mediated knockdown of P-glycoprotein using a transposon-based vector system durably restores imatinib sensitivity in imatinib-resistant CML cell lines. Exp Hematol. 2005; 33(7):767–5 [DOI] [PubMed] [Google Scholar]
- 9.Hughes TP, Branford S, White DL, Reynolds J, Koelmeyer R, Seymour JF, et al. Impact of early dose intensity on cytogenetic and molecular responses in chronic-phase CML patients receiving 600 mg/day of imatinib as initial therapy. Blood. 2008; 112(10):3965–73 [DOI] [PubMed] [Google Scholar]
- 10.Cortes J, Kantarjian H, Goldberg S, Powell BL, Giles FJ, Wetzler M, et al. High dose imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: high rates of rapid cytogenetic and molecular responses. J Clin Oncol. 2009; 27(28):4754–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortes J, Baccarani M, Guilhot F, Druker BJ, Branford S, Kim DW, et al. Phase III, randomized, open-label study of daily imatinib mesylate 400 mg versus 800 mg in patients with newly diagnosed, previously untreated chronic myeloid leukemia in chronic phase using molecular end points: tyrosine kinase inhibitor optimization and selectivity study. J Clin Oncol. 2010; 28(3):424–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baccarani M, Rosti G, Castagnetti F, Haznedaroglu I, Porkka K, Abruzzese E, et al. Comparison of imatinib 400 mg and 800 mg daily in the front-line treatment of high-risk, Philadelphia-positive chronic myeloid leukemia: a European LeukemiaNet study. Blood. 2009; 113(19):4497–504 [DOI] [PubMed] [Google Scholar]
- 13.Hehlmann R, Lauseker M, Jung-Munkwitz S, Leitner A, Müller MC, Pletsch N, et al. Tolerability-adapted imatinib 800 mg/d versus 400 mg/d versus 400 mg/d plus interferon in newly diagnosed chronic myeloid leukemia. J Clin Oncol. 2011; 29(12):1634–42 [DOI] [PubMed] [Google Scholar]
- 14.Preudhomme C, Guilhot J, Nicolini FE, Guerci-Bresler A, Rigal-Huguet F, Maloisel F, et al. Imatinib plus peginterferon alfa-2a in chronic myeloid leukemia. N Engl J Med. 2010; 363(26):2511–21 [DOI] [PubMed] [Google Scholar]
- 15.Lavallade H, Apperley JF, Khorashad JS, Milojkovic D, Reid AG, Bua M, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008; 26(20):3358–63 [DOI] [PubMed] [Google Scholar]
- 16.Quintas-Cardama A, Kantarjian H, Jones D, Shan J, Borthakur G, Thomas D, et al. Delayed achievement of cytogenetic and molecular response is associated with increased risk of progression among patients with chronic myelogenous leukaemia in early chronic phase receiving high-dose or standard-dose imatinib therapy. Blood. 2009; 113(25):6315–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortes J, Talpaz M, O'Brien S, Jones D, Luthra R, Shan J, et al. Molecular responses in patients with chronic myelogenous leukemia in chronic phase treated with imatinib mesylate. Clin Cancer Res. 2005; 11:3425–32 [DOI] [PubMed] [Google Scholar]
- 18.Petzer AL, Wolf D, Fong D, Lion T, Dyagil I, Masliak Z, et al. High-dose imatinib improves cytogenetic and molecular remissions in patients with pretreated philadelphia-positive, bcr-abl-positive chronic phase chronic myeloid leukemia: first results from the randomized CELSG phase III CML 11 “ISTAHIT” study Haematologica. 2010; 95(6):908–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kantarjian HM, Smith TL, O'Brien S, Beran M, Pierce S, Talpaz M, et al. Prolonged survival in chronic myelogenous leukemia after cytogenetic response to interferon-alpha therapy. The Leukemia Service. Ann Int Med. 1995; 122(4):254–61 [DOI] [PubMed] [Google Scholar]
- 20.Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006; 108(6):1809–20 [DOI] [PubMed] [Google Scholar]
- 21.Gabert J, Beillard E, van der Velden VH, Bi W, Grimwade D, Pallisgaard N, et al. Standardization and quality control studies of “real-time” quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia – A Europe Against Cancer Program. Leukemia. 2003; 17(12):2318–57 [DOI] [PubMed] [Google Scholar]
- 22.Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006; 108(1):28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Branford S, Cross NC, Hochhaus A, Radich J, Saglio G, Kaeda J, et al. Rationale for the recommendations for harmonizing current methodology for detecting BCR-ABL transcripts in patients with chronic myeloid leukaemia. Leukemia. 2006; 20(11):1925–30 [DOI] [PubMed] [Google Scholar]
- 24.Branford S, Fletcher L, Cross NC, Müller MC, Hochhaus A, Kim DW, et al. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood.2008; 112(8):3330–8 [DOI] [PubMed] [Google Scholar]
- 25.Deininger M, O'Brien SG, Guilhot F, Goldman JM, Hochhaus A, Hughes TP, et al. International randomized study of interferon vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. Blood. 2009; 114(22):62 [Google Scholar]
- 26.Kantarjian HM, Talpaz M, O'Brien S, Smith TL, Giles FJ, Faderl S, et al. Imatinib mesylate for Philadelphia chromosome-positive, chronic-phase myeloid leukemia after failure of interferon-alpha: follow-up results. Clin Cancer Res. 2002; 8(7):2177–87 [PubMed] [Google Scholar]
- 27.Kantarjian H, O'Brien S, Cortes J, Giles F, Shan J, Rios MB, et al. Survival advantage with imatinib mesylate therapy in chronic-phase chronic myelogenous leukemia (CML-CP) after IFN-alpha failure and in late CML-CP, comparison with historical controls. Clin Cancer Res. 2004; 10(1 Pt 1):68–75 [DOI] [PubMed] [Google Scholar]
- 28.Sacchi S, Kantarjian HM, O'Brian S, Beran M, Koller C, Pierce S, et al. Long-term follow up results of alpha-interferon-based regimens in patients with late chronic phase chronic myelogenous leukemia. Leukemia. 1997; 11(10):1610–6 [DOI] [PubMed] [Google Scholar]
- 29.Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C, et al. for the International STI571 CML Study Group. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002; 346(9):645–52 [DOI] [PubMed] [Google Scholar]
- 30.Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009; 27(35):6041–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006; 355(23):2408–17 [DOI] [PubMed] [Google Scholar]
- 32.Roy L, Guilhot J, Krahnke T, Guerci-Bresler A, Druker BJ, Larson RA, et al. Survival advantage from imatinib compared with the combination interferon-alpha plus cytarabine in chronic-phase chronic myelogenous leukemia: historical comparison between two phase 3 trials. Blood. 2006; 108(5):1478–84 [DOI] [PubMed] [Google Scholar]
- 33.Hochhaus A, O'Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009; 23(6):1054–61 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


