Abstract
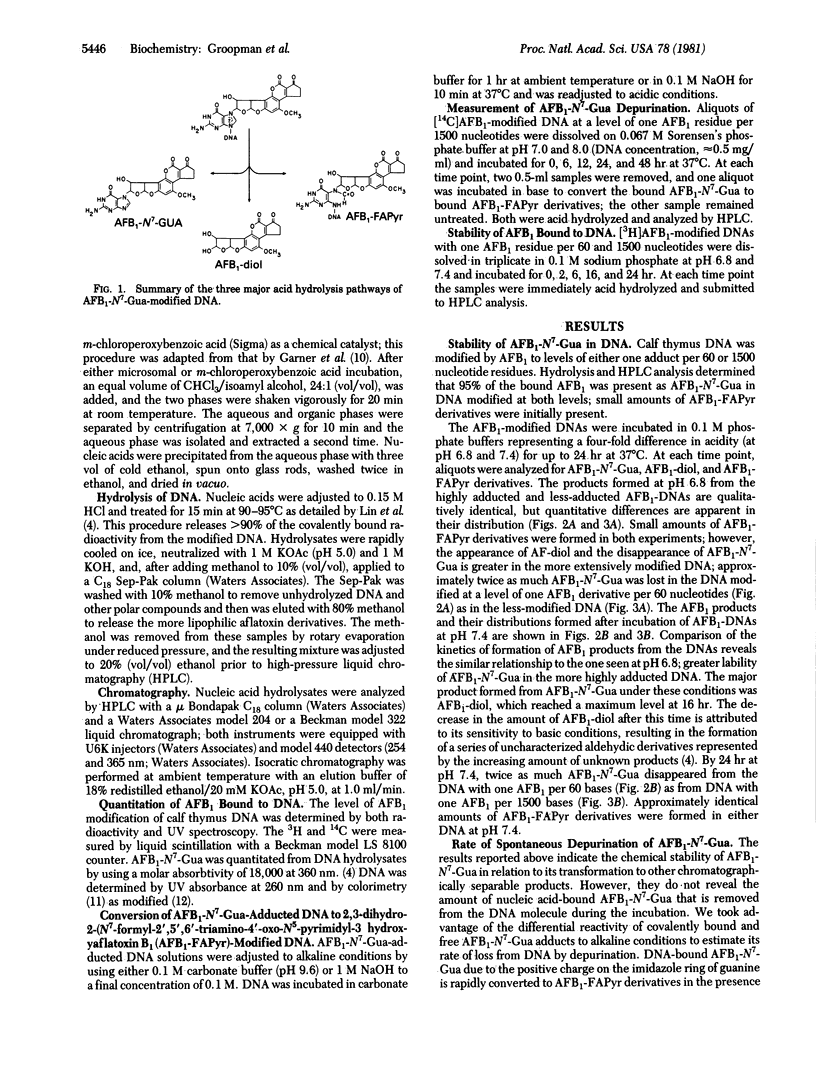
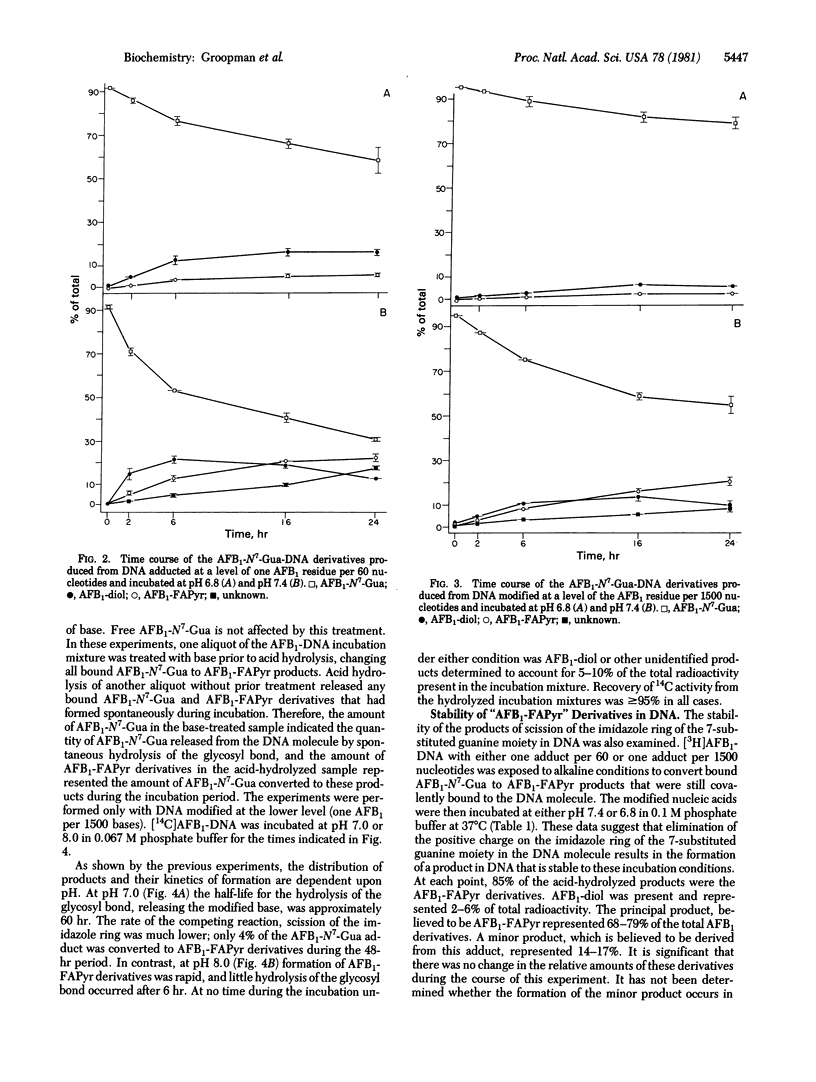
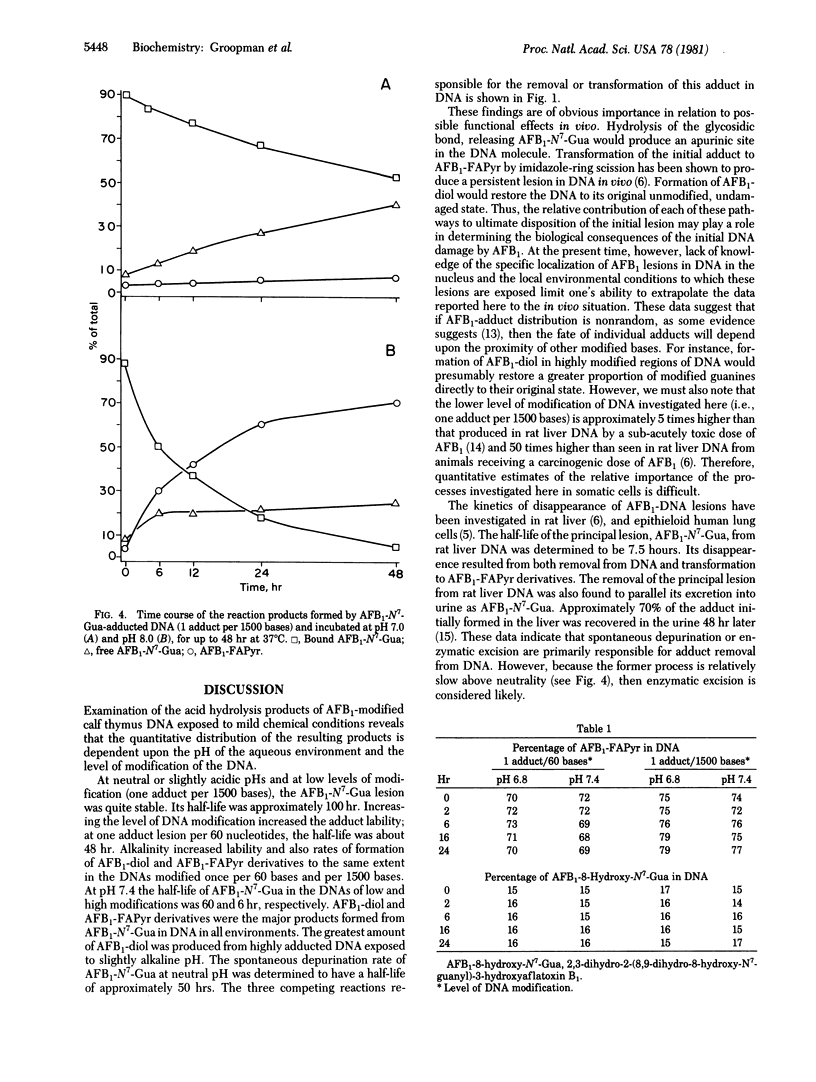
The chemical stability of aflatoxin B1 bound to calf thymus DNA was studied over a 48-hour exposure to phosphate buffers at pH 6.8-8.0 (37 degrees C). During this time, aliquots of the aflatoxin B1-modified DNA were acid-hydrolyzed and analyzed for the presence of 2,3-dihydro-2-(N7-guanyl)-3-hydroxyflatoxin B1, 2,3-dihydro-2,3-dihydroxy-aflatoxin B1, and the tentatively identified, 2,3-dihydro-2-(N5-formyl-2',5',6'-triamino-4'-oxo-N5-pyrimidyl-3-hydroxyflatoxin B1 and 2,3-dihydro-2-(8,9-dihydro-8-hydroxy-N7-guanyl)-3-hydroxyaflatoxin B1. Initial experiments determined the stability of 2,3-dihydro-2-(N7-guanyl)-3-hydroxyaflatoxin B1 in DNA at levels of modification of one residue per 60 and 1500 nucleotides. The acid-hydrolysis products obtained from these modified nucleic acids were qualitatively similar, but their proportional concentrations were different. These quantitative differences were dependent upon both pH and the initial level of modification of the DNA. During the first 6 hr of incubation, under all conditions examined, the formation of 2,3-dihydro-2,3-dihydroxyaflatoxin B1 was responsible for the initial decrease of the 2,3-dihydro-2-(N7-guanyl)-3-hydroxyaflatoxin B1 adduct in DNA. After 48 hr of incubation at pH 7.0, the major reaction of the modified DNA was depurination of the 2,3-dihydro-2-(N7-guanyl)-3-hydroxyaflatoxin B1 adduct. However, at pH 8.0, the predominant reaction product formed in 48 hr was the putative 2,3-dihydro-2-(N5-formyl-2',5',6'-triamino-4'-oxo-N5-pyrimidyl)-3-hydroxy-aflatoxin B1. The putative DNA-bound products resulting from the elimination of the positive charge in the imidazole ring of the aflatoxin-N7-guanine adduct [2,3-dihydro-2-(N5-formyl-2',5',6'-triamino-4'-oxo-N5-pyrimidyl)-3-hydroxy-aflatoxin B1 and 2,3-dihydro-2-(8,9-dihydro-8-hydroxy-N7-guanyl)-3-hydroxyaflatoxin B1] were found to be stable in DNA for at least 24 hr at both pH 6.8 and 7.4. Taken together with observed patterns of stability of aflatoxin B1 adducts in vivo, these observations strongly suggest the involvement of enzymatic repair processes in removal of the N7-guanyl adduct and also emphasize the possible biological significance of the stable imidazole ring-opened adduct.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R. A., Essigmann J. M., Wogan G. N. Excretion of an aflatoxin-guanine adduct in the urine of aflatoxin B1-treated rats. Cancer Res. 1981 Feb;41(2):650–654. [PubMed] [Google Scholar]
- Croy R. G., Wogan G. N. Temporal patterns of covalent DNA adducts in rat liver after single and multiple doses of aflatoxin B1. Cancer Res. 1981 Jan;41(1):197–203. [PubMed] [Google Scholar]
- D'Andrea A. D., Haseltine W. A. Modification of DNA by aflatoxin B1 creates alkali-labile lesions in DNA at positions of guanine and adenine. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4120–4124. doi: 10.1073/pnas.75.9.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essigmann J. M., Croy R. G., Nadzan A. M., Busby W. F., Jr, Reinhold V. N., Büchi G., Wogan G. N. Structural identification of the major DNA adduct formed by aflatoxin B1 in vitro. Proc Natl Acad Sci U S A. 1977 May;74(5):1870–1874. doi: 10.1073/pnas.74.5.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner R. C., Martin C. N., Smith J. R., Coles B. F., Tolson M. R. Comparison of aflatoxin B1 and aflatoxin G1 binding to cellular macromolecules in vitro, in vivo and after peracid oxidation; characterisation of the major nucleic acid adducts. Chem Biol Interact. 1979 Jun;26(1):57–73. doi: 10.1016/0009-2797(79)90093-0. [DOI] [PubMed] [Google Scholar]
- Groopman J. D., Busby W. F., Jr, Wogan G. N. Nuclear distribution of aflatoxin B1 and its interaction with histones in rat liver in vivo. Cancer Res. 1980 Dec;40(12):4343–4351. [PubMed] [Google Scholar]
- Hertzog P. J., Lindsay Smith J. R., Garner R. C. A high pressure liquid chromatography study on the removal of DNA-bound aflatoxin B1 in rat liver and in vitro. Carcinogenesis. 1980 Sep;1(9):787–793. doi: 10.1093/carcin/1.9.787. [DOI] [PubMed] [Google Scholar]
- Lin J. K., Miller J. A., Miller E. C. 2,3-Dihydro-2-(guan-7-yl)-3-hydroxy-aflatoxin B1, a major acid hydrolysis product of aflatoxin B1-DNA or -ribosomal RNA adducts formed in hepatic microsome-mediated reactions and in rat liver in vivo. Cancer Res. 1977 Dec;37(12):4430–4438. [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Wang T. C., Cerutti P. A. Formation and removal of aflatoxin B1-induced DNA lesions in epithelioid human lung cells. Cancer Res. 1979 Dec;39(12):5165–5170. [PubMed] [Google Scholar]
- Wang T. V., Cerutti P. Spontaneous reactions of aflatoxin B1 modified deoxyribonucleic acid in vitro. Biochemistry. 1980 Apr 15;19(8):1692–1698. doi: 10.1021/bi00549a027. [DOI] [PubMed] [Google Scholar]



