Abstract
Infection by some viruses induces immunity to reinfection, providing a means to identify protective epitopes. To investigate resistance to reinfection in an animal model of HIV disease and its control, we employed infection of mice with chimeric HIV, EcoHIV. When immunocompetent mice were infected by intraperitoneal (IP) injection of EcoHIV, they resisted subsequent secondary infection by IP injection, consistent with a systemic antiviral immune response. To investigate the potential role of these responses in restricting neurotropic HIV infection, we established a protocol for efficient EcoHIVexpression in the brain following intracranial (IC) inoculation of virus. When mice were inoculated by IP injection and secondarily by IC injection, they also controlled EcoHIV replication in the brain. To investigate their role in EcoHIV antiviral responses, CD8+ T lymphocytes were isolated from spleens of EcoHIV infected and uninfected mice and adoptively transferred to isogenic recipients. Recipients of EcoHIV primed CD8+ cells resisted subsequent EcoHIV infection compared to recipients of cells from uninfected donors. CD8+ spleen cells from EcoHIV-infected mice also mounted modest but significant interferon-γ responses to two HIV Gag peptide pools. These findings suggest EcoHIV-infected mice may serve as a useful system to investigate the induction of anti-HIV protective immunity for eventual translation to human beings.
Keywords: Mouse model, Chimeric HIV, Superinfection, Adaptive immune responses, HIV neuropathogenesis, Brain
Introduction
Virus infection often elicits adaptive immune responses that protect against subsequent infection by the same virus, indeed this property is deemed highly predictive of the ability to successfully vaccinate against “ordinary viruses” (Hilleman, 1992). With important but rare exceptions, HIV does not naturally induce such protective immunity. To the contrary, in settings in which secondary or superinfection can be clearly documented, infected human beings pose little resistance to HIV superinfection (Chohan et al. 2010).
HIV does induce CD8+ T lymphocyte responses whose efficacy can be inferred by the selection of escape variants in which HIV species increase in prevalence that lack the determinant recognized by functional CD8+ cells (Kearney et al. 2009); unfortunately CD8+ efficacy wanes through multiple mechanisms reflecting both viral diversity and specificity of host responses (Pantaleo et al. 1997). However anti-HIV CD8+ responses appear to underlie the immune state in some sex workers who are frequently exposed to HIV but resist stable infection (Kaul et al. 2000). The simplest explanation for their resistance is that the women experience a transient HIV infection that induces CD8 responses that effectively eliminate HIV-infected cells. In animal models, the salience of CD8-bearing T cells to control HIV disease has been clearly demonstrated in a model of AIDS. Depletion of CD8+ cells using anti-CD8 antibodies allows SIV replication and viremia that resolve with repopulation of CD8+ cells (Schmitz et al. 1999).
We constructed chimeric HIV, EcoHIV, to infect mice to exploit the extensive biological resources in this experimental animal to investigate HIV infection and pathogen-esis (Potash et al. 2005). Vaccination with several HIV DNA vaccines blocked EcoHIV challenge in mice, illustrating that mice can mount protective immune responses against HIV replication (Saini et al. 2007; Roshorm et al. 2009; Im et al. 2011). On this basis we investigated whether EcoHIV infection itself induces such adaptive immunity that protects mice from superinfection by the same virus. The present work shows that mice systemically infected by EcoHIV resist challenge infection both in the periphery and in the brain.
Materials and methods
Mice
All studies using mice in Dr. Volsky's laboratory were approved by the St. Luke's-Roosevelt Institutional Animal Care and Use Committee and were conducted in full compliance with NIH guidelines. Male and female 129×1 and BALB/c mice from 6–16 weeks of age either were purchased from the Jackson Laboratory or bred in Volsky's mouse colony. All animal procedures and care performed in Dr. Hanke's laboratory conformed strictly to the United Kingdom Home Office Guidelines under The Animals (Scientific Procedures) Act 1986. The protocol was approved by the local Research Ethics Committee (Clinical Medicine, University of Oxford). Experiments were carried out under Project License no. 30/2406 held by TH with a strict implementation of the Replacement, Reduction and Refinement (3Rs) principles. Adult female BALB/c mice were purchased from Harlan Laboratory.
EcoHIV and systemic infection
EcoHIV/NDK (EcoHIV) was prepared and euthanasia and tissue collection conducted as described (Potash et al. 2005) at the time points indicated. IP inoculation was conducted as described (Hadas et al. 2007).
Intracranial inoculation
Mice were heavily anesthetized by IP injections of ketamine (100 mg/kg) and xylaxine (20 mg/ kg), and placed into a stereotaxic chamber (Stoetling Co., Wood Dale, IL), and each mouse's head was secured with ear bars and a mouthpiece. A small incision was made along the top of the head, exposing the brain. A small hole was drilled into the skull in the right hemisphere at a distance of 2.5 mm to the right of bregma, a position that allows for injection into the caudate putamen, a region of the basal ganglia. Virus that had been concentrated to a volume of 10 μl was injected using a 25 μl Hamilton syringe with a cemented 31 gauge needle and an Ultra Micropump III (World Precision Instruments) set to inject at a rate of 10 μl/20 min at a depth of 2.5 mm. Mice were then removed from the chamber and the wound sutured. This methodology was adapted from the work of Persidsky and colleagues (Persidsky et al. 1996).
Measurement of EcoHIV burden
RNA isolation and quantitative real-time PCR (QPCR) amplifying HIV-1 vif RNA, standardized by amplification of glyceraldehyde phosphate dehydrogenase, were conducted as described (Hadas et al. 2007). For QPCR amplification of 2-LTR DNA, mouse brain was homogenized in Trizol, and DNA extracted by sequential mixing with DNAzol, ethanol precipitation and washing, and NaOH treatment. DNA was buffered and then employed for QPCR. The forward primer is MH535 5′-AACTAGGGAACCCACTGCTTAAG-3, the reverse primer is MH536 5′-TCCACAGATCAAGGATATCTTGTC-3′, and the probe is MH603 5′-ACACTACTTGAAGCACT-CAAGGCAAGCTTT-3′. DNA input was standardized by amplification of murine β globin (Hadas et al. 2007). A standard curve for quantitation of 2 LTR copy number was constructed using graded numbers of a plasmid containing the LTR-LTR junction as well as the EcoNDK gag amplicon. Every QPCR assay of virus burden included cell extracts from uninfected mice as negative controls.
Adoptive transfer of CD8+ splenic T cells
Spleens were collected from uninfected BALB/c mice or from BALB/c mice six weeks after EcoHIV infection, single cell suspensions were prepared, and CD8+ cells were isolated by negative selection using magnetic beads from Dynabeads according to the manufacturer's protocol. Isolated cells were pooled and CD8 display was confirmed by flow cytometry (not shown). Three million cells from each pool were transferred to each of three BALB/c recipients by intravenous injection one day prior to IP injection of 0.5×106 pg p24 EcoHIV. Spleen cells were harvested one week after infection for measurement of spliced HIV RNA.
Natural immune responses to EcoNDK infection
Groups of five BALB/c mice were injected with 2×106 pg p24 core antigen of EcoNDK or PBS IP. Four weeks post-challenge, animals were euthanized and spleen cells were collected to measure HIV-specific induction of interferon-γ (IFN-γ) expression in CD8+ cells after restimulation with viral peptides in tissue culture. Data shown are responses to two Gag peptides pools from a set of 285 peptides (18-mers overlapping by 10 amino acids) of HIV-1 clade D consensus sequence; peptide pools were obtained from the Center for HIV/AIDS Vaccine Immunology, Oxford. Two million spleen cells were added to each well of a 96-well round-bottomed plate and pulsed with peptide pools or left unstimulated and kept at 37°C, 5% CO2 for 90 min, followed by the addition of GolgiStop™. After a further 5-h incubation, reaction was terminated, the cells washed with FACS wash buffer (PBS, 2% FCS, 0.01% Azide) and blocked with anti-CD16/32 at 4°C for 30 min. All subsequent antibody stains were performed using the same conditions. Cells were then washed and stained with anti-CD8-PerCP, washed again and permeablized using the Cytofix/Cytoperm™ kit. Perm/Wash buffer was used to wash cells before staining with anti-IFN-γ APC. Cells were washed with Perm/Wash buffer and fixed with CellFIXTM and stored at 4°C until analysis. All above regents were obtained from BD Biosciences. Stained cells were analyzed on a four-colour flow cytometer (FacsCaliber, Becton Dickinson) and FlowJo Software (Tree Star, Inc.).
Immunocytochemistry (ICC) of brain sections
ICC was performed on 6 μm coronal sections of paraffin-embedded mouse brains as previously described (Potash et al. 2005). Murine microglial cells were identified using rabbit polyclonal antibodies to ionized calcium-binding adapter molecule 1 (Iba-1, 1:500, Wako, Richmond, VA) using immunoperoxidase method with diaminobenzidine (DAB) and light hematoxylin counterstain. Sections were examined in a Zeiss Photomicroscope III, and images were captured using a Nikon DN100 digital camera.
Statistics
Differences between virus burden or IFN-γ positive cells were tested by Student's t test with p values listed in Figure Legends.
Results
Knowing that HIV DNA vaccines protect mice against EcoHIV infection and that EcoHIV infection itself induces anti-HIV responses (Potash et al. 2005; Saini et al. 2007; Roshorm et al. 2009; Im et al. 2011) we investigated the possibility that responses to EcoHIV infection protect against subsequent infection by the same virus. Mice at three stages of infection were evaluated for the expression of spliced HIV RNA: i) chronic infection (6 weeks after inoculation), ii) acute infection (5 days after inoculation), iii) chronic plus acute infection (6 weeks after primary inoculation, 5 days after secondary inoculation). If EcoHIV induces no protective responses, virus burden in group iii may reach the sum of virus burdens in the other two groups. Alternatively, if virus burden in group iii is less than that sum, we may conclude that prior infection induces resistance to infection (Fig. 1). As Fig. 1 clearly shows, group iii mice carry EcoHIV burdens significantly smaller than group ii mice and similar to group i mice, indicating a profound control of secondary EcoHIV infection. These findings are consistent with the premise that EcoHIV infection primes an adaptive immune response that limits further infection by the same virus. In addition, the observation that EcoHIV burden is much higher 5 days than 6 weeks after infection raises the possibility that responses induced early in infection control subsequent virus expression.
Fig. 1.
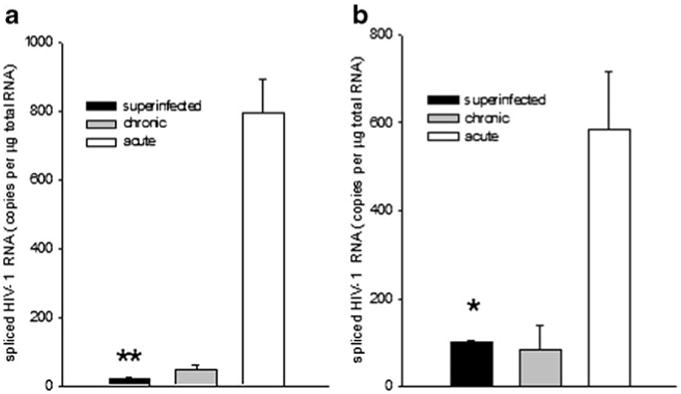
EcoHIV-infected mice resist superinfection. 129×1 mice infected by EcoHIV for either six weeks, five days, or twice for six weeks and five days were euthanized and spleen and peritoneal macrophages collected to measure virus burden by QPCR. a Spliced HIV RNA in spleen cells, b spliced HIV RNA in peritoneal macro-phages; * p<0.05, ** p<0.005
Adaptive immunity plays an important role in SIV infection in the brain in macaques (Williams et al. 2001; Clements et al. 2008). With these precedents in mind, we attempted to test whether immunity induced by EcoHIV infection in the periphery (Fig. 1) also affects EcoHIV infection in the brain. Because peripheral infection of mice with EcoHIV leads to detectable viral infection in the brain in only a fraction of mice (Potash et al. 2005), we first tested whether more efficient and uniform virus replication in the brain can be achieved by administration of virus directly into the brain by IC inoculation (Fig. 2). We adapted the IC inoculation method described by Persidsky and colleagues for implantation of HIV-1 infected human macrophages (Persidsky et al. 1996), including targeting virus inoculum to the basal ganglia region in the right brain hemisphere (Fig. 2a). The inoculation process itself, as verified by injection of saline, had no visible effect on striatal anatomy and lead to minimal activation of resident microglial cells, indicating that brain integrity was preserved (Fig. 2b). We then inoculated concentrated infectious EcoHIV into brains of six 129×1/J mice; 3 mice were sacrificed on Day 3 and the reminder on Day 10 after infection, and expression of HIV-1 Vif RNA was tested in brain quadrants (Fig. 2a) by RT-QPCR as described in Methods. We chose detection of spliced viral RNA because these viral forms have to be synthesized de novo from integrated provirus and therefore they represent new virus expression in the brain. We found robust HIV expression in brains of all infected mice; the table in Fig. 2c lists averages of Vif RNA copies detected in brain quadrants tested. Note that in the time frame tested, virus expression occurred mostly in R2 quadrant containing the inoculation site, with lower virus expression in the adjoining R1 and L2 quadrants and no detectable virus in the frontal left section of the brain (Fig. 2c). These results show that IC inoculation of chimeric HIV into mouse brain permits efficient HIV expression in this tissue.
Fig. 2.
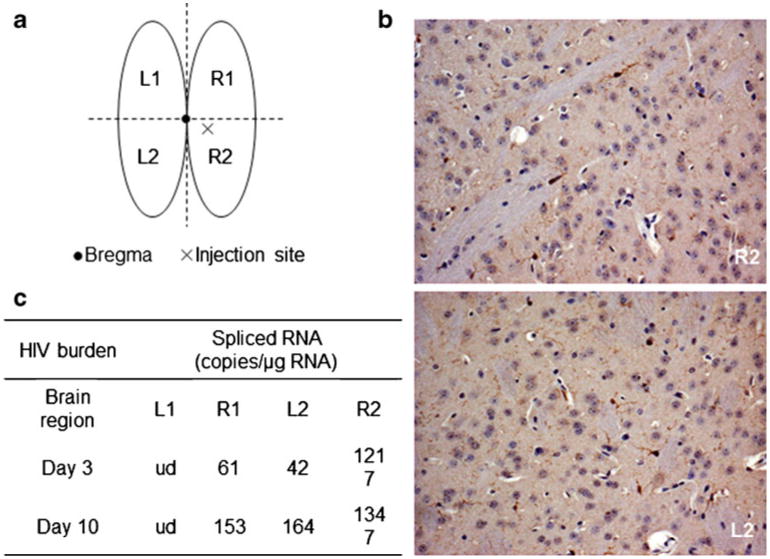
IC inoculation of Eco-HIV into mouse brain. a Schematic view of site of injection and dissected brain regions tested for HIV expression; b ICC for Iba-1 in the right basal ganglia region (R2) beneath and close to the injection site 3 days after IC inoculation of 10 μl of saline; note minimal microglial activation and absence of pathological damage. For comparison, ICC staining for Iba-1 in an analogous basal ganglia region from left (uninjected; L2) hemisphere from the same mouse. 10× magnification. c HIV expression proximal and distal to the injection site 3 days after IC inoculation with EcoHIV
For testing the superinfection resistance paradigm in the brain, four 129×1 mice each were either inoculated with EcoHIV or saline by IP injection; six weeks later the animals were challenged with IC inoculated virus as described above and all mice were euthanized three days later. Brain infection was tested in the right hemisphere by measurement of both viral DNA and spliced RNA (Fig. 3). To measure only newly synthesized DNA and not any potential DNA contamination in the virus stocks themselves, we amplified 2-LTR circular DNA because this genomic form is not produced after transfection. As expected (Fig. 2), IC injection of EcoHIV into control mice established a highly productive infection in the brain. In contrast, mice infected six weeks earlier by IP injection largely resisted the superinfection by EcoHIV in the brain (Fig. 3). This observation indicates that the effector mechanism induced by peripheral EcoHIV infection crosses the blood-brain barrier to control virus replication in the brain. To investigate the kinetics of establishment of protective immunity to EcoHIV infection in the brain, mice were infected by IP inoculation and then superinfected by IC inoculation either three days or three weeks later; positive controls received the IC inoculation only (Fig. 4). The ability to resist superinfection was not established three days after the initial EcoHIV exposure, however after three weeks EcoHIV-infected mice mounted responses that largely protected against superinfection in the brain; these kinetics are consistent with induction of an adaptive immune response.
Fig. 3.
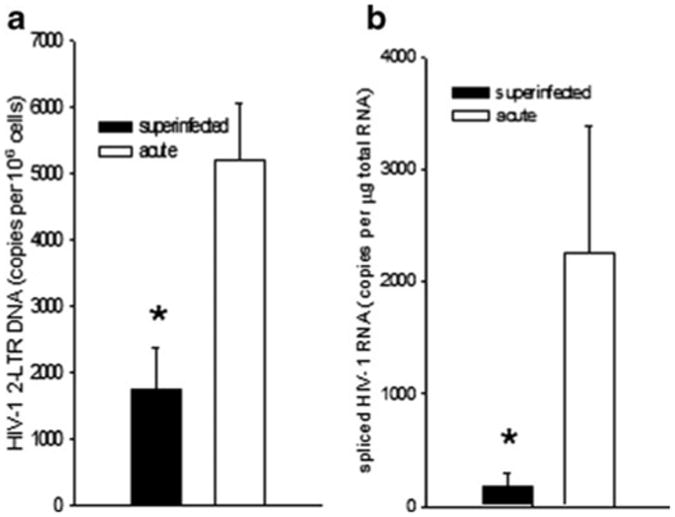
EcoHIV-infected mice resist superinfection in the brain. 129×1 mice were infected by IC injection of EcoHIV (acute) or were infected by IP injection of EcoHIV for six weeks prior to superinfection by IC injection (superinfected). Euthanasia was conducted one week after IC infection and brains were collected for measurement of virus burden by QPCR a 2-LTR circular HIV DNA, b spliced HIV RNA; * p<0.05
Fig. 4.
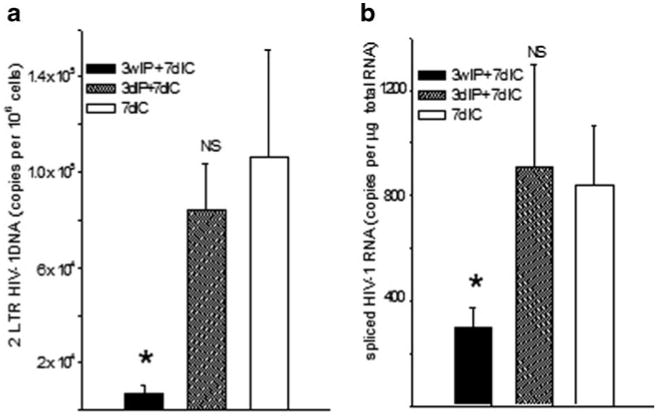
Kinetics of establishment of superinfection resistance in the brain. As indicated, 129×1 mice were infected by IC injection or by IP injection of EcoHIV for 3 days or 3 weeks prior to superinfection by IC injection. Euthanasia, tissue collection, and measurement of brain virus burden were conducted as described in the legend to Fig. 3
Our findings suggest that EcoHIV infection is sufficient to induce an adaptive anti-EcoHIV immune response capable of controlling virus replication. One major effector of antiviral immunity is the CD8+ T lymphocyte. We exploited the ability to transfer cells between inbred mice to assay cell function in control of EcoHIV infection. To determine whether EcoHIV infection induces protective antiviral CD8+ responses, we isolated CD8+ cells from spleens of EcoHIV infected or uninfected mice and transferred them to autologous mice that were subsequently infected by EcoHIV (Fig. 5). The cell recipients were not irradiated for cell transfer since it might influence HIV susceptibility. Recipients of CD8+ cells from EcoHIV-infected donors had significantly lower virus burdens than recipients of cells from uninfected donors. This observation strongly suggests that during EcoHIV infection, mice mount adaptive immune responses through CD8+ T cells that prevent subsequent infection by the same virus.
Fig. 5.
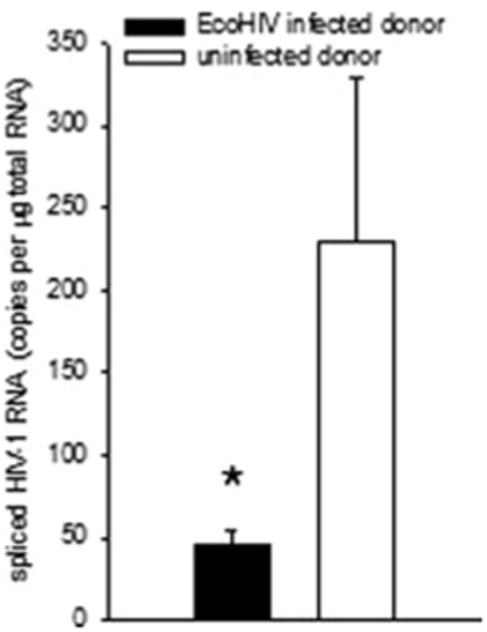
CD8+ cells from EcoHIV-infected mice confer protection against EcoHIV infection. BALB/c mice were left uninfected or were infected for six weeks prior to euthanasia, collection of spleen cells, isolation of CD8+ spleen cells, and transfer to unirradiated BALB/c mice. Cell recipients were infected by IP injection of EcoHIV the day after cell transfer. One week after infection, mice were euthanized, and spliced HIV RNA burden was measured in spleen cells. * p<0.05
To begin to identify protective EcoHIV determinants recognized by antiviral CD8+ T cells during EcoHIV infection, mice were infected for four weeks or left uninfected prior to euthanasia and tissue collection. Spleen cells were tested for activation to IFN-γ production by exposure to Clade D HIV Gag peptide pools in culture; IFN-γ expression in CD8+ cells was determined by flow cytometry (Fig. 6). CD8+ cells from infected mice responded to peptides from HIV Gag more extensively than did cells from uninfected mice. Taken together our results indicate that EcoHIV infection of mice provides a platform to investigate the induction of protective immunity to HIV infection.
Fig. 6.
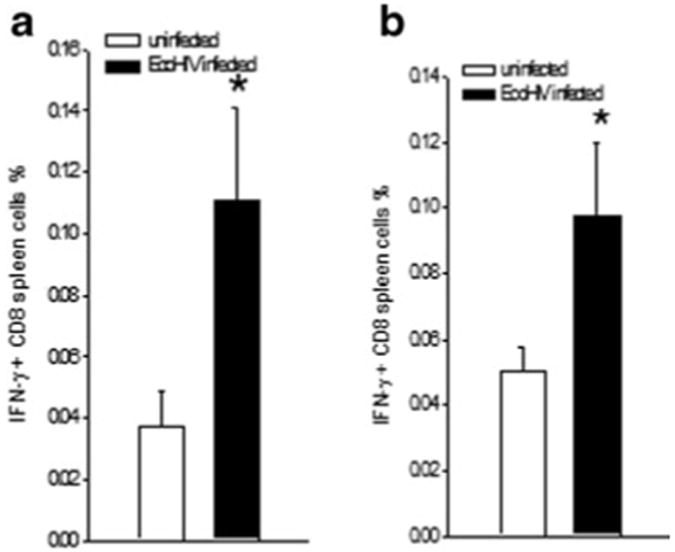
CD8+ cells from EcoHIV-infected mice respond to HIV Gag peptides. Four weeks after EcoHIV infection or PBS injection, BALB/ c mice were euthanized, spleen cells collected, and restimulated with peptides prior to assay of intracellular cytokine expression as described in Materials and Methods. Data shown are% IFN-γ positive CD8+ cells stimulated by two HIV subtype D peptide pools in infected and uninfected mice. * p<0.05
Discussion
Our results demonstrate that EcoHIV-infected mice mount immune responses that protect against subsequent infection in both peripheral tissues and the brain and that the response is mediated, at least in part, by CD8+ lymphocytes. These immune responses may also play a role in the control of EcoHIV expression observed in the decline of virus burden from an early peak as shown in the difference in virus burden in acute and chronically-infected mice (Fig. 1).
Resistance to reinfection is commonly induced during natural infection by viruses like measles and rubella (Hilleman 1992). Indeed, this naturally induced immunity is considered an important predictor of the success of vaccination against virus infection. HIV-specific CD8+ T cell responses are also induced within weeks of infection of human beings and are believed to be essential for the sharp decline in viremia observed then and HIV-infected persons generally mount antiviral antibody responses throughout infection (Pantaleo et al. 1997). Effective immunity can be inferred by the long course of HIV infection in untreated individuals and the selection for variant viral epitopes not recognized by existing responses (Gulzar and Copeland 2004). HIV-induced immune dysfunction ultimately undermines these potentially protective responses.
Experimental models of HIV infection have provided insight into the roles of immunity in the progress or control of disease. Like human beings, chimpanzees are susceptible to superinfection by group M isolates of HIV but they do not succumb to disease in either primary infection or superinfection (Fultz et al. 1987). In contrast, SIV infection of macaques has been extremely informative on viral pathogenesis and immunity. CD8+ T lymphocyte depletion using anti-CD8 antibodies accelerated virus replication in this model, an important indication of the effective antiviral immune responses prior to the onset of immunodeficiency (Schmitz et al. 1999). Moreover, macaques can be immunized with attenuated SIV to resist challenge by pathogenic SIV and antiviral CD8+ cells are concomitantly induced (Genesca et al. 2009). The functionality of such cells was shown by the transient viremia observed when CD8+ cells are depleted after virus challenge (Tsukamoto et al. 2007). More recently, CD8+ depletion was also shown to exacerbate immunopathogenesis in profoundly immuno-deficient mice reconstituted with human CD34+ stem cells prior to HIV infection (Gorantla et al. 2010).
Our studies in conventional mice take advantage of their versatility as experimental animals. When administered to mice peripherally, EcoHIV is not uniformly detected in the brain within weeks of infection (Potash et al. 2005), complicating the study of neuropathogenesis. Solving a similar problem in SIV neuropathogenesis in macaques, two approaches were used to impair immune responses. Williams and colleagues deplete CD8+ cells using anti-CD8 antibody and observe enhanced SIV disease in the brain (Williams et al. 2001). Clements and colleagues achieve brain disease in macaques by infection with mixtures of two SIV strains: one highly immunopathogenic to disable adaptive immunity and the second neurovirulent to reliably enter and replicate in the brain (Clements et al. 2008). In mice another approach is often used, intracranial injection of virus for direct investigation of replication in the brain. Here IC inoculation of EcoHIV revealed robust virus replication in brain cells, the identity of the cells infected is under investigation (He and Volsky, in preparation). We also observed efficient control of this infection through induction of superinfection resistance weeks but not days after peripheral infection. Based on their presumed roles in HIV infection (Pantaleo et al. 1997) and their demonstrated salience in the control of SIV infection in the brain (Williams et al. 2001), we speculated that antiviral CD8+ cells may mediate protection to EcoHIV-infected mice against superinfection.
A simple approach available in inbred animals is the direct assay of function of a cell population by transfer into an isogenic recipient. Using adoptive transfer, we showed that CD8+ Tcells from spleens of EcoHIV-infected mice mediated protection against EcoHIV infection in naive recipients. Preliminary study of the determinants involved indicated that CD8+ cells from EcoHIV-infected mice respond to two pools of Gag peptides, potentially the immunodominant epitopes found in Gag Pool 1, AMQMLKETI, and Gag Pool 2, NPPIPVGEI, respectively in studies of HIV DNA vaccination to protect against EcoHIV infection (Im et al. 2011). By adoptive transfer, we reach a conclusion similar to that of Tsukamoto in the SIV system without the need to create immune deficiency (Tsukamoto et al. 2007).
Our results show that adaptive antiviral responses naturally induced by HIV replication in mice protect against further infection and may contribute to the control of infection observed after its initial peak. Some elite controllers, HIV-infected individuals who remain immune competent with only trace levels of virus, also carry highly effective anti-HIV CD8+ T cells (Saez-Cirion et al. 2007). Antiviral CD8+ cells also have been detected in HIV exposed but uninfected sex workers and may account for their ability to resist infection (Kaul et al. 2000). The results reported here indicate that upon priming mice naturally mount protective responses against EcoHIV infection similar to responses of some human beings. Furthermore, these responses appear to include significant protection against HIV expansion in brain tissue. These observations suggest that EcoHIV-infected mice serve as a useful system to investigate the induction of antiviral immunity for eventual translation to protect human beings against HIV infection.
Acknowledgments
The authors would like to thank Ms. Ilene M. Totillo for manuscript preparation.
Work in this manuscript was supported by grants AI 079792, DA 017618, MH 083627, and NS 061646-01A1 from the National Institutes of Health, Public Health Service.
Contributor Information
Jennifer L. Kelschenbach, Email: jlk2152@columbia.edu, Molecular Virology Division, St. Luke's-Roosevelt Hospital Center, 432 West 58th Street, Antenucci Research Building, Room 709, New York, NY 10019, USA; Department of Pathology & Cell Biology, Columbia University, New York, NY 10032, USA.
Manisha Saini, Email: manisaini@gmail.com, Molecular Virology Division, St. Luke's-Roosevelt Hospital Center, 432 West 58th Street, Antenucci Research Building, Room 709, New York, NY 10019, USA, Department of Pathology & Cell Biology, Columbia University, New York, NY 10032, USA.
Eran Hadas, Email: eran.hadas@gmail.com, Molecular Virology Division, St. Luke's-Roosevelt Hospital, Center, 432 West 58th Street, Antenucci Research Building, Room 709, New York, NY 10019, USA.
Chao-jiang Gu, Email: cg2447@columbia.edu, Molecular Virology Division, St. Luke's-Roosevelt Hospital Center, 432 West 58th Street, Antenucci Research Building, Room 709, New York, NY 10019, USA; Department of Pathology & Cell Biology, Columbia University, New York, NY 10032, USA.
Wei Chao, Email: wei616@verizon.net, Molecular Virology Division, St. Luke's-Roosevelt Hospital Center, 432 West 58th Street, Antenucci Research Building, Room 709, New York, NY 10019, USA.
Galina Bentsman, Email: galina_bentsman@yahoo.com, Molecular Virology Division, St. Luke's-Roosevelt Hospital Center, 432 West 58th Street, Antenucci Research Building, Room 709, New York, NY 10019, USA.
Jessie P. Hong, Email: jessie.hong@gmail.com, Weatherall Institute of Molecular Medicine, University of Oxford, The John Radcliffe, Oxford OX3 9DS, UK.
Tomas Hanke, Email: tomas.hanke@molecular-medicine.oxford.ac.uk, Weatherall Institute of Molecular Medicine, University of Oxford, The John Radcliffe, Oxford OX3 9DS, UK.
Leroy R. Sharer, Email: sharer@umdnj.edu, Department of Pathology, New Jersey Medical School, Newark, NJ 07103, USA.
Mary Jane Potash, Molecular Virology Division, St. Luke's-Roosevelt Hospital, Center, 432 West 58th Street, Antenucci Research Building, Room 709, New York, NY 10019, USA; Department of Pathology & Cell Biology, Columbia University, New York, NY 10032, USA.
David J. Volsky, Email: djv4@columbia.edu, Molecular Virology Division, St. Luke's-Roosevelt Hospital Center, 432 West 58th Street, Antenucci Research Building, Room 709, New York, NY 10019, USA; Department of Pathology & Cell Biology, Columbia University, New York, NY 10032, USA.
References
- Chohan B, Piantadosi A, Overbaugh J. HIV-1 superinfection and its implications for vaccine design. Curr HIV Res. 2010;8:596–601. doi: 10.2174/157016210794088218. [DOI] [PubMed] [Google Scholar]
- Clements JE, Mankowski JL, Gama L, Zink MC. The accelerated simian immunodeficiency virus macaque model of human immunodeficiency virus-associated neurological disease: from mechanism to treatment. J Neurovirol. 2008;14:309–317. doi: 10.1080/13550280802132832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz PN, Srinivasan A, Greene CR, Butler D, Swenson RB, McClure HM. Superinfection of a chimpanzee with a second strain of human immunodeficiency virus. J Virol. 1987;61:4026–4029. doi: 10.1128/jvi.61.12.4026-4029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genesca M, McChesney MB, Miller CJ. Antiviral CD8+ T cells in the genital tract control viral replication and delay progression to AIDS after vaginal SIV challenge in rhesus macaques immunized with virulence attenuated SHIV 89.6. J Intern Med. 2009;265:67–77. doi: 10.1111/j.1365-2796.2008.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorantla S, Makarov E, Finke-Dwyer J, Gebhart CL, Domm W, Dewhurst S, Gendelman HE, Poluektova LY. CD8+ Cell depletion accelerates HIV-1 immunopathology in humanized mice. J Immunol. 2010;184:7082–7091. doi: 10.4049/jimmunol.1000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulzar N, Copeland K. CD8+ T cells: function and response to HIV infection. Curr HIV Res. 2004;2:23–37. doi: 10.2174/1570162043485077. [DOI] [PubMed] [Google Scholar]
- Hadas E, Borjabad A, Chao W, Saini M, Ichiyama K, Potash MJ, Volsky DJ. Testing antiretroviral drug efficacy in conventional mice infected with chimeric HIV-1. AIDS. 2007;21:905–909. doi: 10.1097/QAD.0b013e3281574549. [DOI] [PubMed] [Google Scholar]
- Hilleman MR. The dilemma of AIDS vaccine and therapy. Possible clues from comparative pathogenesis with measles. AIDS Res Hum Retrovir. 1992;8:1743–1747. doi: 10.1089/aid.1992.8.1743. [DOI] [PubMed] [Google Scholar]
- Im EJ, Hong JP, Roshorm Y, Bridgeman A, Létourneau S, Liljeström P, Potash MJ, Volsky DJ, McMichael AJ, Hanke T. Protective efficacy of serially up-ranked subdominant CD8 T cell epitopes against virus challenges. PLoS Pathog. 2011;7:e1002041. doi: 10.1371/journal.ppat.1002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul R, Plummer FA, Kimani J, Dong T, Kiama P, Rostron T, Njagi E, MacDonald KS, Bwayo JJ, McMichael AJ, Rowland-Jones SL. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J Immunol. 2000;164:1602–1611. doi: 10.4049/jimmunol.164.3.1602. [DOI] [PubMed] [Google Scholar]
- Kearney M, Maldarelli F, Shao W, Margolick JB, Daar ES, Mellors JW, Rao V, Coffin JM, Palmer S. Human immunodeficiency virus type 1 population genetics and adaptation in newly infected individuals. J Virol. 2009;83:2715–2727. doi: 10.1128/JVI.01960-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G, Graziosi C, Fauci AS. Virologic and immunologic events in primary HIV infection. Springer Semin Immunopathol. 1997;18:257–266. doi: 10.1007/BF00813497. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Limoges J, McComb R, Bock P, Baldwin T, Tyor W, Patil A, Nottet HS, Epstein L, Gelbard H, Flanagan E, Reinhard J, Pirruccello SJ, Gendelman HE. Human immunodeficiency virus encephalitis in SCID mice. Am J Pathol. 1996;149:1027–1053. [PMC free article] [PubMed] [Google Scholar]
- Potash MJ, Chao W, Bentsman G, Paris N, Saini M, Nitkiewicz J, Belem P, Sharer L, Brooks AI, Volsky DJ. A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proc Natl Acad Sci USA. 2005;102:3760–3765. doi: 10.1073/pnas.0500649102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshorm Y, Hong JP, Kobayashi N, McMichael AJ, Volsky DJ, Potash MJ, Takiguchi M, Hanke T. Novel HIV-1 clade B candidate vaccines designed for HLA-B(*)5101(+) patients protected mice against chimaeric ecotropic HIV-1 challenge. Eur J Immunol. 2009;39:1831–1840. doi: 10.1002/eji.200939309. [DOI] [PubMed] [Google Scholar]
- Saez-Cirion A, Lacabaratz C, Lambotte O, Versmisse P, Urrutia A, Boufassa F, Barre-Sinoussi F, Delfraissy JF, Sinet M, Pancino G, Venet A. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci. 2007;104:6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini M, Hadas E, Volsky DJ, Potash MJ. Vaccine-induced protection from infection of mice by chimeric human immunodeficiency virus type 1, EcoHIV/NL4-3. Vaccine. 2007;25:8660–8663. doi: 10.1016/j.vaccine.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, Ghrayeb J, Forman MA, Montefiori DC, Rieber EP, Letvin NL, Reimann KA. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- Tsukamoto T, Yuasa M, Yamamoto H, Kawada M, Takeda A, Igarashi H, Matano T. Induction of CD8+ cells able to suppress CCR5-tropic simian immunodeficiency virus SIVmac239 replication by controlled infection of CXCR4-tropic simian-human immunodeficiency virus in vaccinated rhesus macaques. J Virol. 2007;81:11640–11649. doi: 10.1128/JVI.01475-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193:905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]


