Abstract
In order to examine the effect of CART (Cocaine and Amphetamine Regulated Transcript) peptide depletion in adult rats, CART shRNAs or scrambled control shRNAs were administered bilaterally into the nucleus accumbens (NAc). There was an increase in body weight of the shRNA injected rats compared to the rats injected with the scrambled RNA. This is compatible with the data showing a role for the peptide in body weight and food intake. Also at this time, there was about a two-and-a-half fold increase in cocaine-mediated locomotion in the shRNA injected rats compared to the control rats. This finding is critical support for the hypothesis that endogenous CART peptides in the NAc inhibit the actions of cocaine and other psychostimulants. In immunohistochemical experiments on these same animals, there was a decrease in the staining density of CART peptide in the NAc of the shRNA injected rats. These data show that shRNA can reduce CART peptides in the NAc and that endogenous CART peptides influence body weight and cocaine-induced locomotor activity (LMA).
Keywords: CART, CART peptide, cocaine, shRNA, locomotor activity, Nucleus accumbens, body weight
1. Introduction
CART(cocaine and amphetamine regulated transcript) peptide neurotransmitters [rlCART 55-102 and 62-102, Rogge et al 2008 (Rogge et al., 2008)] are well known regulators of food intake, body weight, the actions of psychostimulants, other drugs, and other physiologic states (Douglass et al., 1995; Koylu et al., 1998; Kristensen et al., 1998; Lambert et al., 1998; Rogge et al., 2008). The bulk of the evidence for this comes from experiments where exogenous CART peptides are injected into the brains of animals. While the effects of injected, exogenous peptides are clear, the data on the role of endogenous peptides are more limited. Endogenous CART peptides have been examined with injections of anti-CART antibodies and this has supported a role for CART peptides in feeding (Kristensen et al., 1998; Lambert et al., 1998) and anxiety (Dandekar et al., 2008). CART gene knockout mice have also been prepared (Asnicar et al., 2001; Wierup et al., 2005) and the resulting obesity also supports a role for the peptides in body weight regulation. The discovery of a missense mutation resulting in missorting and improper processing of CART peptide in a family with obesity (del Giudice et al., 2001; Yanik et al., 2006) also supports earlier data linking body weight and CART. However, for drug reward, the knock-out data have been conflicting and have not clearly supported a role for CART peptides in cocaine reward (Couceyro et al., 2005; Moffett et al., 2011; Steiner et al., 2006). In these cases though, the gene knockouts and the genetic mutation are present at all times, and abnormal development could play a role in the findings particularly since CART peptides may play a role in development (Brischoux et al., 2001; Brischoux et al., 2002; Dun et al., 2001).
In a larger view of the field, particularly in regard to mechanisms of drug reward, data that are missing include the acute effects of depletion of CART peptides in adult animals. This is important because injected peptides could conceivably act at different brain sites and in a manner that is different from that of endogenous peptides. RNA-interference using small interfering RNAs (siRNAs) and short hairpin RNAs (shRNAs) have been used to knock down peptide expression without the complications of peptide depletions during development. siRNAs have been used to knockdown CART peptides levels in mice (Jean et al., 2007; Jia et al., 2008), but shRNAs usually provide a longer lasting depletion (Dreyer, 2011). This study was undertaken to provide such data and utilizes short-hairpin RNAs (shRNAs) to deplete the CART peptides after direct injection into the Nucleus Accumbens (NAc).
2. Results
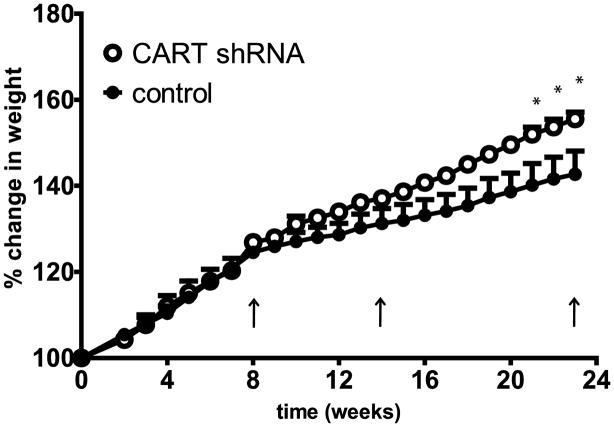
Animals were injected bilaterally with either shRNAs against CART peptides (n=3) or the scrambled shRNA (control) (n=3) (Figure 1), into the Nucleus Accumbens (NAc), as described in Methods. They were returned to the vivarium and their body weights and general conditions were monitored. Because of the extensive, existing data showing that accumbal CART peptide has an effect on body weight (Rogge et al., 2008), this parameter was used as a convenient initial indicator that the shRNA was having an effect. At 8 and 14 weeks there was no significant difference between shRNA and control in percent body weight change from pre-treatment weights, but at 21–23 weeks there was a significant difference between the shRNA and control groups. At 23 weeks, this difference was about 13% (Figure 2, **p<0.01).
Figure 1.
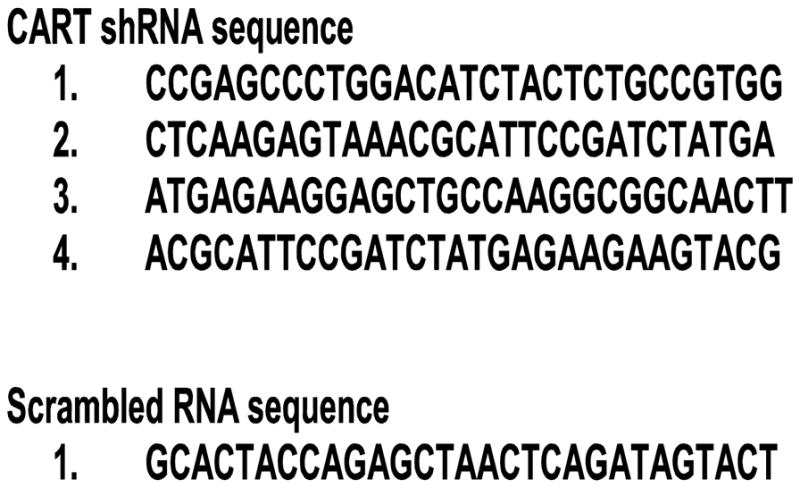
Sequences of shRNAs and Scrambled control RNA.
Figure 2.
Percent change in body weights of rats over time after intra-NAc injections of either CART shRNA or scrambled RNA (control). A 2-way ANOVA shows that there is a significant difference between rats injected with CART shRNA (n = 3) and controls (n = 3) (F 1, 92 = 50.09, *** p < 0.0001). Bonferroni post tests showed significant differences between treatments at 21–23 weeks (*p < 0.05). At 23 weeks, rats treated with CART shRNA or control RNA had gained 208 ± 9 g or 170 ± 22 g respectively over their weight at entry into the vivarium. CART shRNA-injected rats show a significant increase in percent weight gain compared to controls. The arrows show the time points when the rats were administered saline and cocaine (i.e. at 8, 14 and 23 weeks).
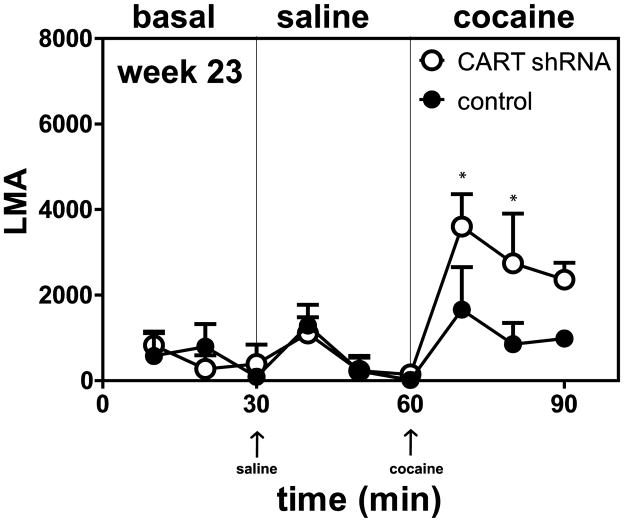
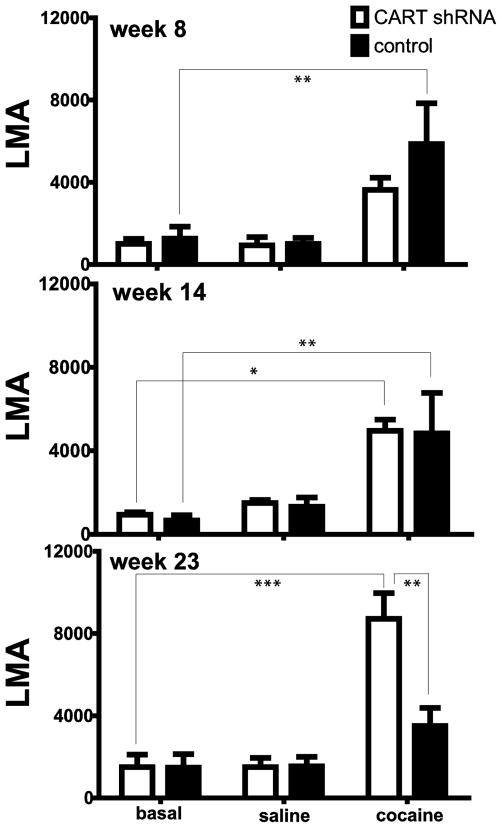
Additional experiments were carried out measuring cocaine-induced locomotor activity (LMA) at 8, 14 and 23 weeks (see Methods). Animals were placed in the activity chambers for 30 min, given a saline injection and monitored for 30 min, and then given a cocaine injection and again monitored for 30 min. The three 30 min periods were referred to as the basal, saline and cocaine periods or components, respectively (see Figure 3). The LMA during these periods were summed and compared (Figure 4). At 8 and 14 weeks (Figure 4) there was no difference in cocaine-induced LMA in the shRNA group vs the scrambled shRNA control group. But at 23 weeks, a time when the body weights were significantly different between the two groups (Figure 2), there was also a difference in the cocaine–induced LMA (Figures 3, 4, *p<0.05, **p<0.01, *** p<0.001). Cocaine-induced LMA was increased in the shRNA group compared to the scrambled RNA control group.
Figure 3.
Time course of the effect of saline or cocaine on locomotor activity in rats 23 weeks after intra-NAc injections of either CART shRNA or scrambled RNA control. The y-axis represents the total distance (LMA) covered by the rat, while the x-axis represents the time points (10 min intervals). The arrows show the times of injections and the thin vertical lines separate the different components (basal, saline, cocaine) of the time course. For analysis, A 2-way ANOVA for repeated measures was used to analyze the different components of the time course. To determine if there was any difference between CART shRNA and controls in basal locomotion, the LMA at times 10–30 min was analyzed. To determine the effect of saline, the LMA (time = 40–60 min) after saline injection was analyzed. To determine the effect of cocaine, the LMA (time = 70–90 min) after cocaine injection was analyzed. There was no significant difference between CART shRNA and control for basal LMA (F 1, 4 = 0.001111, p = 0.9750) or after saline injection (F 1, 4 = 0.004408, p = 0.9503), but there was a significant difference after cocaine injection (F 1, 4 = 11.62, *p = 0.0270). The Bonferroni post hoc tests differences between CART shRNA and controls are shown in the figure at 70 and 80 min: *p < 0.05.
Figure 4.
Basal, saline- or cocaine-mediated LMA in rats 8, 14 and 23 weeks after intra-NAc injections of either CART shRNA or control RNA. The y-axis represents the total LMA by the rat 30 minutes after no injection (basal) or after injections of saline or cocaine (10 mg/kg ip). These data were calculated from time course data (see time course data for week 23 in Figure 3). Basal values are the sum of the activity at 10, 20 and 30 minutes. Similarly, saline values are the sum of activity at 40, 50 and 60 minutes. Also, cocaine values are the sum of the activity at 70, 80 and 90 minutes for all animals. There is no difference in basal, saline-, and cocaine-induced locomotion between CART shRNA and control (n = 3 in each group) at 8 (F 1, 12 = 1.339, p = 0.2697) or 14 weeks (F 1, 12 = 0.07599, p = 0.7875). There is a difference in cocaine-induced locomotion between CART shRNA and control (n = 3 in each group) at 23 weeks (F 1, 12 = 7.576, **p = 0.0175). The differences determined by Bonferroni post hoc tests are shown in the figure: *p < 0.05, **p < 0.01, ***p < 0.001
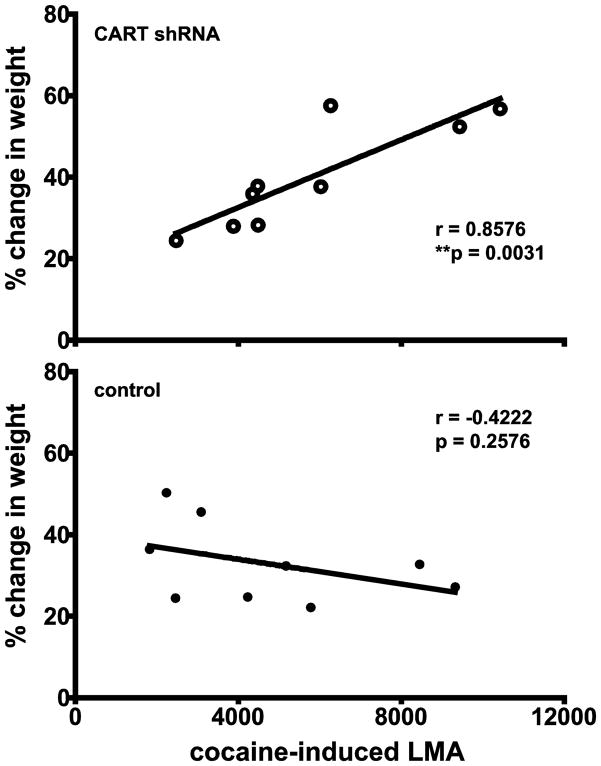
The body weight change and cocaine-induced LMA were correlated using all animals for all the experimental periods: 8, 14, and 23 weeks (there are three data pairs for each animal resulting in n = 9 total, Figure 3). There was a significant correlation for the animals given the shRNAs, but not for the animals given the scrambled shRNA (controls) (Figure 5). This is additional evidence that body weight and cocaine-induced LMA are both regulated by CART peptides.
Figure 5.
Correlation analysis between % weight change and locomotor activity in rats at 8, 14 and 23 weeks after intra-NAc injections of either CART shRNA or scrambled RNA control. The y-axis represents the % weight change compared to weight at pre-treatment, while the x-axis represents the total locomotor activity in 30 minutes after cocaine injection. The analysis shows that there is a correlation between % change in weight (8, 14, 23 weeks) and LMA (8, 14, 23 weeks) for the shRNA treated rats (r = 0.8576, R2 = 0.7354, **p = 0.0031), while there is no correlation for the controls (r = −0.4222, R2 = 0.1783, p = 0.2576).
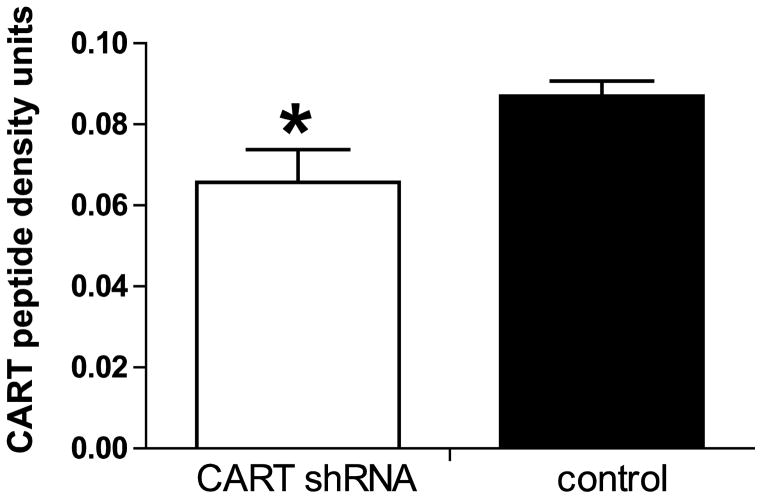
At week 23, after the LMA measurements, the animals were prepared for CART peptide Immunocytochemistry (ICC) as described in Methods. Several regions were examined including the NAc, the dorsal striatum, cerebral cortex and the ventral pallidum (VP). In general, the distribution of CART peptide ICC staining was the same as previously reported in untreated rats (Koylu et al., 1998). There was robust staining in the NAc and VP but little or no staining in the dorsal striatum, and little staining in the cerebral cortex, at the same levels. Non specific staining was assumed to be what was found in the dorsal striatum where there is no CART peptide or mRNA, and this was subtracted from the values from the NAc as described in Methods. CART peptide staining in the NAc was about 25% lower in the shRNA group (Figures 6, 7, *p<0.05) compared to the scrambled shRNA control group. CART peptide staining in the VP was not different in the two groups. Thus, the injections of shRNA into the NAc were effective in reducing CART peptide levels in that region and in revealing functional effects.
Figure 6.
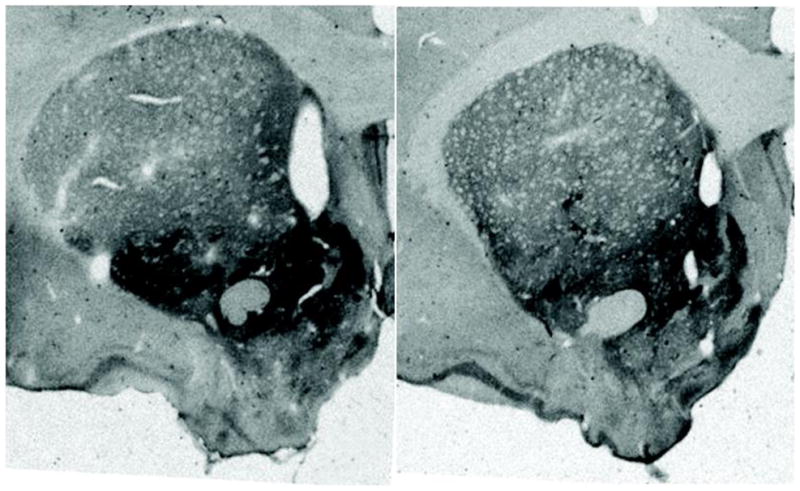
Immunohistochemical localization of CART Peptides in the NAc. The representative section from an animal on the left was injected with the scrambled control RNA, while the representative animal on the right was injected with the shRNA against CART peptide. CART peptide levels are reduced in the animal on the right.
Figure 7.
The effect of intra-accumbal administration of anti-CART shRNA and scrambled shRNA on CART peptide density in the NAc. Immunohistochemical analysis was done 23 weeks after the microinjections of CART shRNA and scrambled controls (Figure 6). Statistical analysis shows that there is a significant difference (* p < 0.05) in CART peptide levels in the nucleus accumbens between CART shRNA and control (n = 6 measurements in each group: each animal had a total of 2 microinjections, 1 microinjection into each hemisphere. therefore 2 measurements corresponding to each hemisphere of the brain were taken/rat). The measurement of the NAc CART peptide density was calculated relative to the dorsal striatum which acts as a background (the dorsal striatum contains no CART peptide.
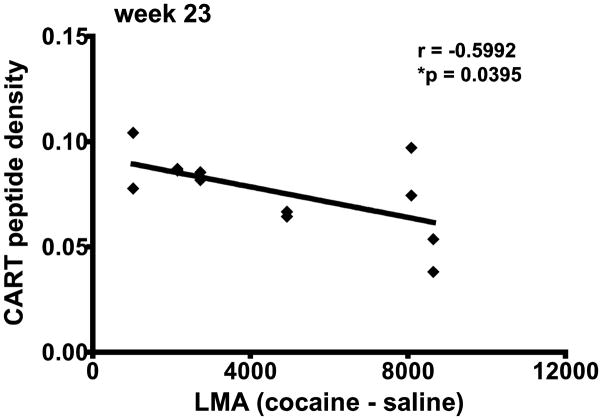
The CART peptide density (Figure 7) and the difference between cocaine- and saline-induced LMA (actual cocaine effect) were correlated for all individual animals (both groups, n=6) at 23 weeks (Figure 8). Each of the 2 hemispheres of the NAc were injected per rat and the CART density in these 2 hemispheres was plotted against cocaine-saline LMA for the rat. This provides two data pairs for each animal, n = 12 total, Figure 8). There was a significant correlation between CART density in the NAc and cocaine-saline LMA (Figure 8). This is additional evidence that as CART peptide levels in the NAc decrease, cocaine-induced LMA increases.
Figure 8.
Correlation analysis between CART density and cocaine-induced LMA in rats at 23 weeks after intra-NAc injections. The data represents individual values for all rats in both the shRNA and scrambled RNA groups. Each animal had a total of 2 microinjections (bilateral), one into each hemisphere and therefore 2 measurements corresponding to each brain were taken/rat for the CART density in the NAc (see Figure 7 legend). The difference between cocaine and saline total LMA (cocaine LMA 30 min – saline LMA 30 min) for each rat was plotted on the x-axis and the CART peptide density for the rat was plotted on the y-axis. The difference between cocaine and saline LMA represents the actual cocaine effect. The analysis shows that there is a correlation between CART density and cocaine-saline LMA (r = −0.5992, R2 = 0.3591, *p = 0.0395).
3. Discussion
This study shows that single, bilateral intra-accumbal injections of shRNAs directed against CART reduced CART peptide levels and increased both body weight and cocaine-induced locomotor activity (LMA) in rats. These novel findings are important for supporting the hypotheses that endogenous CART peptides influence feeding and cocaine-induced actions. These findings agree with previous studies showing that injections of exogenous CART peptides into the NAc inhibit feeding, cocaine-induced LMA (Jaworski et al., 2003; Kim et al., 2003; Kim et al., 2007; Yoon et al., 2007) and cocaine-related reward (Jaworski et al., 2008).
The notion that CART peptides influence the actions of psychostimulants goes back to the discovery of the CART mRNA and gene, and can be summarized briefly. CART mRNA was increased after acute injection of cocaine or amphetamine (Douglass et al., 1995). While this change in levels was not always confirmed (Hunter et al., 2005; Marie-Claire et al., 2003; Vrang et al., 2002), it was later shown that CART neurons in the NAc are activated by cocaine administration (Hubert and Kuhar, 2008). This latter study strongly supports the suggestion by Douglass et al. (1995) that the actions of psychostimulants involve CART peptides. Moreover, CART gene expression is increased in the nucleus accumbens of human cocaine abusers (Albertson et al., 2004; Bannon et al., 2005). Psychostimulants act through mechanisms involving an increase of dopamine (Ritz et al., 1987), and dopamine receptors were found on, and affect, CART neurons in the NAc (Beaudry et al., 2004; Hubert and Kuhar, 2006; Hunter et al., 2006; Smith et al., 1999). CART peptide has an effect on dopamine- or cocaine-induced LMA only when both D1 and D2 receptors are stimulated simultaneously, which is what occurs naturally (Moffett et al., 2011). Also there is evidence of CART projections to and activation of the dopamine neurons in the ventral tegmental area (VTA) (Dallvechia-Adams et al., 2002; Kimmel et al., 2000; Shieh, 2003). Finally, direct injections of CART peptides into the NAc reduced the actions of psychostimulants (Jaworski et al., 2003; Jaworski et al., 2008; Kim et al., 2003; Kim et al., 2007; Yoon et al., 2007). Additional details can be found in a recent review (Rogge et al., 2008). Taken together, these finding suggest that CART peptidergic neurons in the NAc are homeostatic regulators of psychostimulant and dopamine, and tend to suppress the results of excessive dopamine. Administration of other drugs including methamphetamine, MDMA and ethanol have also been found to increase CART mRNA levels in the NAc, suggesting the involvement of CART with other drugs as well (Jean et al., 2007; Ogden et al., 2004; Salinas et al., 2006).
The effects of CART shRNA on body weight required a surprisingly long time (21 – 23 weeks) after injection to become significant. This could be for either technical or biological reasons or both. For example, the efficiency of incorporation of the plasmid and expression of the shRNA may have been low. Or, endogenous CART peptide may have only a small influence on body weight such that a long time is required to produce cumulative and measurable effects. Earlier published findings showed that an increase in body weight was not found in knock-out mice until they were 40 weeks of age or fed a high fat diet (Asnicar et al., 2001; Wierup et al., 2005). This supports the idea of a slower biological mechanism rather than a technical problem. In any case, having established that shRNAs can produce functional effects, future experiments will examine a variety of additional variables.
Other investigators have used siRNAs, rather than shRNAs, against CART peptides in the NAc (Jean et al., 2007) and have found increases in food intake and reductions in CART mRNA in the NAc due to the siRNA administration. The increases in the food intake were observed within 3 hours after siRNA injection into the NAc. Also, the increases in the food intake were observed every day (3 day study) that the CART siRNA was injected. After three days of daily intra-NAc CART siRNA injections, immunocytochemistry showed that CART mRNA had reduced in the NAc. Thus, depletion of endogenous CART peptides in the NAc had biological effects, although the use of siRNAs produced effects much more quickly in mice (within 3 hours) than the shRNAs in rats (several weeks) as expected. One explanation is that shRNAs produce siRNAs but only after incorporation and expression. The study by Jean et al (2007) did not examine the effects of CART depletion in the NAc on psychostimulant drugs.
In summary, CART is clearly involved in the regulation of food intake and body weight as originally proposed by Koylu et al (1997;1998) and in the regulation of psychostimulant effects (Rogge et al., 2008). This study showed that shRNA administration led to an increase in body weight and supports, albeit in rats, the finding that depletion of CART peptides by siRNAs enhanced feeding in mice (Jean et al., 2007). The major new finding of this study was that reduction of the levels of endogenous CART peptides in the NAc by shRNA increased the LMA due to cocaine. This is consistent with the observation that the opposite occurs when exogenous CART peptide is injected into the NAc (Jaworski et al., 2003; Jaworski et al., 2008; Kim et al., 2003; Kim et al., 2007; Yoon et al., 2007). Overall, these findings are in strong support of the idea that CART peptides in the NAc regulate the effects of cocaine and other psychostimulants (Rogge et al., 2008).
4. Methods and Materials
Animals
Six male, Sprague–Dawley rats (Charles River Laboratories, Wilmington, MA) 12 weeks old and weighing 365–425 grams at the time of surgery were used. The rats were housed at 25°C on a 12 hr light/dark schedule with ad libitum access to food and water. All procedures were carried out in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Emory University. While the n of 3 for both our groups may be considered low, that number produced statistically significant differences and it is difficult to justify a larger n given current guidelines to reduce, refine and replace the numbers of animals.
shRNAs and Microinjections
shRNA sequences were obtained in conjunction with ORIGENE (HuSH shRNA-construct against CART peptide, cat number TF709920, Origene Technologies, Inc., Rockville MD). Four different shRNA sequences were selected along with a scrambled sequence as a control (Figure 1). The scrambled sequence had no significant homology with any known sequence. Every shRNA vector was cloned in pRFP-C-RS plasmids under U6 promoter control (Origene Technologies, Inc., Rockville MD). An injection cocktail was prepared that contained equal amounts (62.5 ng/μL) of the four shRNAs such that a total of 250 ng/μL was used for bilateral injection. The scrambled control shRNA was also prepared as a 250 ng/μL solution for injection. Solutions were made using nuclease free water. The bilateral injections were made in a total volume of 1 μL (250 ng) delivered at 0.2 μL per min, for a total delivery time of 5 min.
Surgery and Microinjection
Rats were anesthetized with isoflurane (4%) gas anesthesia for stereotaxic surgical procedure for bilateral microinjection into the NAc. Injections were performed bilaterally at the following target coordinates for the NAc (from bregma): A/P +1.6, M/L ± 1.5, DV −7.7 according to Paxinos and Watson (1998) (Paxinos, 1998.). Microinjection was done with 5 μL Hamilton syringes (MicroLiter 75N; Hamilton Company, Reno, NV) with 26-gauge tip needle attached to microsyringe pumps (Ultra MicroPump II, World precision Instruments (WPI), Sarasota FL) and controlled by the microsyringe pump controller (Micro4, WPI). The microinjection parameters were set so as to deliver a total volume of 1 μL at 0.2 μL per min, for a total delivery time of 5 min. After the injections, the syringes were left in place for another 5 min to allow diffusion of injection solution, and withdrawn slowly from the brain to prevent backflow of injection solutions and to minimize damage. Only one injection/side was given per animal, and both sides were infused with the same injection solution. The injection solutions were shRNAs (as a cocktail) or scrambled shRNA controls (see details above). After the injections, the rats were then sutured and allowed to recover for 8 weeks before the first locomotor experiment. During recovery, weights of rats were taken weekly and rats were habituated to the locomotor chambers.
Locomotor Experiments
Overall, the procedures were those used by Jaworski et al (2003). Locomotor Activity (LMA) was measured in a photocell cage (40 × 40 × 30 cm) testing chamber (Omnitech Electronics, Columbus, Ohio) equipped with 32 photobeams. Experimental data were collected using an IBM computer equipped with Digipro* software (Omnitech Electronics). Each cage was placed within a separate isolated stainless steel box. On experimental days, the rats were placed into the testing chambers for 30 minutes to measure baseline LMA. After this, rats were given intraperitoneal (i.p.) injections of saline and returned to the testing chambers for another 30 minutes. Then, rats were given cocaine (10 mg/kg i.p., 1 mL/kg) and returned to the testing chambers for another 30 minutes.
Immunohistochemistry
After the LMA experiments were completed, rats were prepared for CART immunohistochemistry as previously described (Hubert and Kuhar, 2008; Koylu et al., 1997). The primary antibody was the one designated C4 by Koylu et al (1997), and the overall procedure is as follows. The sections were preincubated in a solution containing 10% normal goat serum (NGS), 1.0% BSA, and 0.3% Triton X-100 in PBS for 1 h. They were then incubated overnight with the primary antisera diluted at 1–15,000 in a solution containing 1.0% NGS, 1.0% BSA and 0.3% Triton X-100 in PBS. Next, the sections were rinsed in PBS and transferred for 1 h to a secondary antibody solution containing biotinylated goat-anti-rabbit IgGs (Vector, Burlingame, CA, USA) diluted 1:200 in the primary antibody diluent solution. After rinsing, sections were put in a solution containing 1:100 avidin-biotin-peroxidase complex (ABC, Vector, Burlingame, CA, USA). The tissue was then washed in PBS and 0.05 M TRIS buffer before being transferred to a solution containing 0.01 M imidazole, 0.0005% hydrogen peroxide, and 0.025% 3,3′-diaminobenzidine tetrahydrochloride (DAB, Sigma, St. Louis, MO, USA) in TRIS for 7–10 min. Sections were then mounted on gelatin-coated slides and dried, and a coverslip was applied with permount. Quantitative analysis of CART peptide content was done using MCID Basic Imaging Analysis Software which analyzed the ICC staining density over the NAc, cerebral cortex, ventral pallidum (VP) and the dorsal striatum. For Figure 7, dorsal striatum density values were averaged and used as a blank value which was subtracted from values generated for NAc.
Data Analysis
All analyses were performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego California USA, www.graphpad.com). For weight change data (Figure 2), weight change is expressed as percent of weight before administration of shRNA or control. A 2-way ANOVA with Bonferroni post test was used to analyze weight change throughout the course of the experiment (up to 23 weeks).
LMA data were acquired in 10-minute bins and the time course data for week 23 (Figure 3) and total LMA data (for weeks 8, 14 and 23; Figure 4) were analyzed. The total LMA data is derived from the time course data by summation of individual time point locomotor activity values (three-10 min samples) for 30 min before saline injection (basal), post-saline and post-cocaine LMA. For analysis of the time course data (Figure 3), a 2-way Repeated Measures ANOVA with Bonferroni post test was used to analyze each component (basal, saline, cocaine) of the time course. For analysis of the total locomotor activity data (Figure 4), a 2-way ANOVA with Bonferroni post test was used.
The percent body weight change and cocaine-induced LMA were correlated for each animal (Figure 5). To do this, weight change data and cocaine-induced LMA data for each animal from the three time periods of experimentation – 8, 14 and 23 weeks were correlated (we have three data pairs (weight change, cocaine-induced LMA) for each animal, Figure 3).
For immunocytochemistry data (Figure 7), statistical analysis was done using an unpaired t-test (with Welch’s correction). The CART density (Figure 7) and cocaine effect on LMA were correlated for each animal at 23 weeks (Figure 8). To do this, the CART density values in each hemisphere of the NAc were plotted against the cocaine effect (total cocaine LMA – total saline LMA) for each rat. All rats, both shRNA and controls, were included in a single graph (Figure 8). For all analyses, data values are reported as mean ± SEM and type I error was set to P<0.05 (*p<0.05, **p<0.01, *** p<0.001).
shRNAs can be used to deplete CART peptides
shRNA administration results in increases in body weight and cocaine –induced locomotion
This is needed evidence to support the hypothesis that accumbal CART peptides regulate the actions of psychostimulants.
Acknowledgments
The authors acknowledge the support of NIH grants, DA15162, and DA15040. Also, this project was funded by the National Center for Research Resources P51RR165 and is currently supported by the Office of Research Infrastructure Programs/OD P51OD11132.
Footnotes
None of the authors have a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albertson DN, Pruetz B, Schmidt CJ, Kuhn DM, Kapatos G, Bannon MJ. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J Neurochem. 2004;88:1211–9. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnicar MA, Smith DP, Yang DD, Heiman ML, Fox N, Chen YF, Hsiung HM, Koster A. Absence of cocaine- and amphetamine- regulated transcript results in obesity in mice fed a high caloric diet. Endocrinology. 2001;142:4394–400. doi: 10.1210/endo.142.10.8416. [DOI] [PubMed] [Google Scholar]
- Bannon M, Kapatos G, Albertson D. Gene expression profiling in the brains of human cocaine abusers. Addict Biol. 2005;10:119–26. doi: 10.1080/13556210412331308921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudry G, Zekki H, Rouillard C, Levesque D. Clozapine and dopamine D3 receptor antisense reduce cocaine- and amphetamine-regulated transcript expression in the rat nucleus accumbens shell. Synapse. 2004;51:233–40. doi: 10.1002/syn.10302. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Fellmann D, Risold PY. Ontogenetic development of the diencephalic MCH neurons: a hypothalamic ‘MCH area’ hypothesis. Eur J Neurosci. 2001;13:1733–44. doi: 10.1046/j.0953-816x.2001.01552.x. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Griffond B, Fellmann D, Risold PY. Early and transient ontogenetic expression of the cocaine- and amphetamine-regulated transcript peptide in the rat mesencephalon: correlation with tyrosine hydroxylase expression. J Neurobiol. 2002;52:221–9. doi: 10.1002/neu.10077. [DOI] [PubMed] [Google Scholar]
- Couceyro PR, Evans C, McKinzie A, Mitchell D, Dube M, Hagshenas L, White FJ, Douglass J, Richards WG, Bannon AW. Cocaine-and amphetamine-regulated transcript (CART) peptides modulate the locomotor and motivational properties of psychostimulants. J Pharmacol Exp Ther. 2005;315:1091–100. doi: 10.1124/jpet.105.091678. [DOI] [PubMed] [Google Scholar]
- Dallvechia Adams S, Kuhar MJ, Smith Y. Cocaine- and amphetamine-regulated transcript peptide projections in the ventral midbrain: colocalization with gamma-aminobutyric acid, melanin-concentrating hormone, dynorphin, and synaptic interactions with dopamine neurons. J Comp Neurol. 2002;448:360–72. doi: 10.1002/cne.10268. [DOI] [PubMed] [Google Scholar]
- Dandekar MP, Singru PS, Kokare DM, Lechan RM, Thim L, Clausen JT, Subhedar NK. Importance of cocaine- and amphetamine-regulated transcript peptide in the central nucleus of amygdala in anxiogenic responses induced by ethanol withdrawal. Neuropsychopharmacology. 2008;33:1127–36. doi: 10.1038/sj.npp.1301516. [DOI] [PubMed] [Google Scholar]
- del Giudice EM, Santoro N, Cirillo G, D’Urso L, Di Toro R, Perrone L. Mutational screening of the CART gene in obese children: identifying a mutation (Leu34Phe) associated with reduced resting energy expenditure and cosegregating with obesity phenotype in a large family. Diabetes. 2001;50:2157–60. doi: 10.2337/diabetes.50.9.2157. [DOI] [PubMed] [Google Scholar]
- Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15:2471–81. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer JL. Lentiviral vector-mediated gene transfer and RNA silencing technology in neuronal dysfunctions. Mol Biotechnol. 2011;47:169–87. doi: 10.1007/s12033-010-9334-x. [DOI] [PubMed] [Google Scholar]
- Dun SL, Castellino SJ, Yang J, Chang JK, Dun NJ. Cocaine- and amphetamine-regulated transcript peptide-immunoreactivity in dorsal motor nucleus of the vagus neurons of immature rats. Brain Res Dev Brain Res. 2001;131:93–102. doi: 10.1016/s0165-3806(01)00267-x. [DOI] [PubMed] [Google Scholar]
- Hubert GW, Kuhar MJ. Colocalization of CART peptide with prodynorphin and dopamine D1 receptors in the rat nucleus accumbens. Neuropeptides. 2006;40:409–15. doi: 10.1016/j.npep.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Hubert GW, Kuhar MJ. Cocaine administration increases the fraction of CART cells in the rat nucleus accumbens that co-immunostain for c-Fos. Neuropeptides. 2008;42:339–43. doi: 10.1016/j.npep.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RG, Vicentic A, Rogge G, Kuhar MJ. The effects of cocaine on CART expression in the rat nucleus accumbens: a possible role for corticosterone. Eur J Pharmacol. 2005;517:45–50. doi: 10.1016/j.ejphar.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Hunter RG, Jones D, Vicentic A, Hue G, Rye D, Kuhar MJ. Regulation of CART mRNA in the rat nucleus accumbens via D3 dopamine receptors. Neuropharmacology. 2006;50:858–64. doi: 10.1016/j.neuropharm.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Kozel MA, Philpot KB, Kuhar MJ. Intra-accumbal injection of CART (cocaine- amphetamine regulated transcript) peptide reduces cocaine-induced locomotor activity. J Pharmacol Exp Ther. 2003;307:1038–44. doi: 10.1124/jpet.103.052332. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Hansen ST, Kuhar MJ, Mark GP. Injection of CART (cocaine- and amphetamine-regulated transcript) peptide into the nucleus accumbens reduces cocaine self-administration in rats. Behav Brain Res. 2008;191:266–71. doi: 10.1016/j.bbr.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean A, Conductier G, Manrique C, Bouras C, Berta P, Hen R, Charnay Y, Bockaert J, Compan V. Anorexia induced by activation of serotonin 5-HT4 receptors is mediated by increases in CART in the nucleus accumbens. Proc Natl Acad Sci U S A. 2007;104:16335–40. doi: 10.1073/pnas.0701471104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Chen X, Zhu W, Luo Y, Hua Z, Xu Y. CART protects brain from damage through ERK activation in ischemic stroke. Neuropeptides. 2008;42:653–61. doi: 10.1016/j.npep.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Kim JH, Creekmore E, Vezina P. Microinjection of CART peptide 55-102 into the nucleus accumbens blocks amphetamine-induced locomotion. Neuropeptides. 2003;37:369–73. doi: 10.1016/j.npep.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Kim S, Yoon HS, Kim JH. CART peptide 55-102 microinjected into the nucleus accumbens inhibits the expression of behavioral sensitization by amphetamine. Regul Pept. 2007;144:6–9. doi: 10.1016/j.regpep.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Gong W, Vechia SD, Hunter RG, Kuhar MJ. Intra-ventral tegmental area injection of rat cocaine and amphetamine-regulated transcript peptide 55-102 induces locomotor activity and promotes conditioned place preference. J Pharmacol Exp Ther. 2000;294:784–92. [PubMed] [Google Scholar]
- Koylu EO, Couceyro PR, Lambert PD, Ling NC, DeSouza EB, Kuhar MJ. Immunohistochemical localization of novel CART peptides in rat hypothalamus, pituitary and adrenal gland. J Neuroendocrinol. 1997;9:823–33. doi: 10.1046/j.1365-2826.1997.00651.x. [DOI] [PubMed] [Google Scholar]
- Koylu EO, Couceyro PR, Lambert PD, Kuhar MJ. Cocaine- and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J Comp Neurol. 1998;391:115–32. [PubMed] [Google Scholar]
- Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, Larsen PJ, Hastrup S. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–6. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- Lambert PD, Couceyro PR, McGirr KM, Dall Vechia SE, Smith Y, Kuhar MJ. CART peptides in the central control of feeding and interactions with neuropeptide Y. Synapse. 1998;29:293–8. doi: 10.1002/(SICI)1098-2396(199808)29:4<293::AID-SYN1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Marie-Claire C, Laurendeau I, Canestrelli C, Courtin C, Vidaud M, Roques B, Noble F. Fos but not Cart (cocaine and amphetamine regulated transcript) is overexpressed by several drugs of abuse: a comparative study using real time quantitative polymerase chain reaction in rat brain. Neurosci Lett. 2003;345:77–80. doi: 10.1016/s0304-3940(03)00307-0. [DOI] [PubMed] [Google Scholar]
- Moffett MC, Song J, Kuhar MJ. CART peptide inhibits locomotor activity induced by simultaneous stimulation of D1 and D2 receptors, but not by stimulation of individual dopamine receptors. Synapse. 2011;65:1–7. doi: 10.1002/syn.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CA, Rich ME, Schork NJ, Paulus MP, Geyer MA, Lohr JB, Kuczenski R, Niculescu AB. Candidate genes, pathways and mechanisms for bipolar (manic-depressive) and related disorders: an expanded convergent functional genomics approach. Mol Psychiatry. 2004;9:1007–29. doi: 10.1038/sj.mp.4001547. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Burlington MA: 1998. [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–23. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Rogge G, Jones D, Hubert GW, Lin Y, Kuhar MJ. CART peptides: regulators of body weight, reward and other functions. Nat Rev Neurosci. 2008;9:747–58. doi: 10.1038/nrn2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas A, Wilde JD, Maldve RE. Ethanol enhancement of cocaine- and amphetamine-regulated transcript mRNA and peptide expression in the nucleus accumbens. J Neurochem. 2006;97:408–15. doi: 10.1111/j.1471-4159.2006.03745.x. [DOI] [PubMed] [Google Scholar]
- Shieh KR. Effects of the cocaine- and amphetamine-regulated transcript peptide on the turnover of central dopaminergic neurons. Neuropharmacology. 2003;44:940–8. doi: 10.1016/s0028-3908(03)00095-9. [DOI] [PubMed] [Google Scholar]
- Smith Y, Kieval J, Couceyro PR, Kuhar MJ. CART peptide-immunoreactive neurones in the nucleus accumbens in monkeys: ultrastructural analysis, colocalization studies, and synaptic interactions with dopaminergic afferents. J Comp Neurol. 1999;407:491–511. doi: 10.1002/(sici)1096-9861(19990517)407:4<491::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Steiner RC, Hsiung HM, Picciotto MR. Cocaine self-administration and locomotor sensitization are not altered in CART knockout mice. Behav Brain Res. 2006;171:56–62. doi: 10.1016/j.bbr.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Vrang N, Larsen PJ, Kristensen P. Cocaine-amphetamine regulated transcript (CART) expression is not regulated by amphetamine. Neuroreport. 2002;13:1215–8. doi: 10.1097/00001756-200207020-00029. [DOI] [PubMed] [Google Scholar]
- Wierup N, Richards WG, Bannon AW, Kuhar MJ, Ahren B, Sundler F. CART knock out mice have impaired insulin secretion and glucose intolerance, altered beta cell morphology and increased body weight. Regul Pept. 2005;129:203–11. doi: 10.1016/j.regpep.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Yanik T, Dominguez G, Kuhar MJ, Del Giudice EM, Loh YP. The Leu34Phe ProCART mutation leads to cocaine- and amphetamine-regulated transcript (CART) deficiency: a possible cause for obesity in humans. Endocrinology. 2006;147:39–43. doi: 10.1210/en.2005-0812. [DOI] [PubMed] [Google Scholar]
- Yoon HS, Kim S, Park HK, Kim JH. Microinjection of CART peptide 55-102 into the nucleus accumbens blocks both the expression of behavioral sensitization and ERK phosphorylation by cocaine. Neuropharmacology. 2007;53:344–51. doi: 10.1016/j.neuropharm.2007.05.014. [DOI] [PubMed] [Google Scholar]