Abstract
Pediatric choroid plexus carcinomas (CPC) and adrenocortical carcinomas (ACC) are exceedingly rare tumors, each occurring at an annual rate of 0.3 cases per million children or less. Although both tumor types are associated with Li-Fraumeni Syndrome (LFS), the penetrance of germline TP53 mutations in CPC remains to be established. We report here a young boy without a family history of cancer who presented with CPC and subsequently ACC. Genetic testing revealed a novel de novo germline TP53 mutation (E285V). Neither tumor underwent loss of heterozygosity. Consistent with this observation, functional analyses demonstrated that E285V acts as a dominant-negative mutant that is defective in regulating target gene expression, growth suppression and apoptosis. These results further strengthen the association between germline TP53 mutations and childhood CPC, even when occurring in the absence of familial tumor susceptibility.
Keywords: p53, mutation, germline, choroid plexus, adrenal cortex
INTRODUCTION
Choroid plexus carcinomas (CPC) are rare, epithelial brain tumors that are highly-vascularized, invasive and associated with a poor prognosis (5–year survival, ~40%) (1). Pediatric adrenocortical carcinomas (ACC) are equally rare and often fatal (2). Little is known regarding the etiology of CPC and ACC, although both tumor types can arise within the context of Li Fraumeni Syndrome (LFS). LFS is usually associated with germline TP53 mutations that predispose carriers to diverse tumor types at a young age (3). However, pediatric ACC can also occur in association with a germline TP53 mutation without a family history of cancer, suggesting the existence of de novo and/or low penetrance TP53 mutations (4, 5).
To our knowledge only two pediatric cases of combined CPC and ACC have been previously reported, one of which is believed to be related to a germline TP53 mutation based on strong nuclear staining of p53 in the tumors and surrounding normal tissue (6, 7). In addition, a third child who developed a choroid plexus papilloma and ACC was found to carry the common germline TP53-R248W mutation (8). We report here a young boy with no family history of cancer who was diagnosed with CPC and ACC, and harbored a novel de novo germline TP53 mutation (E285V). Detailed molecular and biochemical analyses demonstrated that TP53-E285V has lost tumor suppressor activity, and functions as a dominant-negative mutant. These results further implicate constitutional TP53 mutations in the genesis of CPC, as well as childhood ACC, and support the genetic testing of individuals for TP53 alterations in these settings regardless of family cancer history.
MATERIALS & METHODS
Subject Enrollment
T.D was the second child born at term to parents without a medical history suggestive of familial cancer predisposition. He was diagnosed, late in pregnancy, with a left congenital diaphragmatic hernia and underwent an uncomplicated repair on day of life three. An ultrasound of the brain at birth revealed grade I bilateral germinal matrix hemorrhages, without any evidence of tumor on re-analysis. He was well until five months of age when he presented with a short duration of irritability and was evaluated for sepsis by lumbar puncture, which was hemorrhagic. Subsequent neural-imaging noted bilateral intraventricular (lateral ventricles) gadolinium enhancing masses. He underwent partial resection of the left ventricular tumor and was diagnosed with metastatic CPC. He was treated with multi-agent chemotherapy according to a Pediatric Brain Tumor Consortium protocol for embryonal CNS tumors (PBTC-001), and had a partial response to therapy with residual persistent disease bilaterally, but was otherwise asymptomatic and developing well. On routine surveillance imaging (MRI brain and spine), he was found to have a large (6.5×6.1×5.7cm) enhancing mass of the right adrenal gland. Laboratory testing revealed marked elevation of andrenal androgens. He underwent complete resection and pathology was consistent with ACC. He had a locally metastatic relapse of the ACC three months after surgery and died of disease (ACC) five months after diagnosis. Tumor material at each surgical resection was obtained for tumor banking under an IRB-approved protocol with parental consent, and retained for research purposes after appropriate material for pathologic diagnoses was obtained.
DNA Isolation and Sequencing
DNA was extracted from peripheral blood lymphocytes (PBLs) using a Puregene Blood DNA Kit (Gentra Systems, Minneapolis, MN) and from the ACC and CPC paraffin-embedded samples as previously described (9). The entire coding region of TP53 (exons 2-11) was PCR-amplified and analyzed by high-throughput DNA sequencing (Hartwell Center, St. Jude Children’s Research Hospital). Primer sequences are available upon request.
Tissue Preparation and Immunohistochemistry
ACC and CPC tumor samples were fixed in 10% neutral phosphate buffered formalin, processed and stained for p53 expression using the primary p53 antibody (DAKO clone DO-7) as previously described (9). Positive samples were identified by the nuclear deposition of a permanent brown precipitate that is readily detected by light microscopy.
Cell Culture
Human SaOS2 osteosarcoma cells were plated in 10 cm2 tissue culture dishes at approximately 1.5 × 106 cells per dish, and grown at 37°C under 5% CO2 in Dulbecco’s Minimum Eagle’s Medium (DMEM) containing 10% fetal bovine serum, 2.5 mM glutamine, penicillin (50 µg/ml) and streptomycin (100 units/ml). For apoptosis assays, SaOS2 and human H1299 lung adenocarcinoma cells were plated in 35 mm glass coverslip-embedded dishes at 2.4 × 104 and 4 × 103 cells per dish, respectively. Both SaOS2 and H1299 are TP53-null cell lines.
Colony Reduction
SaOS2 cells were transfected in duplicate with 1 µg CMV-Neo-Bam (CMV; empty vector) or CMV-expressing wild-type p53 (WT), E285V or R175H and selected in medium containing 800 ng/ml G418 antibiotic (Invitrogen, Carlsbad, CA) for up to 21 days as previously described (9). The colonies were washed with Dulbecco’s Phosphate Buffered Saline (D-PBS), fixed with methanol, and stained with 1:20 Giemsa stain (Sigma-Aldrich). The colonies were counted and photographed.
Transactivation
SaOS2 cells were transfected in duplicate with 150 ng wild-type p53-responsive p50-2Luc promoter-reporter (13) and 1 µg CMV or WT, E285V, R175H, R273H or R248W as previously described (9). After 48 hours, cells were harvested and lysed in M-PER buffer containing proteinase inhibitors (PIC) (Pierce, Rockford, IL). Protein yields were quantified and normalized using the BCA protein assay (Pierce). Relative light units were determined by the Single Luciferase Assay (Promega, Madison, WI) according to the manufacturer’s protocol.
Western Analysis
Proteins (40 µg/sample) were separated on 12.5% Tris-HCl precast Criterion gels (Biorad, Hercules, CA), transferred to nitrocellulose filters and probed with mouse monoclonal anti-human p53 DO-1 antibody (Calbiochem, San Diego, CA), sheep polyclonal anti-p53 antibody Ab-7 (Calbiochem) or mouse monoclonal β-Actin antibody (Sigma-Aldrich, St. Louis, MO) as previously described (9). Secondary antibodies included sheep anti-mouse IgG horseradish peroxidase (GE Healthcare, UK) and rabbit anti-sheep IgG horseradish peroxidase. Proteins were detected using SuperSignal West Pico Chemiluminescent substrate or SuperSignal West Dura Extended substrate (Pierce).
RESULTS
Association of a Novel Germline TP53-E285V Mutation with ACC and CPC
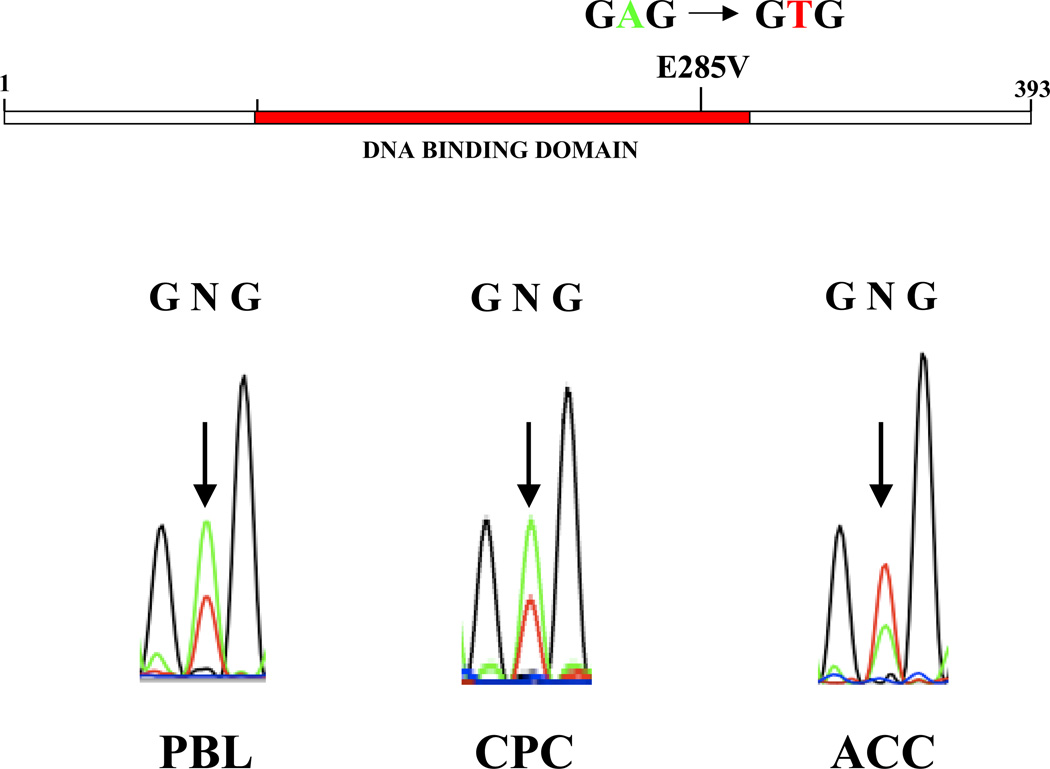
DNA sequence analysis of genomic PBL DNA identified a single point mutation in one TP53 allele (codon 285; GAG→GTG), resulting in a glutamic acid to valine substitution (Fig. 1). The ACC and CPC samples remained heterozygous for the TP53-E285V mutation, indicating that there was no selection against the wild-type allele (Fig. 1). No mutation was detected in either parent, suggesting that the E285V mutation arose de novo in the proband (data not shown).
Figure 1. Identification of a novel germline TP53-E285V mutation.
Sequence analysis of genomic DNA identified a heterozygous missense mutation in the DNA binding domain at codon 285 (H2 α helix), resulting in a glutamic acid to valine substitution (E285V). The adrenal (ACC) and brain (CPC) tumors retained both the wild-type and mutant TP53 alleles.
Nuclear Accumulation of Mutant TP53
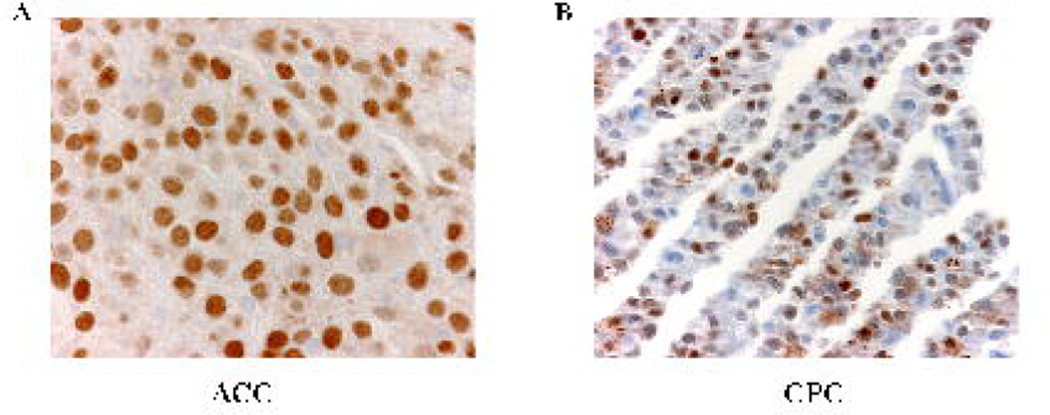
Immunohistochemical analysis showed strong positive staining for p53 in the nuclei of both the ACC and CPC cells, which is consistent with these tumors expressing mutant TP53-E285V (Fig. 2).
Figure 2. CPC and ACC overexpress p53.
Expression of p53 was determined by immunohistochemical analysis. A) ACC, strong to moderate staining for p53 in most tumor nuclei, as expected for cells expressing mutant TP53 protein B) CPC, strong to moderate p53 nuclear staining.
Functional Characterization of E285V
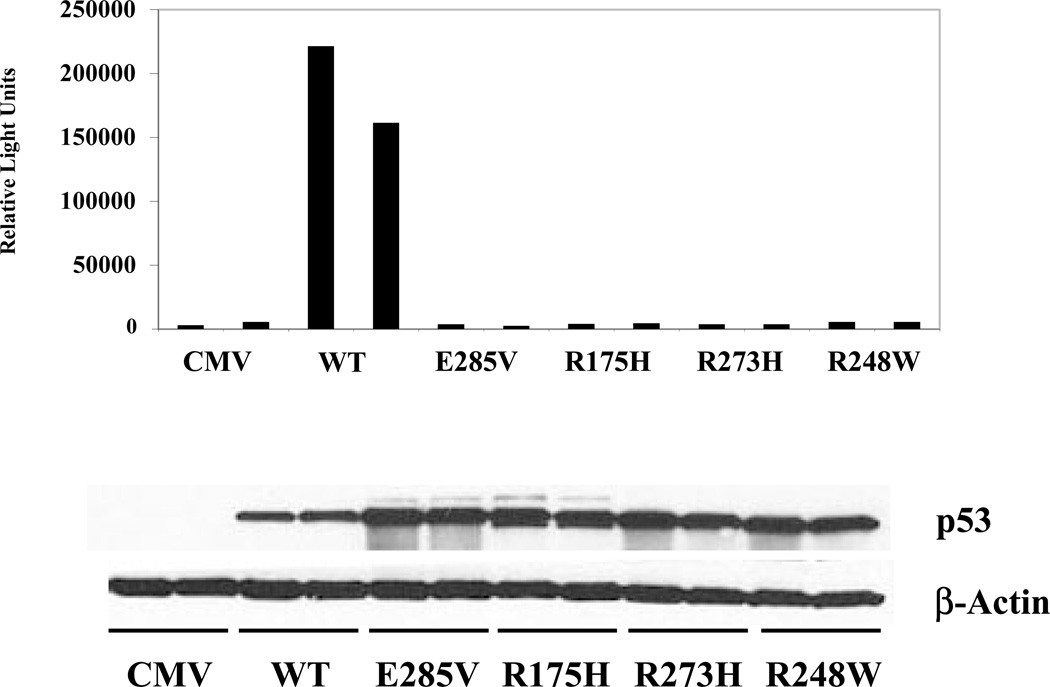
The capacity of the E285V mutant to regulate gene expression was tested in a standard promoter-reporter assay (13). As shown in Fig. 3, wild-type p53 (WT) efficiently induced promoter activity, whereas each of the established mutants R175H, R273H and R248W, as well as the novel mutant E285V, was inactive (upper panel). Each of the mutants were well-expressed based on Western blot analysis (Fig. 3, lower panel), demonstrating that their inability to induce promoter activity is due to an inherent functional defect.
Figure 3. The E285V mutant is defective in transactivation.
SaOS-2 cells were cotransfected in duplicate with the wild-type p53 responsive p50-2 Luc promoter-reporter and p53 expression vectors. Promoter-reporter activity was determined by measuring relative light units (upper panel). p53 protein expression was determined by Western blot analysis (lower panel). Data are representative of two independent experiments.
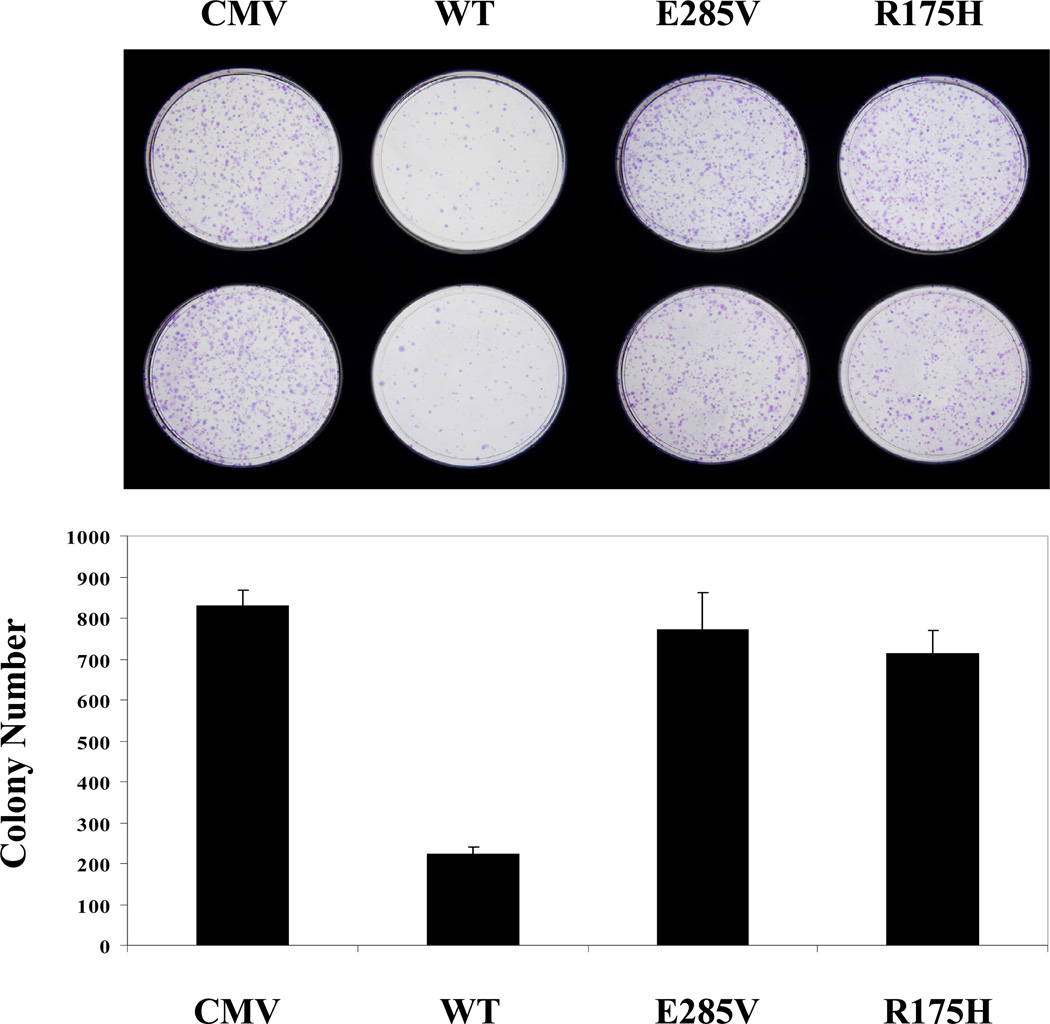
In addition, colony growth was significantly inhibited by wild-type p53, but not the R175H and E285V mutants (Fig. 4). Therefore, the E285V mutant is defective in regulating promoter activity and in repressing tumor cell growth.
Figure 4. The E285V mutant is defective in tumor cell growth suppression.
SaOS-2 cells were transfected with p53 expression vectors and selected in medium containing G418. Resulting colonies were stained, counted (lower panel) and photographed (upper panel). Data are representative of two independent experiments.
DISCUSSION
Pediatric CPC and ACC are exceptionally rare tumors. The identification of a child who developed both tumor types by 1.5 years of age is remarkable and points to an underlying genetic predisposing factor. Indeed, DNA sequencing analysis revealed a germline TP53 mutation corresponding to a single amino acid substitution (E285V). Although the TP53-E285V mutant has not been previously reported as a germline mutation (www-p53.iarc.fr), it has been infrequently observed in sporadic tumors of the liver, bladder, lung, ovary, brain, colon, blood and skin (14). Interestingly, the only other reported germline mutation at codon 285, a glutamic acid to glutamine substitution, was identified in child who developed ACC and osteosarcoma (16).
Functional analyses of E285V revealed significant defects in its ability to regulate promoter activity (Fig. 3), suppress tumor cell growth (Fig. 4), and trigger apoptosis (Supplemental Fig. S1). Although some mutant p53 proteins exhibit a gain-of-function that induces oncogenic target genes to promote malignancy (12), the E285V mutant is inactive in this assay (Supplemental Fig. S2). We also determined that E285V efficiently functions as a dominant negative regulator that neutralizes wild-type p53 activity (Supplemental Fig. S3). These results are consistent with the observation that the CPC and ACC tumors express elevated levels of nuclear TP53 (Fig. 2) while maintaining both wild-type and mutant TP53 alleles (Fig. 1). Therefore, E285V is severely compromised as a tumor suppressor in terms of failing to induce wild-type p53 responsive genes, blocking tumor cell growth and activating tumor cell death.
The proband carries a germline TP53 mutation, but does not have a strong family history of cancer. Genetic testing of both parents failed to detect any TP53 alterations, indicating that the E285V mutation arose de novo. This finding is in keeping with other studies of childhood ACC where de novo and low penetrant germline TP53 mutations have been reported (4, 5). It also highlights the need to consider constitutional TP53 mutations in children who present with CPC and/or ACC, even outside the context of tumor prone families. At present there is no effective strategy to correct a mutant TP53 allele. However, the identification of carriers provides an opportunity for closer clinical follow-up, which should lead to earlier interventions and better prognosis. It also becomes important to establish structure-function relationships between TP53 mutations and tumor suppressor activity, as it is becoming increasingly clear that different p53 mutants retain different degrees of function (15). Determining these genotype-phenotype associations will be important for genetic counseling purposes. Studying rare tumors, such as ACC and CPC, provides a powerful, natural approach to identify clinically relevant mutants and to study their consequence on tumor susceptibility.
Supplementary Material
ACKNOWLEDGMENTS
We thank JinLing Wang, Simon Moshiach, Jill Lahti and the St. Jude Hartwell Center for their technical assistance. We also thank Dr. Suzanne Gronemeyer and the Pediatric Oncology Education Program (NIH/NCI CA23944) for their continued support of A.R-S. This study was funded by grants from the NIH/NCI CA63230 (G.P.Z.), Cancer Center CORE Grant CA21765, and by the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
The authors have no competing interest to declare. The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in JMG and any other BMJPGL products to exploit all subsidiary rights, as set out in the journal’s license (http://jmg.bmj.com/ifora/licence.pdf).
REFERENCES
- 1.Wolff JE, et al. Choroid plexus tumors. Br J Cancer. 2002;87:1086–1091. doi: 10.1038/sj.bjc.6600609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez-Galindo C, et al. Biology, clinical characteristics, and management of adrenocortical tumors in children. Pediatr Blood Cancer. 2005;45:265–273. doi: 10.1002/pbc.20318. [DOI] [PubMed] [Google Scholar]
- 3.Malkin D, et al. Germ Line p53 Mutations in a Familial Syndrome of Breast Cancer, Sarcomas, and other Neoplasms. Science. 1990;250:1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- 4.Varley JM, et al. Are there low-penetrance TP53 alleles? Evidence from childhood adrenocortical tumors. Am J Hum Genet. 1999;65:995–1006. doi: 10.1086/302575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribeiro RC, et al. An inherited p53 mutation that contributes in a tissue-specific manner to pediatric adrenal cortical carcinoma. PNAS. 2001;98(16):9330–9335. doi: 10.1073/pnas.161479898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandrini R, Ribiero RC, DeLacerda L. Childhood Adrenocortical Tumors. J Clin Endocrinol Metab. 1997;82:2027–2031. doi: 10.1210/jcem.82.7.4057. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Cornford ME. Coincident choroid plexus carcinoma and adrenocortical carcinoma with elevated p53 expression. Arch Pathol Lab Med. 2002;126:70–72. doi: 10.5858/2002-126-0070-CCPCAA. [DOI] [PubMed] [Google Scholar]
- 8.Vital A, et al. Astrocytomas and choroid plexus tumors in two families with identical p53 germline mutations. J Neuropath Exp Neurol. 1998;57:1061–1069. doi: 10.1097/00005072-199811000-00009. [DOI] [PubMed] [Google Scholar]
- 9.West AN, et al. Identification of a novel germ line variant hotspot mutant p53-R175L in pediatric adrenal cortical carcinoma. Cancer Res. 2006;66:5056–5061. doi: 10.1158/0008-5472.CAN-05-4580. [DOI] [PubMed] [Google Scholar]
- 10.Kleihues P, et al. Tumors associated with p53 germline mutations: a synopsis of 91 families. Am J Pathol. 1997;150:1–13. [PMC free article] [PubMed] [Google Scholar]
- 11.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 12.Cadwell C, Zambetti GP. The effects of wild-type p53 tumor suppressor activity and mutant p53 gain of function on cell growth. Gene. 2001;277:15–30. doi: 10.1016/s0378-1119(01)00696-5. [DOI] [PubMed] [Google Scholar]
- 13.Zambetti GP, et al. Wild-type p53 mediates positive regulation of gene expression through a specific DNA sequence element. Genes Dev. 1992;6:1143–1152. doi: 10.1101/gad.6.7.1143. [DOI] [PubMed] [Google Scholar]
- 14.Petitjean A, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28:622–629. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- 15.Zambetti GP. The p53 Mutation “Gradient Effect” and its Clinical Implications. J Cell Physiol. 2007;213:370–373. doi: 10.1002/jcp.21217. [DOI] [PubMed] [Google Scholar]
- 16.Yonemoto T, et al. Two Cases of Osteosarcoma Occurring as Second Malignancy of Childhood Cancer. Anticancer Research. 1999;19:5563–5566. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.