Abstract
Prior studies suggest that autism spectrum disorders (ASD) are associated with a domain-specific memory impairment for faces. The underlying cause of this problem and its relation to impaired visual scanning of faces—particularly of the eyes—remains to be determined. We recorded eye movements while 22 high-functioning ASD and 21 typically developing (TD) adolescents encoded and later recognized faces and objects from a single, nonsocial object category (electric fans). Relative to TD subjects, ASD individuals had poorer memory for faces, but not fans. Correlational analyses showed significant relationships between recognition memory and fixations. Eye tracking during encoding revealed that TD subjects made more fixations to faces than fans, whereas ASD individuals did not differ in number of fixations made to each stimulus type. Moreover, although both the TD and ASD groups showed a strong preference for fixating the eyes more than the mouth, the ASD subjects were less likely than TD subjects to scan regions of the face outside of the primary facial features (i.e., eyes, nose, and mouth). We concluded that ASD individuals have a domain-specific memory impairment for faces relative to mechanical objects and that this impairment may be related to abnormal scanning during encoding.
Keywords: Autistic disorder, Asperger’s syndrome, Face processing, Memory, Eye tracking, Domain-specificity
INTRODUCTION
Numerous studies of autism spectrum disorders (ASD) have focused on evaluating face processing and memory abilities to better understand their social deficits. These studies have commonly found that ASD individuals have a domain-specific memory impairment defined by poor recognition of faces relative to other object categories (Blair, Frith, Smith, Abell, & Cipolotti, 2002; Boucher & Lewis, 1992; Hauck, Fein, Maltby, Waterhouse, & Feinstein, 1998; Williams, Goldstein, & Minshew, 2005). The underlying cause of this domain-specific memory deficit remains to be determined.
One possibility is that ASD subjects fail to adequately encode faces during learning due to an aversion to looking at the eyes of others. Indeed, poor eye contact in social interactions, such as during a clinical interview, is a key diagnostic criterion for ASD (APA, 2000). Experimental support for this claim comes from studies that have evaluated looking preferences while ASD subjects view faces and other objects (for a detailed review of eye-tracking studies in ASD, see Boraston & Blakemore, 2007; Dawson et al., 2002; Grelotti, Gauthier, & Schultz, 2002). For example, Osterling and Dawson (1994) analyzed first birthday party videotapes and found that children with autism were less likely than typically developing (TD) children to look at the face of another individual. Recent studies have recorded eye movements under more controlled conditions to provide direct evidence for reduced attention to the eye region, and typically greater attention to the mouth region, in ASD (e.g., Klin, Jones, Schultz, Volkmar, & Cohen, 2002; Pelphrey, Sasson, Reznick, Paul, & Goldman, 2002; Rutherford, Clements, & Sekuler, 2007; Spezio, Adolphs, Hurley, & Piven, 2006).
Nevertheless, these findings have not gone unchallenged. Bar-Haim, Shulman, Lamy, and Reuveni (2006) reported that ASD and TD individuals did not differ in their attention to the eyes versus the mouth. Similarly, Anderson, Colombo, and Shaddy (2006) failed to find a difference between young ASD and TD individuals in duration of fixations to internal (eye, nose, and mouth) as opposed to external (e.g., hair and chin) features, while Rutherford and Towns (2008) did not find a difference in scan time to eyes and mouth based on diagnosis (although there was an emotion-complexity by diagnosis interaction). Sterling et al. (2008) similarly reported that both ASD and TD individuals made more and longer fixations on the eyes than the mouth, although TD subjects spent a greater percentage of time than the ASD individuals looking at the eyes. In addition, Speer, Cook, McMahon, and Clark (2007) reported that, whereas fixation duration in the eye region differed between groups for social-dynamic movie stimuli, no differences were found for static faces. Finally, both TD and ASD individuals were found to be faster and more accurate at detecting eye-gaze changes than control changes during a change-blindness task (Fletcher-Watson, Leekam, Findlay, & Stanton, 2008), and ASD individuals did not differ from TD subjects with regard to the total number of fixations to people relative to other types of objects (Fletcher-Watson, Leekam, Benson, Frank, & Findlay, 2009).
Another, and perhaps related possibility is that face processing difficulties and subsequent poor face memory in ASD are attributable to more general attentional impairments. ASD has been linked with attentional abnormalities, including overfocus or a narrowed attentional spotlight (Mann & Walker, 2003). These idiosyncrasies may play a critical role in face processing deficits in ASD individuals. Atypically focused attention could result in abnormally long fixations on the central features of a face (i.e., eye, nose, and mouth) and/or diminished scanning of other facial features.
Based on these findings, we predicted that (1) high-functioning adolescents with ASD will have a domain specific memory impairment defined by poorer recognition memory for faces, relative to memory for a single category of mechanical objects (electric fans), and worse memory for faces, but not for electric fans, relative to TD subjects; (2) TD, but not ASD, individuals will have a greater number of and longer fixations to faces than fans; (3) TD, but not ASD, individuals will have more fixations to the eyes than other parts of the face; and (4) ASD subjects will show evidence of a narrowed attentional spotlight (e.g., more fixations and longer fixation duration to central face features, and fewer and shorter fixations to other facial features) relative to TD individuals.
METHOD
Subjects
Thirty subjects with ASD and 22 TD controls participated in the study. Subjects with ASD were recruited primarily from a hospital clinic specializing in the diagnosis and treatment of these disorders, while TD controls were recruited from the community. ASD diagnoses were given based on clinical impression using the DSM-IV (APA, 2000) as well as the ADI/ADI-R (Autism Diagnostic Interview) (Le Couteur et al., 1989; Lord, Rutter, & Le Couteur, 1994) and/or the ADOS (Autism Diagnostic Observation Schedule) (Lord, Rutter, DiLavore, & Risi, 1999). Because the ADI and ADOS do not provide a diagnostic algorithm for Asperger’s syndrome, we used criteria developed by the NICHD/NIDCD Collaborative Programs for Excellence in Autism (see Lainhart et al., 2006) to define “broad ASD,” if subjects: (1) meet the ADI cutoff for autism in the social domain and at least one other domain or (2) meet the ADOS cutoff for the combined social and communication score. Written consent from parents (and participants when they were 18 years or older) and verbal and written assent from participants under age 18 were obtained in accordance with an institutional review board-approved National Institutes of Health (NIH) protocol. Exclusion criteria for the ASD group included any known co-morbid medical conditions, genetic disorder (e.g., fragile X syndrome), or neurological disorder that may affect cognitive functioning. TD subjects were excluded from participation if they had ever received mental health treatment for anxiety, depression, or any other psychiatric condition, taken psychiatric medications, required special services in school, had a first-degree relative with an ASD diagnosis, or had trauma/injury that could potentially affect cognitive functioning and/or brain development. All subjects in both groups had a Full Scale IQ (FSIQ) above 80, as measured by the Wechsler Abbreviated Scale of Intelligence, Wechsler Adult Intelligence Scale-III, Wechsler Intelligence Scale for Children-III, or Wechsler Intelligence Scale for Children-IV.
Eight of the 30 ASD subjects were excluded (three because of equipment failure; two because of poor calibration, excessive movement, or somnolence; and three because of poor comprehension of task directions). The ASD subjects who were excluded were similar to the included ASD subjects in terms of age, IQ, and ASD symptoms. One TD control subject was excluded because of poor comprehension of task directions. A final sample of 21 TD adolescents (17 males, 2 left-handed) between 13 and 20 years of age and 22 high-functioning adolescents with ASD (21 males, 2 left-handed) between 12 and 23 years of age were included in the analyses. Of the ASD subjects, four were diagnosed with high-functioning autism, 14 with Asperger’s syndrome, and four with Pervasive Developmental Disorder—Not Otherwise Specified. Subjects were group-matched on age and IQ scores (Table 1). All subjects had normal or corrected vision.
Table 1.
Characteristics of ASD and TD groups
| ASD (n = 22)
|
TD (n = 21)
|
|||
|---|---|---|---|---|
| M | (SD) | M | (SD) | |
| Age | 15.96 | (2.44) | 16.81 | (1.90) |
| IQ | 111.50 | (17.57) | 110.33 | (10.06) |
| ADI-Social | 19.90 | (4.38) | – | – |
| ADI-Verbal Comm. | 14.63 | (4.48) | – | – |
| ADI-SRIB | 6.14 | (3.01) | – | – |
| ADOS-Social | 8.27 | (3.01) | – | – |
| ADOS-Comm. | 4.27 | (1.78) | – | – |
| ADOS-SB | 1.68 | (1.84) | – | – |
Note. ASD = autism spectrum disorder; TD = typically developing; ADI = Autism Diagnostic Interview; ADOS = Autism Diagnostic Observation Schedule; Comm. = Communication; SRIB = Stereotyped Repetitive Interests and Behaviors; SB = Stereotyped Behavior.
Data Acquisition
Eye gaze position data were collected during encoding trials of a face recognition task. A video-based eye-tracking system with remote pan tilt optics (Model 504, Applied Science Laboratories [ASL], Bedford, MA) was used. This system uses bright pupil technology to acquire horizontal and vertical coordinates of eye position. The eye tracking application removed blinks and smoothed the data thus reducing artifacts.
Subjects were seated in front of an LCD display. The display height was adjusted for each subject so that the center of the display was level with the subject’s eyes. The viewing distance to the screen was set at 57 cm and a chinrest minimized head movement. At this viewing distance, 1 cm on the display was equivalent to 1° of visual angle. The accuracy of the eye tracker was better than 0.5°, which is typical of video-based systems. The eye-tracking camera was centered and its height adjusted so that it was just below the stimulus display. The room lighting directly over the subject was dimmed to reduce glare and produce a measurable pupil size.
Vertical and horizontal pupil positions were recorded at the camera’s frame rate of 60 Hz. Before beginning the trials, a calibration run was performed using an evenly distributed 9-point stimulus grid. The calibration established individual subject gaze maps, which joined the known display positions to the location of the subject’s gaze. Calibration verification was performed by asking the subject to rescan the 9-point grid. The ASL software determined gaze position by computing distances between the corneal reflection and the pupil center. To smooth the recorded data, each eye position value was averaged with its three preceding values.
Face-Fan Memory Task
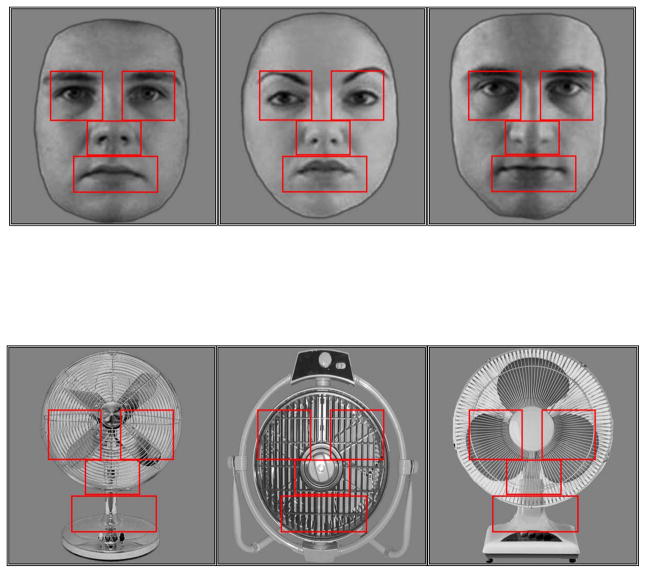
Before starting the task, subjects were instructed that they would be shown pictures and that they should study them for a later memory test. Stimuli were blocked by category (face, fan). Electric fans were chosen because of ASD individuals’ well-documented fascination with mechanical objects with rapidly moving parts (American Psychiatric Association, 2000). Moreover, like faces, they constitute a single, basic level category (Rosch, 1978), are round, and have constituent parts (propeller, grating, etc.) (Figure 1).
Fig. 1.
Examples of faces and fans used in the experiment. Red lines indicate the location of the AOIs used for analyses of the eye tracking data. These red lines were not present during either encoding or recognition phases of the experiment.
In addition, fans were chosen as the comparison stimuli to provide a more stringent test of the domain-specificity hypothesis than typically encountered in the literature. A strong version of the domain-specific argument would hold if, and only if, the ASD subjects had worse memory for faces than fans, and relative to TD controls, the ASD subjects had impaired memory for faces, while showing equivalent—neither greater nor worse—memory performance for fans. If, for example, the ASD subjects showed a greater memory impairment for faces than fans, but also had impaired memory, relative to TD controls, for the electric fans, it would argue against strong claims of domain-specificity.
Luminance levels were adjusted to make all pictures equiluminant. The face stimuli were all front views with a neutral expression and were carefully edited to remove non-facial features (hair, neck, ears) (Figure 1). The face stimuli were from the Karolinska Directed Emotional Faces set (Lundqvist, Flykt, & Ohman, 1998), and from a set developed at the National Institute of Mental Health (NIMH) (Ishai, Pessoa, Bikle, & Ungerleider, 2004). The fan stimuli were obtained from the Internet and from a set developed at the NIMH for this study. Fan photographs were carefully edited to remove any extraneous marks that could be used to uniquely identify the stimuli. The faces subtended an average visual angle of 14.0° horizontal and 17.7° vertical at their respective widest point. The fans subtended an average visual angle of 13.7° horizontal and 18.9° vertical at their respective widest point.
Order of presentation of the category blocks was counterbalanced across subjects. Each block consisted of an encoding phase followed by a recognition phase. During each encoding phase subjects viewed six grayscale faces, or fans, one at a time for 2500 ms, preceded by a centrally located fixation cross displayed for 1000 ms. A total of five encoding blocks were presented for each stimulus type. Thus, over the course of the experiment subjects viewed and attempted to learn 30 faces and 30 fans. Each encoding phase was followed by a recognition phase. During the recognition phase, subjects viewed 12 pictures individually, of which six had been presented during encoding (targets). Subjects were instructed to indicate by button press whether or not they had previously seen the stimuli during the encoding phase. The subjects’ button presses determined the display duration of recognition items. Each recognition item was preceded by a 1000 ms fixation cross. Thus, during the retrieval phases, subjects viewed 60 faces and 60 fans, half of which were targets.
Data Analysis
Using ASL’s EYENAL analysis application, fixations were located according to the following algorithm: the start of a fixation occurs when six consecutive samples (100 ms) have a deviation of no more than 0.5° visual angle from the first sample; a fixation ends when three consecutive samples are farther than 1° from the initial fixation position, with any isolated gaze coordinates that are farther than 1.5° from the initial fixation not included in the calculation of average gaze position for that fixation.
During data analysis, a fixed set of individual areas of interest (AOI) maps were overlaid on the stimuli (face and fan) corresponding to the location of each of the eyes, the nose, and the mouth (see Figure 1). The face AOIs were used for analysis of the fan stimuli to control for generally biased looking. Placing the AOIs in the identical position on the faces and fans allows us to determine whether, for example, the ASD subjects looked less at the eyes, versus whether they simply had a tendency to focus their attention below the centrally located fixation cross, irrespective of stimulus type. For each of the face/fan stimuli the number and duration of fixations occurring within each of the four AOIs as well as the total for the entire screen were calculated. The number of fixations for each subject was determined by calculating the average number of fixations across trials. Similarly, fixation duration for each subject was defined as the average amount of time fixating across trials.
An eyeblink was identified when the eye tracker recorded a pupil diameter that equaled zero for up to a maximum of 12 samples (200 ms), during which time there is no position or pupil data available. Therefore, for the fixation analyses, 200 ms is the maximum blink duration. The responses during the recognition phase were coded as a “hit” if the subject responded to a previously presented stimulus (target) and as a “false alarm” if the subject responded to a stimulus that was not previously presented (distracter). D prime (d′) was used to determine how well each subject discriminated between targets and distracters. We calculated the value of d′ by subtracting the normalized false alarm rate (using the inverse of the cumulative density function of the standard normal distribution) from the normalized hit rate [d′ = Z(hit rate)-Z(false alarm rate)]. In the event that an individual had no misses or false alarms, the hit rate or false alarm rate was substituted with a value of 0.983 or 0.016, respectively, corresponding to 29.5/30 and 0.5/30 (with 30 being the number of targets and distracters administered to each subject).
Statistical analyses of results primarily involved mixed (both between and within subjects factors) ANOVAs. Interaction effects were followed up with pairwise comparisons (one-way ANOVAs). Pearson product-moment coefficients were used to determine the correlation of eye movement data with memory performance.
RESULTS
Face-Fan Memory
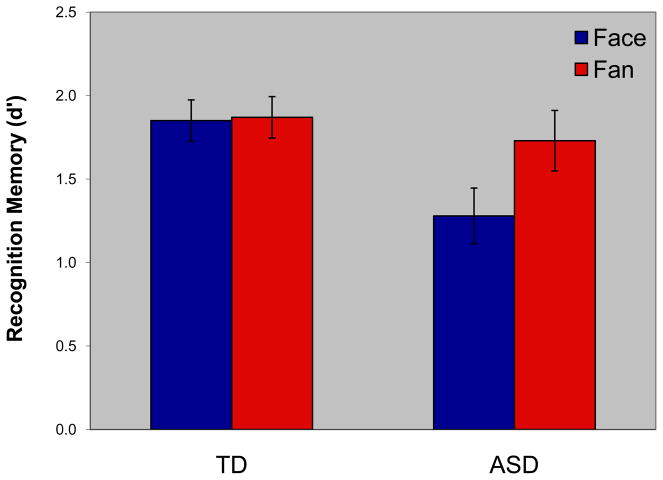
A Stimulus by Diagnosis mixed-model ANOVA on recognition memory accuracy (d′) yielded a main effect of Stimulus, with poorer memory for faces (d′ = 1.56) than fans (d′ = 1.80) (F(1,41) = 4.98; p < .05; partial η2 = .108). The memory disadvantage for faces relative to fans, however, was due entirely to the ASD subjects (Stimulus by Diagnosis interaction (F(1,41) = 4.19; p < .05; partial η2 = .093; see Table 2 and Figure 2). For the ASD group, memory for faces was poorer than fans (fans, mean d′ = 1.73; SD = 0.85; faces, mean d′ = 1.28; SD = 0.78) (p < .01; partial η2 = .186), whereas the TD group showed equivalent memory for the two stimulus types (faces, mean d′ = 1.85; SD = 0.57; fans, mean d′ = 1.87; SD = 0.57) (p > .90; partial η2 < .001; thereby confirming that we were successful in equating the memory tasks for difficulty). Moreover, relative to the TD group, the ASD subjects had worse memory for faces (p = .01; partial η2 = .150), but not for fans (p > .50; partial η2 = .009). Thus, ASD subjects had a domain-specific memory impairment for faces defined by poorer memory for faces than fans, and impaired face, but not fan, memory relative to the age and IQ matched control group.
Table 2.
Percent correct for recognition memory
| ASD (n = 22)
|
TD (n = 21)
|
|||
|---|---|---|---|---|
| M | (SD) | M | (SD) | |
| Percent correct | ||||
| Face | 71.59 | (10.62) | 80.24 | (6.71) |
| Fan | 77.58 | (10.92) | 80.87 | (7.00) |
Note. ASD = autism spectrum disorder; TD = typically developing
Fig. 2.
Recognition memory (d′) for faces and fans for the typically developing (TD) and autism spectrum disorders (ASD) groups.
Face-Fan Eye Movements During Encoding: Number of fixations
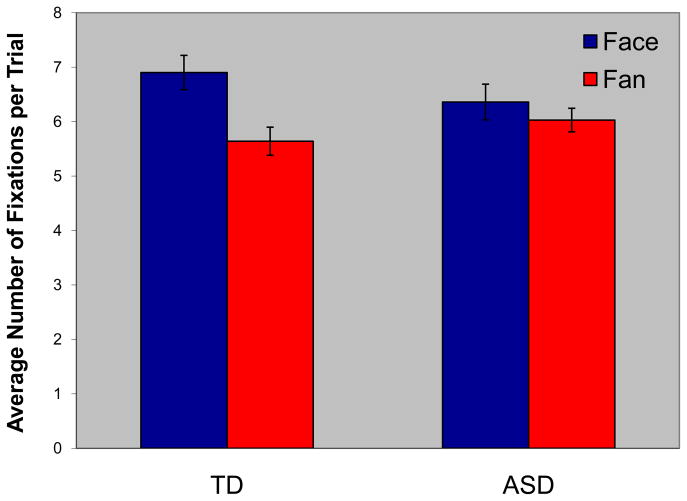
A three-way ANOVA of the number of fixations [Stimulus (face, fan), Recognition Accuracy (hit, miss), and Diagnosis (ASD, TD)] revealed main effects of Accuracy (F(1,40) = 18.28; p < .0001; partial η2 = .314; with more eye movements to stimuli later recognized, than to those that were not) and Stimulus (F(1,40) = 39.84; p < .0001; partial η2 = .499; more eye movements to faces than fans). Importantly, there also was a Stimulus by Diagnosis interaction (F(1,40) = 14.48; p < .0001; partial η2 = .266; see Figure 3). Pairwise comparisons revealed that TD individuals made significantly more fixations to faces than fans (p < .0001; partial η2 = .561, whereas ASD individuals did not (faces vs. fans; p = .084; partial η2 = .073). These findings cannot be attributed to differences in total gaze time as the average time for ASD subjects was 2427 ms and for TD subjects was 2398 ms, a nonsignificant difference (p > .23; partial η2 = .035). Group differences, however, were not observed for either faces (p = .192; partial η2 = .042) or fans (p = .185; partial η2 = .043), and neither the main effect of Diagnosis, nor the other interactions were significant. Correlation analyses revealed a significant relationship between d′ recognition memory scores for faces and the number of fixations during encoding of faces (r = .48; p < .05) and recognition memory scores for fans and the number of fixations during encoding fans (r = .47; p < .05) for the ASD subjects. For TD subjects there were no significant relationships between number of fixations and recognition memory (all ps > .05).
Fig. 3.
Number of fixations for faces and fans for the typically developing (TD) and autism spectrum disorders (ASD) groups.
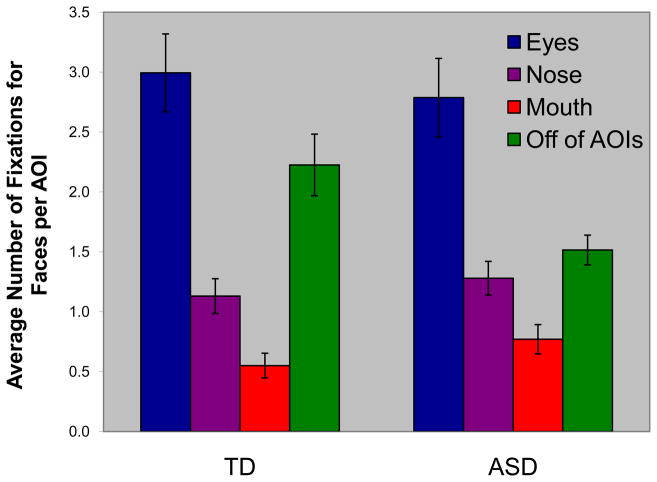
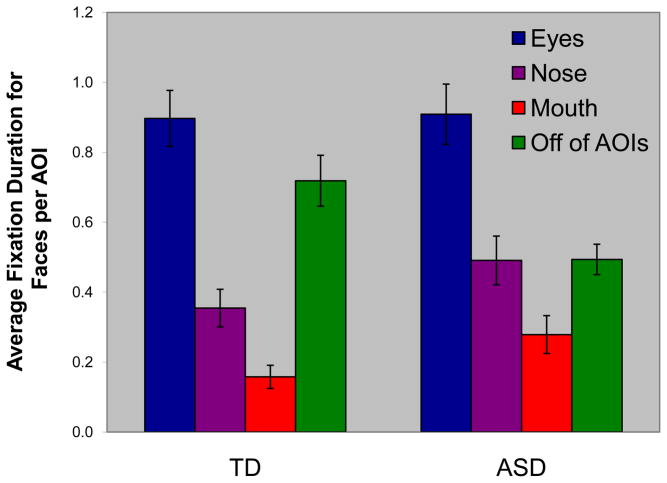
A three-way ANOVA (Diagnosis by Stimuli by AOI) of the total number of fixations did not produce a Diagnosis main or interaction effects (Fs < 1.0). There was, however, a significant Stimulus by AOI interaction effect, F(2,82) = 14.10; p < .001, partial η2 = .256 (characterized by a disproportionately greater number of fixations in the eye AOIs for the face stimuli). The significant Stimulus by AOI interaction supports the notion that differential rates of fixations in certain areas are not due to location on the screen, but rather the particular content (faces or fans) on the screen. There were also significant main effects of Stimulus (face> fan; F(1,41) = 113.45; p < .0001; partial η2 = .735) and AOI (eyes > nose > mouth; F(2,82) = 86.38; p < .0001; partial η2 = .678). An analysis of the number of fixations on just face stimuli as a function of gaze location revealed a main effect of AOI (eyes > nose > mouth; F(2,82) = 53.05; p < .001; partial η2 = .564; see Figure 4), but no main effect of Diagnosis (F(1,41) = 0.13; p > .05; partial η2 = .003) nor Diagnosis by AOI interaction (F(2,82) = 0.52; p > .05; partial η2 = .013). Thus, both TD and ASD subjects made more fixations to the eyes than to the other prominent facial features (see Figure 5 for examples of typical ASD and TD scanpaths to faces, as well as to fans). Given this somewhat surprising finding, we computed the percentage of individuals who had a larger number of face fixations for the eye than for the mouth region. The difference between TD and ASD individuals was significant (χ2(2, n = 43) = 4.21; p = .04; φ = .313). Specifically, 4 of 22 ASD (18%) subjects, but none of the TD subjects, had more fixations on the mouth than the eyes. These four ASD subjects did not differ from the other ASD subjects on measures of face and fan memory (ps > .30), or on measures of autism symptomatology (i.e., ADI and ADOS; all ps > .20). These subjects, however, did make fewer fixations to faces than did the other ASD subjects (Mann-Whitney U = 11.00; p < .05; r = .454).
Fig. 4.
Number of fixations by area of interest (AOI) for faces for the typically developing (TD) and autism spectrum disorders (ASD) groups.
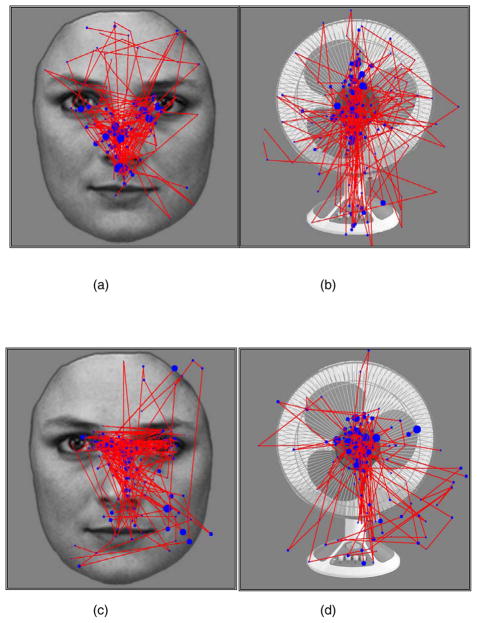
Fig. 5.
Examples of scanpaths to face and fan stimuli for one autism spectrum disorders (ASD) and one typically developing (TD) subject superimposed on a sample face and on a sample fan. (a) Scanpaths of a subject with ASD for all 30 face encode trials. (b) Scanpaths of the same subject with ASD for all 30 fan encode trials. (c) Scanpaths of a TD subject for all 30 face encode trials. (d) Scanpaths of the same TD subject for all 30 fan encode trials. The blue circles indicate the location of the fixations. Their relative size indicates their duration.
Finally, to determine if fixation patterns outside of the central facial features differed between groups, we evaluated the number of fixations that did not fall within the eye, nose, or mouth AOIs. A three-way ANOVA [Stimulus (face, fan) by Diagnosis (ASD, TD) by AOI (in, out)] for the number of fixations failed to reveal a main effect of Diagnosis (F(1,41) = 0.04; p = .84; partial η2 = .001), but there was a significant Diagnosis by AOI interaction (F(1,41) = 13.11; p < .01; partial η2 = .242). Inspection of the data for faces suggested that whereas TD and ASD individuals did not differ in the number of fixations within the AOIs, TD individuals produced more fixations outside the AOIs. This impression was confirmed by pairwise comparisons, which revealed that for faces, TD controls made more fixations outside the eye, nose, and mouth regions than the ASD subjects (F(1,41) = 6.37; p < .05; partial η2 = .135; see Figure 4). A pairwise comparison of TD to ASD individuals for number of fixations on the eye, nose, and mouth regions of faces was not significant (F(1,41) = 0.13; p > .05; partial η2 = .003).
Face-Fan Eye Movements During Encoding: Fixation Duration
Analysis of the total length of time that a subject fixated for a particular trial (i.e., fixation duration) failed to reveal significant main effects or interactions. In addition, an analysis of face fixation duration as a function of gaze location (Stimulus by AOI by Diagnosis) failed to reveal a Diagnosis by AOI interaction or three-way interaction effect (Fs < 1.0). However, the effect of AOI was significant (F(2,82) = 83.55; p < .001; partial η2 = .671) and the Stimulus by AOI interaction approached significance (F(2,82) = 2.92; p = .06; partial η2 = .066), suggesting that the duration of fixations were not simply due to their location, but rather varied as a function of stimulus type (face, fan). The Diagnosis by Stimulus interaction was also significant (F(1,41) = 4.69; p < .05; partial η2 = .103; ASD individuals had longer duration on AOIs than TD only for faces, p < .05, partial η2 = .145). Similarly, an analysis of fixation duration on just faces as a function of gaze location (AOI by Diagnosis) revealed a main effect of AOI (eyes > nose > mouth; F(2,82) = 44.13; p < .001; partial η2 = .518), and a main effect of Diagnosis (ASD subjects maintained their fixations longer on face AOIs than TD individuals; F(1,41) = 6.94; p < .01; partial η2 = .145; see Figure 6).
Fig. 6.
Fixation duration by area of interest (AOI) for faces for the typically developing (TD) and autism spectrum disorders (ASD) groups.
Although the Diagnosis by AOI interaction was not significant, an analysis of the number of individuals who had longer fixations on the mouth than eye regions yielded a trend in the same direction as the analysis of number of fixations described above. Specifically, five of the of the ASD subjects (23%), but only one of the TD subjects (5%) gazed longer at the mouth than the eye region (χ2(2, n = 43) = 2.89; p = .09; φ = .259). Four of these five ASD subjects were the previously reported four ASD subjects that had more fixations on the mouth than the eyes. Thus, this group of five ASD subjects gazed longer and, in all but one of these subjects, also made more fixations at the mouth than eyes.
To determine if fixation duration outside of the main facial features differs between groups, we conducted a three-way ANOVA [Stimulus (face, fan) by Diagnosis (ASD, TD) by AOI (in, out)], which revealed a significant three-way interaction (F(1,41) = 4.02; p = .05). Again, the main effect of Stimulus (F(1,41) = 28.58; p < .001; partial η2 = .411) and the Stimulus by AOI interaction (F(1,41) = 139.43; p < .001; partial η2 = .773) effects were significant, again suggesting that the duration of fixations were not simply due to their location, but rather varied as a function of stimulus type. An analysis of fixation durations on just face stimuli revealed a main effect of AOI (in > out; F(1,41 = 106.15; p < .001; partial η2 = .721) and a significant AOI by Diagnosis interaction (F(1,41) = 7.37; p < .01, partial η2 = .152). Pairwise comparisons revealed that the TD controls had longer fixations outside the eye, nose, and mouth regions than the ASD subjects (F(1,41) = 7.21; p < .01; partial η2 = .150; with a mean duration of 0.72 ± 0.33 vs. 0.49 ± 0.20 for the TD and ASD subjects, respectively). The reverse was true for fixation duration in the AOIs, with TD subjects having shorter fixations in the AOIs than the ASD subjects (F(1,41) = 6.94; p < .05; partial η2 = .145; with a mean duration of 1.41 ± 0.42 vs. 1.68 ± 0.23 for the TD and ASD subjects, respectively).
DISCUSSION
Our findings provided support for a strong version of the domain-specific face memory impairment in ASD. Specifically, ASD subjects were less accurate at recognizing previously studied photographs of faces than electric fans, and, relative to TD individuals, ASD subjects were less accurate at recognizing the faces, but not the fans.
We also found evidence to support the possibility that this recognition memory impairment was due to abnormal attention/encoding of the faces as indexed by the number of eye movements to faces during the encoding phase of the experiment. This conclusion is based first on the expected link that was demonstrated in the current study between recognition memory and the encoding process. That is, we found that subjects in general made more eye movements to stimuli later recognized than to those that were not (the main effect of accuracy on number of fixations) and that for ASD subjects in particular there was a significant correlation between the number of fixations and accuracy of memory performance. This link between encoding and recognition as well as our finding that TD individuals make more fixations to faces than fans whereas ASD individuals demonstrated no attentional/encoding preference (reflected in differences in fixation counts), suggests that poor memory for faces in ASD may be related to reduced scanning of the faces during encoding. This lack of attentional preference to social stimuli in ASD is consistent with previous studies (Fletcher-Watson et al., 2009; Klin et al., 2002).
We had further hypothesized, based on several previous reports, that ASD individuals would display fewer fixations to eyes than to other parts of the face. In the current study, however, this was not the case. Both ASD and TD individuals showed a strong preference for the eyes over other facial features. Moreover, although an individual subject analysis revealed that a small subgroup of ASD individuals showed a diminished preference for the eyes, these subjects did not differ from the other ASD subjects with regard to either subsequent face memory performance, or with regard to ASD symptomatology. Thus, whether these subjects represent a true subgroup within the ASD population remains to be determined.
We also found evidence that the reduced number of eye movements to faces by the ASD individuals was due to fewer and shorter fixations outside of the main constituent face parts (eyes, nose, mouth), and longer fixations within the AOIs. This pattern of performance is consistent with an impairment in attentional focus or a narrowed attentional spotlight (Mann & Walker, 2003), albeit limited to faces, rather than other object types. That is, the ASD subjects may have been overly focused on specific aspects of the eyes, nose, and mouth region (as indexed by longer fixation durations), at the expense of other aspects of the face, resulting in poorer recognition memory. Alternatively, it may be that the TD subjects are more adept at coming up with a successful strategy for encoding faces for recognition performance. For example, the TD subjects may have been more proficient at finding subtle, but distinct, facial characteristics, outside of the eye, nose, and mouth regions, to aid later recognition.
Our finding of a domain-specific memory impairment for social (i.e., faces) as opposed to nonsocial (i.e., fans) objects replicates that of other investigators (Blair et al., 2002; Boucher & Lewis, 1992; Hauck et al., 1998; Williams et al., 2005). Our findings also extend and strengthen previous reports in two ways. First, our face and fan memory tasks were carefully constructed to produce equivalent levels of performance in the TD group, thereby mitigating problems from ceiling effects and/or asymmetrical memory performance in the control subjects. Second, in contrast to previous studies, our control condition used a single object category –electric fans – that were similar to faces in their overall shape, and, like faces, had component parts and were strongly associated with motion. Use of a single object category as a control, however, raises questions and concerns about the generality of our findings. Thus, while our data support a domain-specific deficit in ASD, the extent to which the memory impairment in ASD is limited exclusively to faces and perhaps other socially-relevant stimuli remains to be fully determined (Blair et al., 2002).
The lack of an overall group difference in attention to eyes versus nose versus mouth is consistent with some reports, but not others (see Introduction). It is likely that there are multiple reasons for the discrepancy in the literature, including patient population differences. It is also likely that task demands and stimulus characteristics play crucial roles (for review, see Harms, Martin, & Wallace, 2010). For example, Speer et al. (2007) and Rutherford and Towns (2008) failed to find eye-tracking differences between ASD and TD individuals for static, neutral faces, but did find differences for more complex face depictions. That is, in the study by Rutherford and Towns (2008), ASD individuals looked less than TD individuals at the eyes only when viewing complex emotions (e.g., arrogant) during an emotion recognition task. Similarly, Speer et al. (2007) demonstrated that fixation duration in the eye region differed between groups for dynamic, but not static, social material. Thus, studies with either emotional or dynamic stimuli may better draw out differences between ASD and TD individuals’ attention to various face regions. Another possible explanation for the discrepancy in findings is intervention and/or maturation effects. It may be that all or some portion of the adolescents with ASD who took part in our study once exhibited a tendency to look less at eyes that either responded well to interventions designed to improve eye contact or simply dissipated over time. The generalizability of the current findings are also limited in that subjects in both of our groups have above average IQs and thus may not be representative of the broader population.
In addition, it should be noted that, whereas the ASD subjects were permitted to exhibit symptoms of anxiety and depression, the TD subjects were not. Symptoms of anxiety and depression were an exclusion only for the TD group. We are unaware of reports that such symptoms can affect recognition memory for faces, but not objects. Nevertheless, to address this possibility we examined the correlation between the Child Behavior Checklist (Achenbach & Rescorla, 2001) Internalizing Problems score and number and duration of fixation to faces or face AOIs, and did not find any significant relationships. Finally, in comparing the present investigation to prior studies, it is important to note that the paradigm of the current study involved the scanning of stimuli for the purpose of later recognizing them, unlike the task instructions for (most) previous eye tracking studies of face stimuli. As Yarbus (1967) demonstrated in an early eye tracking study, the nature of the task given to individuals has a strong influence on their eye movements. Therefore, it is possible that the current study’s task demands contributed to both comparable attention to eyes between individuals with ASD and TD controls as well as the bias for attending to central facial features in ASD.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Mental Health. There are no financial or other relationships that could be interpreted as a conflict of interest affecting this manuscript. We thank the participants and their families for the time and energy they gave in completing this research. We also thank Laura Dolan and Madeline Harms for their assistance with data collection and management.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms & profiles: An integrated system of multi-informant assessment. Burlington, VT: ASEBA; 2001. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders-Text Revision (DSM-IV-TR) 4. Washington DC: APA; 2000. [Google Scholar]
- Anderson CJ, Colombo J, Shaddy JD. Visual scanning and pupillary responses in young children with Autism Spectrum Disorder. Journal of Clinical and Experimental Neuropsychology. 2006;28(7):1238–1256. doi: 10.1080/13803390500376790. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Shulman C, Lamy D, Reuveni A. Attention to the eyes and mouth in high-functioning children with autism. Journal of Autism and Developmental Disorders. 2006;36(1):131–137. doi: 10.1007/s10803-005-0046-1. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Frith U, Smith N, Abell F, Cipolotti L. Fractionation of visual memory: Agency detection and its impairment in autism. Neuropsychologia. 2002;40(1):108–118. doi: 10.1016/s0028-3932(01)00069-0. [DOI] [PubMed] [Google Scholar]
- Boraston Z, Blakemore SJ. The application of eye-tracking technology in the study of autism. Journal of Physiology. 2007;581(3):893–898. doi: 10.1113/jphysiol.2007.133587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher J, Lewis V. Unfamiliar face recognition in relatively able autistic-children. Journal of Child Psychology and Psychiatry. 1992;33(5):843–859. doi: 10.1111/j.1469-7610.1992.tb01960.x. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb S, Schellenberg GD, Dager S, Friedman S, Aylward E, Richards T. Defining the broader phenotype of autism: Genetic, brain, and behavioral perspectives. Development and Psychopathology. 2002;14(3):581–611. doi: 10.1017/s0954579402003103. [DOI] [PubMed] [Google Scholar]
- Fletcher-Watson S, Leekam SR, Benson V, Frank MC, Findlay JM. Eye-movements reveal attention to social information in autism spectrum disorder. Neuropsychologia. 2009;47(1):248–257. doi: 10.1016/j.neuropsychologia.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Fletcher-Watson S, Leekam SR, Findlay JM, Stanton EC. Brief report: Young adults with autism spectrum disorder show normal attention to eye-gaze information-evidence from a new change blindness paradigm. Journal of Autism Developmental Disorders. 2008;38(9):1785–1790. doi: 10.1007/s10803-008-0548-8. [DOI] [PubMed] [Google Scholar]
- Grelotti DJ, Gauthier I, Schultz RT. Social interest and the development of cortical face specialization: What autism teaches us about face processing. Developmental Psychobiology. 2002;40(3):213–225. doi: 10.1002/dev.10028. [DOI] [PubMed] [Google Scholar]
- Harms MB, Martin A, Wallace GL. Facial emotion recognition in autism spectrum disorders: A review of behavioral and neuroimaging studies. Neuropsychology Review. 2010;20(3):290–322. doi: 10.1007/s11065-010-9138-6. [DOI] [PubMed] [Google Scholar]
- Hauck M, Fein D, Maltby N, Waterhouse L, Feinstein C. Memory for faces in children with autism. Child Neuropsychology. 1998;4(3):187–198. [Google Scholar]
- Ishai A, Pessoa L, Bikle PC, Ungerleider LG. Repetition suppression of faces is modulated by emotion. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(26):9827–9832. doi: 10.1073/pnas.0403559101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry. 2002;59(9):809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Bigler ED, Bocian M, Coon H, Dinh E, Dawson G, Volkmar F. Head circumference and height in autism: A study by the Collaborative Program of Excellence in Autism. American Journal of Medical Genetics A. 2006;140(21):2257–2274. doi: 10.1002/ajmg.a.31465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur A, Rutter M, Lord C, Rios P, Robertson S, Holdgrafer M, McLennan J. Autism diagnostic interview: A standardized investigator-based instrument. Journal of Autism and Developmental Disorders. 1989;19(3):363–387. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autism diagnostic observation schedule-WPS edition. Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Ohman A. The Karolinska Directed Emotional Faces—KDEF, CD Rom from Department of Clinical Neurosciences, Psychology section. Stockholm: Karolinska Institute; 1998. [Google Scholar]
- Mann TA, Walker P. Autism and a deficit in broadening the spread of visual attention. Journal of Child Psychology and Psychiatry. 2003;44(2):274–284. doi: 10.1111/1469-7610.00120. [DOI] [PubMed] [Google Scholar]
- Osterling J, Dawson G. Early recognition of children with autism: A study of first birthday home videotapes. Journal of Autism and Developmental Disorders. 1994;24(3):247–257. doi: 10.1007/BF02172225. [DOI] [PubMed] [Google Scholar]
- Pelphrey K, Sasson N, Reznick J, Paul G, Goldman B. Visual scanning of faces in autism. Journal of Autism and Developmental Disorders. 2002;32(4):249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- Rosch EH. Principles of categorization. In: Rosch E, Lloyd B, editors. Cognition and categorization. Hillsdale, NJ: Erlbaum Associates; 1978. pp. 27–48. [Google Scholar]
- Rutherford MD, Clements KA, Sekuler AB. Differences in discrimination of eye and mouth displacement in autism spectrum disorders. Vision Research. 2007;47(15):2099–2110. doi: 10.1016/j.visres.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Rutherford MD, Towns AM. Scan path differences and similarities during emotion perception in those with and without autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38(7):1371–1381. doi: 10.1007/s10803-007-0525-7. [DOI] [PubMed] [Google Scholar]
- Speer LL, Cook AE, McMahon WM, Clark E. Face processing in children with autism: Effects of stimulus contents and type. Autism. 2007;11(3):265–277. doi: 10.1177/1362361307076925. [DOI] [PubMed] [Google Scholar]
- Spezio M, Adolphs R, Hurley R, Piven J. Analysis of face gaze in autism using “Bubbles”. Neuropsychologia. 2006;45(1):144–151. doi: 10.1016/j.neuropsychologia.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Sterling L, Dawson G, Webb S, Murias M, Munson J, Panagiotides H, Aylward E. The role of face familiarity in eye tracking of faces by individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38:1666–1675. doi: 10.1007/s10803-008-0550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Goldstein G, Minshew NJ. Impaired memory for faces and social scenes in autism: Clinical implications of memory dysfunction. Archives of Clinical Neuropsychology. 2005;20(1):1–15. doi: 10.1016/j.acn.2002.08.001. [DOI] [PubMed] [Google Scholar]
- Yarbus AL. Eye movements during perception of complex objects. Eye Movements and Vision. 1967;7:171–196. [Google Scholar]