Abstract
Semipermeable cell walls or apoplastic “membranes” have been hypothesized to be present in various plant tissues. Although often associated with suberized or lignified walls, the wall component that confers osmotic semipermeability is not known. In muskmelon (Cucumis melo L.) seeds, a thin, membranous endosperm completely encloses the embryo, creating a semipermeable apoplastic envelope. When dead muskmelon seeds are allowed to imbibe, solutes leaking from the embryo are retained within the envelope, resulting in osmotic water uptake and swelling called osmotic distention (OD). The endosperm envelope of muskmelon seeds stained with aniline blue, which is specific for callose (β-1,3-glucan). Outside of the aniline-blue-stained layer was a Sudan III- and IV-staining (lipid-containing) layer. In young developing seeds 25 d after anthesis (DAA) that did not exhibit OD, the lipid layer was already present but callose had not been deposited. At 35 DAA, callose was detected as distinct vesicles or globules in the endosperm envelope. A thick callose layer was evident at 40 DAA, coinciding with development of the capacity for OD. Removal of the outer lipid layer by brief chloroform treatment resulted in more rapid water uptake by both viable and nonviable (boiled) seeds, but did not affect semipermeability of the endosperm envelope. The aniline-blue-staining layer was digested by β-1,3-glucanase, and these envelopes lost OD. Thus, apoplastic semipermeability of the muskmelon endosperm envelope is dependent on the deposition of a thick callose-containing layer outside of the endosperm cell walls.
The presence of semipermeable apoplastic “membranes” has been reported in sugarcane stems (Welbaum et al., 1992), developing seeds (Bradford, 1994), and roots (Steudle and Peterson, 1998). For example, sugarcane stems accumulate high apoplastic Suc concentrations but the xylem stream within the vascular bundles is virtually free of solutes (Welbaum and Meinzer, 1990). Welbaum et al. (1992) demonstrated that a semipermeable apoplastic barrier exists between the vascular bundles and storage parenchyma apoplast. Solutes and water are transported into developing seeds primarily through the phloem, and high apoplastic solute concentrations are involved in maintaining phloem unloading in sink tissues (Wolswinkel, 1992). At the same time, excess water delivered via the phloem is recycled back to the mother plant via an apoplastic pathway (Oparka and Gates, 1981; Peoples et al., 1985). Strategically located semipermeable apoplastic membranes may prevent solute movement by mass flow back to the mother plant, retaining solutes within the unloading regions of developing seeds (Bradford, 1994). The tetrazolium ion, which is used for vital staining, does not penetrate into most grass seeds (Brown, 1907) or through the inner coat of watermelon (Thornton, 1968), tomato, or pepper seeds (Beresniewicz et al., 1995b). Similarly, the lanthanum ion, a water-soluble heavy metal, is accumulated at the inner seed coat adjacent to the endosperm in tomato and pepper (Beresniewicz et al., 1995b; Taylor et al., 1997). In an examination of seeds of 500 species from more than 40 families, semipermeability was very common, except in the Leguminosae and some genera of Cistaceae and Cruciferae (Gola, 1905, cited by Kotowski, 1927). The Casparian strip in the root endodermis has been considered to be essentially impermeable to both water and solutes, but Steudle and Peterson (1998) have proposed that a semipermeable Casparian strip is required to explain water and solute movement through roots.
Suberized or lignified walls are often associated with apoplastic permeability barriers (O'Brien and Carr, 1970; Cochran, 1983; Welbaum and Bradford, 1990; Jacobsen et al., 1992; Welbaum et al., 1992). Suberin has been suggested as the semipermeable material in seeds of corn (Johann, 1942), Johnsongrass (Harrington and Crocker, 1923), tomato, and pepper (Beresniewicz et al., 1995a), whereas cutin may serve this role in leek seeds (Taylor et al., 1997). In pollen grains, Heslop-Harrison (1964) suggested that callose in the pollen mother cells acts as a “molecular sieve” to isolate the pollen cells from maternal compounds. Nonetheless, although many histochemical studies have attempted to relate the composition of semipermeable cell walls to their function, there is no direct demonstration that specific wall components are responsible for semipermeability.
In muskmelon (Cucumis melo L.) seeds the embryo is completely enclosed by a membranous envelope that has been described as consisting of a layer of endosperm cells and several layers of thick-walled perisperm cells (originating from the nucellus); it was therefore termed the “perisperm envelope” (Singh, 1953; Welbaum and Bradford, 1990). However, as will be shown here, the envelope surrounding the muskmelon embryo is composed entirely of a single layer of endosperm cells covered by a thick, noncellular external layer. We will therefore refer to this tissue as the “endosperm envelope.” When dead muskmelon seeds are allowed to imbibe, solute leakage from the embryo generates an osmotic gradient across the semipermeable endosperm envelope, resulting in water uptake and swelling, known as OD (Welbaum and Bradford, 1990). The intact endosperm envelope can shrink and swell repeatedly in response to external osmotic conditions without significant loss of solutes (Welbaum and Bradford, 1990). A semipermeable envelope is present in many species of Cucurbitaceae and Compositae (e.g. lettuce), as shown by the development of OD in hydrated dead seeds (Hill and Taylor, 1989).
In muskmelon, endosperm envelope semipermeability was maintained after various treatments that killed the embryos (freezing and thawing the hydrated seeds, boiling, autoclaving, aging, and 100% methanol), whereas the semipermeability was lost after strong acid or alkaline treatments (Welbaum and Bradford, 1990). A Sudan IV-staining (lipid-containing) layer was detected on the outer surface of the endosperm envelope and was assumed to be involved in semipermeability, in analogy with the presumed role of suberized cell walls in other tissues (Welbaum and Bradford, 1990). However, no direct evidence was available to determine which component of the endosperm envelope is responsible for semipermeability. We demonstrate here that an extracellular layer composed primarily of callose is entirely responsible for the semipermeable properties of the muskmelon endosperm envelope.
MATERIALS AND METHODS
Muskmelons (Cucumis melo L. cv Top Mark) were field grown at the University of California (Davis) in 1995 and 1996, and seeds were harvested, dried, and stored at −20°C (Welbaum and Bradford, 1988). For the seed-development study, flowers were tagged at anthesis and harvested at 5-d intervals from 25 to 60 DAA.
Anatomy
Intact seeds were hand sectioned, and decoated seeds were embedded in paraffin (Jensen, 1962) and thin sectioned (10 μm) with a microtome. Decoating was done manually with forceps without damaging the endosperm envelope or embryo. Sections on glass slides were stained with 0.05% aniline blue in 0.1 m phosphate buffer (pH 8.2) or a Sudan III and IV mixture (equal volumes of saturated solutions in 70% ethanol) for approximately 5 min and observed using light microscopy. To decrease unspecific staining by aniline blue, toluidine blue O (0.5% in 0.1 m phosphate buffer, pH 7.0) was used after aniline blue staining. To confirm the specificity of the blue staining for callose, mature seeds were hand sectioned, stained with 0.001% synthetic aniline blue fluorochrome (Sirofluor, Biosupplies Australia, Parkville, Australia) for 20 min, and observed with a fluorescence microscope (Axiovert 100, Zeiss) with a fluorescein isothiocyanate filter (excitation, 470 nm; emission, 515 nm; beam splitter, 505DCLP).
For SEM, seeds were freeze-dried for 3 d and mounted on aluminum stubs. For cross-sectional views, endosperm envelopes fractured during freeze-drying were positioned with a fracture plane on an aluminum stub. Samples were sputter coated with 30-nm gold (SEM coating system, Bio-Rad) and observed with a scanning electron microscope (model DS 130, International Scientific Instruments, Top Con Technologies, Inc., Paramus, NJ) at 10 kV.
OD and Water-Imbibition Kinetics
To induce OD, decoated seeds were killed by boiling them in water for 3 min, and were then incubated on water-saturated blotter paper. To observe the imbibition kinetics, viable or boiled decoated seeds with or without chloroform treatment (see below) were incubated on water-saturated blotter paper in Petri dishes at 30°C. At frequent intervals, seeds were briefly blotted on lint-free tissues for 30 s, weighed, and returned to the Petri dish. Observations were terminated when live seeds completed germination (radicle emergence).
Chloroform and Enzyme Treatment
To remove the outer lipid-containing layer, decoated seeds were dipped in chloroform for 3 min, rinsed in water, and incubated on water-saturated germination paper overnight. To treat with β-1,3-glucanase, decoated seeds exhibiting OD (with or without chloroform treatment) were incubated on germination paper saturated with 3 × 10−4 units of endo-β-1,3-glucanase (purified from Helix pomatia; Fluka, Buchs, Switzerland) in 0.1 m citrate-phosphate buffer (pH 5.5). After the seeds had lost OD, they were examined using light microscopy or SEM as described above.
RESULTS
Anatomy of the Endosperm Envelope
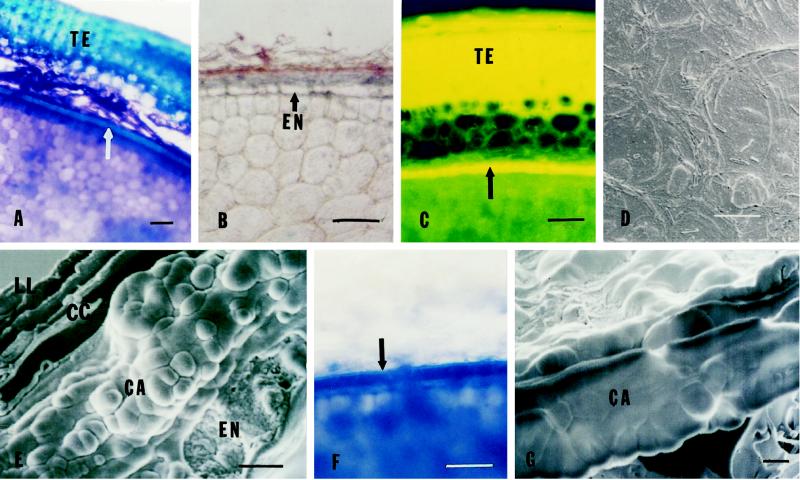
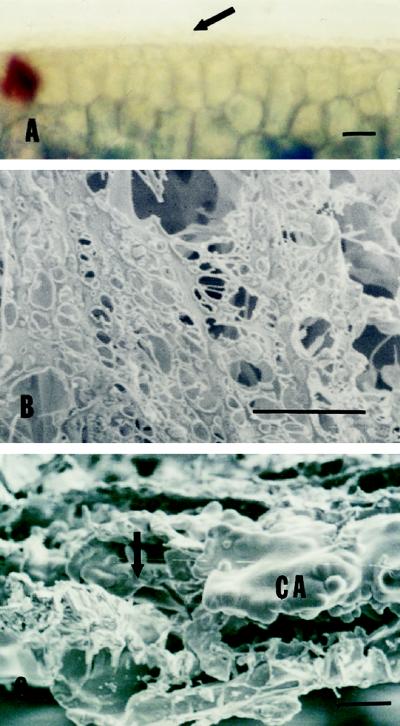
Hand-sectioned muskmelon seeds showed dark-blue staining (aniline blue) of the endosperm envelope below the spongy tissue of the seed coat (Fig. 1A, arrow). Thin sections of decoated, paraffin-embedded seeds showed specific gray-blue staining with aniline blue associated with the endosperm envelope outside of a single layer of rectangular endosperm cells (Fig. 1B). The specificity of the blue staining for callose observed by light microscopy was confirmed by fluorescence microscopy using synthetic aniline blue fluorochrome (Stone et al., 1984). The endosperm envelope showed yellow fluorescence only when stained with this fluorochrome (Fig. 1C, arrow), whereas the testa and thin lipid layer of the endosperm envelope showed autofluorescence in the presence or absence of the dye (data not shown). Only triploid nuclei were found when the nDNA contents of entire envelope tissues were analyzed using flow cytometry, demonstrating that all living cells originated from the endosperm (data not shown). When viewed using SEM, the surface of the endosperm envelope was smooth and waxy in appearance without obvious cellular structure (Fig. 1D), in contrast to the cellular outlines clearly visible in back-illuminated light-microscope surface views (Welbaum and Bradford, 1990). In cross-view (Fig. 1E), the outer lipid layer appeared to consist of plate-like or waxy sheets and possibly one or two layers of crushed cells. Beneath this was a thick layer composed of globules that had a “foamy” appearance and no cellular compartmentation or contents. Below the globular layer was a single layer of rectangular endosperm cells.
Figure 1.
A, Cross-section of a dry muskmelon seed stained with 0.05% aniline blue and 0.5% toluidine blue O. The testa (TE) is at the top, covering a spongy layer. The endosperm envelope (arrow) below the spongy layer shows an outer blue-stained layer. The bar represents 10 μm. B, Thin section of paraffin-embedded, decoated dry muskmelon seed stained with 0.05% aniline blue and a saturated mixture of Sudan III and IV. An aniline blue-staining layer (gray-blue) is adjacent to a layer of rectangular endosperm cells (EN), and a Sudan-staining layer (orange-red) is present on top of the aniline- blue-staining layer. The bar represents 10 μm. C, Similar to B, but stained with synthesized aniline blue fluorochrome and viewed by fluorescence microscopy with a fluorescein isothiocyanate filter. The bright-yellow fluorescent band (arrow) identifies the location of callose in the endosperm envelope. The testa (TE) shows strong autofluorescence that was not dependent on the presence of the fluorochrome (data not shown). The bar represents 10 μm. D, Surface of the endosperm envelope of muskmelon seeds with OD viewed by SEM. The bar represents 10 μm. E, Freeze-fractured cross-section of the muskmelon endosperm envelope viewed by SEM. Above the inner rectangular endosperm cells (EN) is a globular, callosic layer having a “foamy” appearance (CA). Some crushed cells (CC) appear to be present in the outer lipid-containing layer (LL). The bar represents 2 μm. F, Removal of the lipid-containing layer by chloroform treatment. Decoated muskmelon seeds were dipped in chloroform for 3 min and hand sectioned. Sections were stained with aniline blue and Sudan III and IV. The Sudan-staining layer was removed but the aniline-blue-staining layer was intact after chloroform treatment (compare with Fig. 2D). The bar represents 10 μm. G, Freeze-fractured cross-section of the chloroform-treated muskmelon endosperm envelope viewed by SEM. The globular layer remained but the waxy outer layer was removed by the chloroform treatment. The bar represents 2 μm.
Because the outermost layer of the envelope stained orange-red with Sudan III and IV, indicating a lipid-containing component (Fig. 1B), we tested whether this lipid layer could be removed by dipping decoated seeds in chloroform. This treatment removed the Sudan-staining layer (Fig. 1F, arrow; compare with Fig. 2D, arrow), but did not affect aniline blue staining of the callose layer (Fig. 1F). This was also evident in SEM, in which the chloroform treatment removed the outer layers and exposed the globular, foam-like layer covering the endosperm cells (Fig. 1G; compare surface with that in Fig. 1D). Because suberin is not soluble in chloroform, the Sudan-staining material is apparently not composed of suberin. Thus, the endosperm envelope of muskmelon contains a single line of endosperm cells covered by a thick, globular callosic layer, which in turn is covered by what appears to be a waxy outer coating and some crushed cell remnants possibly derived from the perisperm or testa.
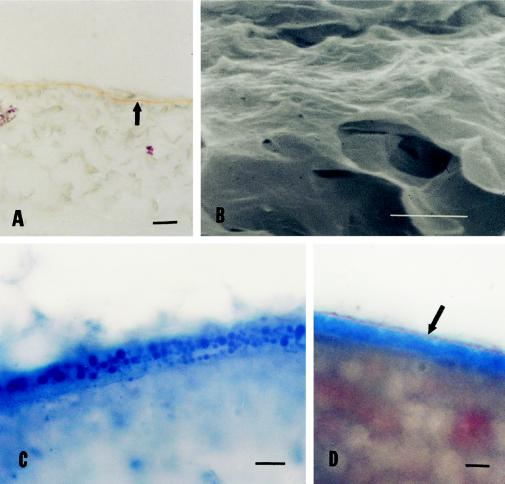
Figure 2.
A, Cross-section of developing muskmelon seeds at 25 DAA. The seeds were decoated carefully, sectioned, and stained with aniline blue and Sudan III and IV. Only Sudan staining (arrow) was detected on the endosperm envelope. B, Freeze-fractured cross-section of endosperm envelope 25 DAA viewed by SEM. Only the plate-like or waxy appearance was evident; the globular layer present in mature envelopes was absent. C, Cross-section of developing muskmelon seeds at 35 DAA. Decoated seeds were hand sectioned and stained with aniline blue. Aniline-blue-staining vesicles were observed in the endosperm envelope. These vesicles were not present in other developmental stages. D, Cross-section of mature muskmelon seeds (55 DAA). Decoated seeds were hand sectioned and stained with aniline blue and Sudan III and IV. The orange Sudan-staining lipid layer (arrow) was observed on top of the thick, aniline-blue-staining layer. The bar in each panel represents 5 μm.
Relationship between Semipermeability and Callose Deposition in Developing Muskmelon Seeds
Early in development (25 DAA), muskmelon seeds are not capable of OD, but their endosperm envelopes become semipermeable at approximately 40 DAA (Welbaum and Bradford, 1990; data not shown). However, endosperm envelopes at 25 DAA can be stained with Sudan III and IV (Fig. 2A, arrow) but not with aniline blue (Fig. 2A). Using SEM, the globular layer was not observed at 25 DAA (Fig. 2B). At 30 DAA, the endosperm envelope did not stain with aniline blue (data not shown), but at 35 DAA, aniline blue-staining vesicles were evident (Fig. 2C). By 40 DAA, a thick aniline blue-staining callosic layer was present (data not shown), coinciding with the acquisition of semipermeability, and at 50 DAA, virtually all seeds exhibited both semipermeability and the anatomical characteristics of mature seeds (Fig. 2D).
Role of the Lipid Layer in Water Absorption and Semipermeability
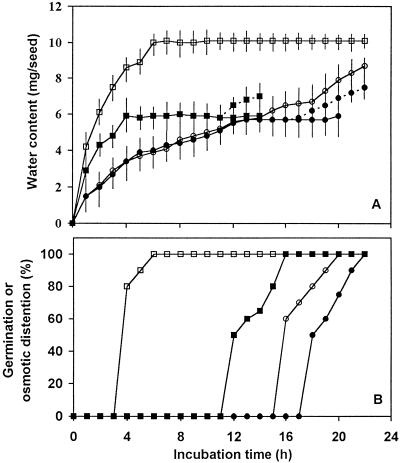
Decoated muskmelon seeds showed a typical triphasic imbibition time course, with an initial phase of rapid water uptake (0–12 h) followed by a plateau phase of relatively constant water content (12–18 h) (Fig. 3A). Radicle emergence occurred between 18 and 22 h (Fig. 3B), and was accompanied by additional water uptake associated with embryo growth (Fig. 3A). Boiled seeds exhibited identical initial water- absorption kinetics, but did not attain a water-content plateau; instead, they continued to absorb water and became osmotically distended (Fig. 3). Removal of the outer lipid layer from viable decoated seeds by dipping them in chloroform hastened the initial uptake of water and subsequent radicle emergence (Fig. 1, F and G), but did not affect the plateau water content (Fig. 3). When boiled seeds were treated with chloroform, water absorption was even more rapid and OD was achieved within 4 to 6 h, 12 h earlier than for boiled seeds with the lipid layer present (Fig. 3). Thus, the outer lipid layer slows the rate of initial water uptake, but is not required for semipermeability of the endosperm envelope.
Figure 3.
A, Water-absorption kinetics of muskmelon seeds. Decoated seeds were incubated on water-saturated blotters at 30°C with or without chloroform treatment (3-min dip) and/or boiling. Dotted lines for control and chloroform-treated seeds indicate germinated seeds. •, Control seeds; ▪, chloroform-treated seeds; ○, boiled seeds; □, boiled and chloroform-treated seeds. The bars represent ± se (n = 20). B, Percentages of germination (control [•] and chloroform-treated [▪]) and OD (boiled [○] and boiled and chloroform-treated [□]) of decoated muskmelon seeds.
Role of the Callose Layer in Semipermeability
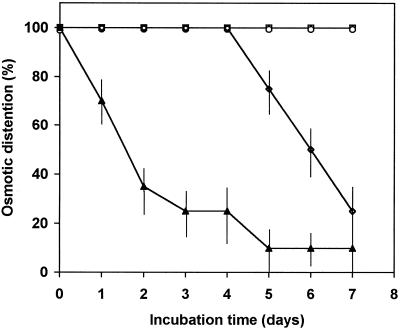
Callose (β-1,3-glucan) is hydrolyzed by endo-β-1,3-glucanase, so seeds exhibiting OD were incubated in commercially purified enzyme to determine whether the callose layer would be degraded and whether this would affect semipermeability. Decoated melon seeds were boiled and incubated on water-saturated blotting paper to induce OD, which was maintained for at least 7 d regardless of whether the outer lipid layer was present (Fig. 4). When allowed to imbibe on solutions containing β-1,3-glucanase, seeds began to lose OD after 5 d, and by 7 d of incubation, only 20% of the seeds maintained semipermeability (Fig. 4). A chloroform dip before β-1,3-glucanase treatment accelerated the loss of OD (Fig. 4), suggesting that the lipid layer restricts access by the enzyme to the callose layer.
Figure 4.
Loss of OD after β-1,3-glucanase treatment. Decoated muskmelon seeds were killed in boiling water for 3 min and incubated overnight on blotters saturated with distilled water to induce OD. For chloroform treatments, boiled seeds with OD were dipped into a chloroform solution for 3 min and rinsed with water. Seeds in buffer (□) or in buffer after chloroform treatments (○) maintained OD for at least 7 d. The presence of β-1,3-glucanase in the buffer (⋄) initiated the loss of OD at 5 d of incubation or after only 1 d in seeds pretreated in chloroform (▴). The bars represent ± se from three independent experiments of 20 seeds each.
Endosperm envelopes from seeds that had been treated with both chloroform and β-1,3-glucanase and had lost OD did not stain with aniline blue or Sudan III and IV (Fig. 5A; compare with Fig. 2D). The β-1,3-glucanase treatment digested the globular layer outside of the endosperm cells, leaving only a thin, porous network in the surface view (Fig. 5B). A fractured cross-section of the β-1,3-glucanase-treated endosperm envelope showed that the callose layer had essentially disappeared (Fig. 5C, arrow), leaving only a few isolated regions where the globular material could still be found (Fig. 5C, CA). The effects of the enzyme treatment on OD (Fig. 4) and on callose degradation (compare Fig. 1, A, B, E, and G, with Fig. 5, A–C) leave little doubt that the callose layer is responsible for the semipermeability of the endosperm envelope.
Figure 5.
A, Cross-section of chloroform- and β-1,3-glucanase-treated muskmelon seeds. Seeds with OD were treated with chloroform and incubated for 36 h on blotters saturated with solution containing β-1,3-glucanase. Seeds that lost OD were hand sectioned and stained with aniline blue and Sudan III and IV. The Sudan-staining lipid layer and the aniline-blue-staining callose layer were removed (arrow; compare with Fig. 2D). B, Surface of chloroform- and β-1,3-glucanase-treated muskmelon endosperm envelope viewed by SEM (compare with Fig. 1G). C, Freeze-fractured cross-section of chloroform- and β-1,3-glucanase-treated muskmelon endosperm envelope viewed by SEM. The cross-section shows that the globular layer is removed (arrow; compare with Fig. 1G), leaving only a few remnants in some areas (CA). The bar in each panel presents 5 μm.
DISCUSSION
Although it was hypothesized long ago that callose may act as a “molecular filter” in plants by altering the gel-filtration properties of the cell wall (Heslop-Harrison, 1964), no direct in vivo evidence to support this hypothesis has been reported. Extracted callose has a high water-holding capacity (Barskaya and Balina, 1971; Vithanage et al., 1980), and low permeability to small molecules of water-swollen callose (Eschrich and Eschrich, 1964, cited by Stone and Clarke, 1992). Here we present evidence that a thick deposit of callose covering the endosperm envelope of muskmelon seeds serves as a semipermeable molecular filter that readily allows movement of water but not of solutes.
The thick outer wall of the muskmelon endosperm envelope can be stained with both aniline blue dye and aniline blue fluorochrome (Fig. 1, A–C, F), which are specific for β-1,3-glucans, and is also virtually completely digested by β-1,3-glucanase (Fig. 5, A–C). Thus, the thick globular layer of the outer endosperm wall (Fig. 1, E and G) is composed largely of callose, although the presence of other components in addition to callose cannot be excluded. The β-1,3-glucanase treatment also causes loss of OD (Fig. 4), providing direct evidence that the callose layer is responsible for semipermeability. This conclusion is further supported by the simultaneous deposition of callose, the development of semipermeability (Welbaum and Bradford, 1990), and the increase in the energy required to penetrate the endosperm envelope (Oluoch, 1996) at around 40 DAA during seed development.
Callose generally occurs in plant cells as a component of specialized wall or wall-associated structures at certain stages of growth. Callose has been detected in or on cell walls of various tissues, including cell plates, cotton seed fibers, pollen grain cell walls, innermost pollen tube walls, endosperms, sieve plates, and abscission zones (Esau, 1948; Currier, 1957; Heslop-Harrison, 1964; Scott et al., 1967; Morrison and O'Brien, 1976; Waterkeyn, 1981; Stone and Clarke, 1992). Callose deposition is also induced locally by wounding, stress, and fungal or viral infection (Currier, 1957; Esau and Cronshaw, 1967; Coffey, 1976). Despite the widespread occurrence of callose, its general function is not well understood (for review, see Stone and Clarke, 1992). It may serve as a matrix for deposition of other cell wall materials, as in developing cell plates and sieve-plate pores; as a cell wall-strengthening material, as in cotton seed hairs (Maltby et al., 1979) and pollen; as a sealing or plugging material at the plasma membrane of pit fields, plasmodesmata, and sieve-plate pores (Eschrich, 1975); as a mechanical obstruction to growth of fungal hyphae; or as a special permeability barrier, as in pollen mother cell walls and muskmelon endosperm envelopes (Heslop-Harrison, 1964; this report). In addition, the degree of polymerization, age, and thickness of the deposits may vary the physical properties of callose (Stone and Clarke, 1992). For example, callose deposits in clover seed coats apparently prevent water absorption (Bhalla and Slattery, 1984), in contrast to the high water permeability of the muskmelon endosperm envelope (Fig. 3A).
Lipid-containing or suberized cell walls (stained with Sudan dye) are frequently associated with semipermeable regions (Fig. 1B; Harrington and Crocker, 1923; Johann, 1942; O'Brien and Carr, 1970; Cochran, 1983; Welbaum and Bradford, 1990; Jacobsen et al., 1992; Welbaum et al., 1992; Beresniewicz et al., 1995a). However, the lipid-containing outer layer of the melon endosperm envelope slows water uptake but is not responsible for semipermeability (Fig. 3). Whether callose is also associated with other “suberized” apoplastic membranes, such as the Casparian strip of the root endodermis or the sheaths of sugarcane vascular bundles, is unknown. Cucumber, zucchini, watermelon, and barley seeds, all of which exhibit semipermeability, also have a thick aniline-blue-staining layer inside of the seed coat (data not shown). However, some seeds have semipermeable layers that do not contain callose (Beresniewicz et al., 1995a; K.-O. Yim and K.J. Bradford, unpublished results for lettuce endosperm envelopes). Because embryos often leak solutes upon initial imbibition (Simon and Mills, 1983), a semipermeable envelope would prevent loss of solutes to the environment until the embryo is capable of reabsorbing them before initiation of radicle growth. There are many additional locations in the plant where the ability to restrict solute movement in the apoplast while permitting water continuity and flow would be advantageous, particularly in developing seeds (Bradford, 1994), in the root (Steudle and Peterson, 1998), and in the vascular system (Canny, 1995).
The large amounts of callose present and its specific deposition only on the outer side of the endosperm envelope (Fig. 1, B, C, and E) raise intriguing questions about the mechanism of callose synthesis in these cells. Synthesis of callose via a well-characterized plasma membrane-bound callose synthase is usually activated temporally by specific signals such as wounding, infection, or other stresses (for review, see Delmer and Amor, 1995). The plasma membrane callose synthase is activated by micromolar concentrations of Ca2+ and β-glucoside (Hayashi et al., 1987), suggesting that transient increases in Ca2+ concentration caused by cell perturbation, such as by microbe infection or wounding, induce callose deposition outside of the plasma membrane. Although less well characterized, there is some evidence for a Golgi-vesicle-mediated callose-synthesis system in developmentally regulated callose-rich tissues such as pollen tube walls, pollen tube plugs, and developing cell plates. In pollen tubes callose synthase activity was associated with Golgi vesicles (Helsper et al., 1977) and showed little dependence on Ca2+ (Schlüpmann et al., 1993; Li et al., 1997). Callose was detected by immunogold labeling in Golgi vesicles, which were concentrated at the tips of germinating pollen tubes of camellia (Hasegawa et al., 1996). However, Li et al. (1997) proposed that inactive callose synthase is secreted in vesicles at the tips of tobacco pollen tubes and is activated upon insertion into the plasma membrane. When callose accumulation occurred at about 35 DAA in the muskmelon endosperm envelope, discrete vesicles or globules were stained with aniline blue (Fig. 2C), consistent with the globular appearance of the callose layer (Fig. 1, E and G). However, more detailed studies of the callose synthesis and deposition process in muskmelon endosperm envelopes are required to determine the mechanism by which the callose layer is formed.
In conclusion, we have demonstrated that the semipermeability of the muskmelon endosperm envelope is caused by a callose-containing layer deposited outside of the outer walls of the endosperm cells. A chloroform-soluble waxy layer outside of the callosic layer delays water uptake but is not required for semipermeability. These results directly confirm, for the first time to our knowledge, the long-standing hypothesis that callose may act as a molecular sieve. They also show that the presence of Sudan-stainable material does not in itself imply impermeability to water. The existence of semipermeable apoplasts resulting from the deposition of callose (or other materials) in the cell wall provides options for controlling water and solute transfer in many parts of the plant and at critical stages of development.
ACKNOWLEDGMENTS
The authors express their appreciation to Dr. Deborah Delmer (University of California, Davis) for critical reading and constructive comments on the manuscript and for technical assistance with the fluorescence microscopy. The assistance of Dr. Sunitha Gurusinghe with the flow cytometry of endosperm nuclei is gratefully acknowledged.
Abbreviations:
- DAA
days after anthesis
- OD
osmotic distention
- SEM
scanning electron microscopy
Footnotes
This work was supported by the U.S. Department of Agriculture Binational Agricultural Research and Development Fund (grant no. US-2422-94).
LITERATURE CITED
- Barskaya EI, Balina NV. The role of callose in plant anthers. Fiziol Rast. 1971;18:716–721. [Google Scholar]
- Beresniewicz MM, Taylor AG, Goffinet MC, Koeller WD. Chemical nature of a semipermeable layer in seed coats of leek, onion (Liliaceae), tomato and pepper (Solanaceae) Seed Sci Technol. 1995a;23:135–145. [Google Scholar]
- Beresniewicz MM, Taylor AG, Goffinet MC, Terhune BT. Characterization and location of a semipermeable layer in seed coats of leek and onion (Liliaceae), tomato and pepper (Solanaceae) Seed Sci Technol. 1995b;23:123–134. [Google Scholar]
- Bhalla PL, Slattery HD. Callose deposits make clover seeds impermeable to water. Ann Bot. 1984;53:125–128. [Google Scholar]
- Bradford KJ. Water stress and the water relations of seed development: a critical review. Crop Sci. 1994;34:1–11. [Google Scholar]
- Brown R. On the existence of a semi-permeable membrane enclosing the seeds of some of the Gramineae. Ann Bot. 1907;21:79–87. [Google Scholar]
- Canny MJ. Apoplastic water and solute movement: new rules for an old space. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:215–236. [Google Scholar]
- Cochran MP. Morphology of the crease region in relation to assimilate uptake and water loss during caryopsis development in barley and wheat. Aust J Plant Physiol. 1983;10:473–491. [Google Scholar]
- Coffey MD. Flax rust resistance involving the K gene: an ultrastructural survey. Can J Bot. 1976;54:1443–1457. [Google Scholar]
- Currier HB. Callose substance in plant cells. Am J Bot. 1957;44:478–488. [Google Scholar]
- Delmer DP, Amor Y. Cellulose biosynthesis. Plant Cell. 1995;7:987–1000. doi: 10.1105/tpc.7.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau K. Phloem structure in the grapevine, and its seasonal changes. Hilgardia. 1948;18:217–296. [Google Scholar]
- Esau K, Cronshaw J. Relation of tobacco mosaic virus to the host cells. J Cell Biol. 1967;33:665–678. doi: 10.1083/jcb.33.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschrich W. Sealing systems in phloem. In: Zimmermann MH, Milburn JA, editors. Encyclopedia of Plant Physiology, Vol 1: Transport in Plants. I. Phloem Transport. Berlin: Springer-Verlag; 1975. pp. 39–56. [Google Scholar]
- Eschrich W, Eschrich B. Das verhalten isolierter callose gegenüber wässrigen lösungen. Ber Dtsch Bot Ges. 1964;77:329–331. [Google Scholar]
- Gola G. Richerche sui rapporti tra i tegumenti seminali e le soluzioni saline. Annali di Botanica. 1905;3:59–100. [Google Scholar]
- Harrington GT, Crocker W. Structure, physical characteristics, and composition of the pericarp and integument of Johnson grass seed in relation to its physiology. J Agric Res. 1923;23:193–222. [Google Scholar]
- Hasegawa Y, Nakamura S, Nakamura N. Immunocytochemical localization of callose in the germinated pollen of Camellia japonica. Protoplasma. 1996;194:133–139. [Google Scholar]
- Hayashi T, Read SM, Bussell J, Thelen M, Lin FC, Brown RM, Delmer DP. UDP-glucose:(1,3)-β-glucan synthases from mung bean and cotton. Differential effects of Ca2+ and Mg2+ on enzyme properties and on macromolecular structure of the glucan product. Plant Physiol. 1987;83:1054–1062. doi: 10.1104/pp.83.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsper JPFG, Veerkamp JH, Sassen MMA. β-Glucan synthetase activity in Golgi vesicles of Petunia hybrida. Planta. 1977;133:303–308. doi: 10.1007/BF00380693. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J (1964) Cell walls, cell membranes and protoplasmic connections during meiosis and pollen development. In HF Linskens, ed, Pollen Physiology and Fertilization. North- Holland, Amsterdam, pp 29–47
- Hill HJ, Taylor AG. Relationship between viability, endosperm integrity and imbibed lettuce seed density and leakage. HortScience. 1989;24:814–816. [Google Scholar]
- Jacobsen JS, Fisher DG, Maretzki A, Moore PH. Anatomy of sugarcane stem in relation to phloem unloading and sucrose storage. Bot Acta. 1992;18:959–969. [Google Scholar]
- Jensen WA (1962) Botanical Histochemistry: Principles and Practice. WH Freeman, San Francisco, CA, pp 35–99
- Johann H. Origin of the suberized semipermeable membrane in the caryopsis of maize. J Agric Res. 1942;64:275–281. [Google Scholar]
- Kotowski F. Semipermeability of seed coverings and stimulation of seeds. Plant Physiol. 1927;2:177–186. doi: 10.1104/pp.2.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Bacic A, Read SM. Activation of pollen tube callose synthase by detergents. Evidence for different mechanisms of action. Plant Physiol. 1997;114:1255–1265. doi: 10.1104/pp.114.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltby D, Carpita NC, Montezinos D, Kulow C, Delmer DP. β-1,3-Glucan in developing cotton fibers. Structure, localization and relationship of synthesis to that of secondary wall cellulose. Plant Physiol. 1979;63:1158–1164. doi: 10.1104/pp.63.6.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison IN, O'Brien TP. Cytokinesis in the developing wheat grain: division with and without a phragmoplast. Planta. 1976;130:57–67. doi: 10.1007/BF00390845. [DOI] [PubMed] [Google Scholar]
- O'Brien TP, Carr DJ. A suberized layer in the cell walls of the bundle sheath of grasses. Aust J Biol Sci. 1970;23:275–287. [Google Scholar]
- Oluoch MO (1996) Effects of priming and stage of development on vigor and longevity of muskmelon (Cucumis melo L.) seeds. PhD dissertation. Virginia Polytechnic Institute and State University, Blacksburg, Virginia
- Oparka KJ, Gates P. Transport of assimilates in the developing caryopsis of rice (Oryza sativa L.): the pathways of water and assimilated carbon. Planta. 1981;152:388–396. doi: 10.1007/BF00385354. [DOI] [PubMed] [Google Scholar]
- Peoples MB, Pate JS, Atkins CA, Murray DR. Economy of water, carbon and nitrogen in the developing cowpea fruit. Plant Physiol. 1985;77:142–147. doi: 10.1104/pp.77.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüpmann H, Bacic A, Read SM. Planta. 1993;191:470–481. [Google Scholar]
- Scott PC, Miller LW, Webster BD, Leopold AC. Structural changes during bean leaf abscission. Am J Bot. 1967;54:730–734. [Google Scholar]
- Simon EW, Mills LK (1983) Imbibition, leakage and membranes. In C Nozzolillo, PJ Lea, FA Loewus, eds, Mobilization of Reserves in Germination. Plenum Press, New York, pp 9–27
- Singh B. Studies on the structure and development of seeds of Cucurbitaceae. Phytomorphology. 1953;3:224–239. [Google Scholar]
- Steudle E, Peterson CA. How does water get through roots? J Exp Bot. 1998;49:775–788. [Google Scholar]
- Stone BA, Clarke AE (1992) Chemistry and Biology of (1→3)-β-Glucans. La Trobe University Press, Victoria, Australia, pp 355–429
- Stone BA, Evans NA, Bonig I, Clarke AE. The application of Sirofluor, a chemically defined fluorochrome from aniline blue for the histochemical detection of callose. Protoplasma. 1984;122:191–195. [Google Scholar]
- Taylor AG, Beresniewicz MM, Goffinet MC. Semipermeable layer in seeds. In: Black EM, Murdoch AJ, Hong TD, editors. Basic and Applied Aspects of Seed Biology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1997. pp. 429–436. [Google Scholar]
- Thornton ML. Seed dormancy in watermelon Citrullus vulgaris Shrad. Proceedings of the Association of Official Seed Analysts. 1968;58:80–84. [Google Scholar]
- Vithanage HIMV, Gleeson PA, Clarke AE. The nature of callose produced during self-pollination in Secale cereale. Planta. 1980;148:498–509. doi: 10.1007/BF00552666. [DOI] [PubMed] [Google Scholar]
- Waterkeyn L. Cytochemical localization and function of the 3-linked glucan callose in the developing cotton fibre cell wall. Protoplasma. 1981;106:49–67. [Google Scholar]
- Welbaum GE, Bradford KJ. Water relations of seed development and germination in muskmelon (Cucumis melo L.) I. Water relations of seed and fruit development. Plant Physiol. 1988;86:406–411. doi: 10.1104/pp.86.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welbaum GE, Bradford KJ. Water relations of seed development and germination in muskmelon (Cucumis melo L.) IV. Characteristics of the perisperm during seed development. Plant Physiol. 1990;92:1038–1045. doi: 10.1104/pp.92.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welbaum GE, Meinzer FC. Compartmentation of solutes and water in developing sugarcane stalk tissue. Plant Physiol. 1990;93:1147–1153. doi: 10.1104/pp.93.3.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welbaum GE, Meinzer FC, Grayson RL, Thornham KD. Evidence for and consequences of a barrier to solute diffusion between the apoplast and vascular bundles in sugarcane stalk tissue. Aust J Plant Physiol. 1992;19:611–623. [Google Scholar]
- Wolswinkel P. Transport of nutrients into developing seeds: a review of physiological mechanisms. Seed Sci Res. 1992;2:59–73. [Google Scholar]