Abstract
GnRH, produced in the hypothalamus, acts on pituitary gonadotropes to stimulate release of the gonadotropins LH and FSH. Reduced responsiveness of gonadotropes to GnRH is a primary cause of hypogonadotropic hypogonadism (HH), a disease characterized by gonadal dysfunction and low blood levels of gonadotropins. Loss-of-function mutations in the gene encoding the receptor for GnRH (GNRHR) are a common cause of HH. Sequencing of the GNRHR gene in patients with HH revealed mainly point mutations producing single amino acid substitutions that cause misfolding and misrouting of this G protein-coupled receptor. To generate a mouse model that mimics the human disease, we introduced a single amino acid substitution (E90K) into the mouse Gnrhr gene, which is identical to a known human recessive mutation. In humans, E90K causes severe HH by preventing formation of the E90-K121 salt bridge, which is essential for correct folding. In cell cultures, E90K causes misfolding that leads to almost complete retention by the protein quality control system and subsequent degradation. Here we report that the primary phenotype of mice homozygous for E90K is female infertility due to ovulation failure. Mutant males are fertile despite reduced gonadotropin levels and smaller testes. These results suggest decreased GnRH receptor signaling in the mutant animal, compared with wild type. Our findings suggest that a threshold level of GnRH receptor activity is required for ovulation.
Hypogonadotropic hypogonadism (HH) is a reproductive disease characterized by impaired gonadal function due to low blood levels of LH and FSH. Patients present with low serum testosterone or estrogen, infertility, and absence or reduction in secondary sexual characteristics. The reported incidence of idiopathic HH ranges from one in 10,000 to one in 86,000 (1, 2). Kallmann syndrome, in which HH is accompanied by anosmia, accounts for about 60% of cases. Mutations in the GnRH receptor (GnRHR) gene (GNRHR) are estimated to account for 40% of cases with familial HH and normal sense of smell. GNRHR mutations are also implicated in 16% of sporadic cases (3, 4).
The GnRHR is a seven-transmembrane G protein-coupled receptor (GPCR) expressed by the gonadotropes of the anterior pituitary. Sequencing of the GNRHR gene in patients with HH revealed that point mutations were the most common genetic lesion. These mutations produce changes in protein charge, gain or loss of Cys bridges, loss of a Pro residue, or premature truncation. GPCR harboring these types mutations are frequently misfolded and retained by the quality control system (QCS) in the endoplasmic reticulum (5). Interestingly, species-specific differences exist in the percentage of GnRHR that is expressed on the plasma membrane. For example, almost 100% of rodent GnRHR is expressed on the plasma membrane, whereas only about half of human GnRHR does so (6). This is due to specific amino acid sequence differences that either promote or inhibit correct folding of the GPCR. The 50% of human GnRHR that fails to fold correctly is captured by the QCS.
We previously reported the phenotype of mice harboring GnrhrL117P (7). The point mutation that resulted in the L117P amino acid substitution was generated in a random N-ethyl-N-nitrosourea mutagenesis screen. Because position 117 lies in the third transmembrane helix, introduction of the rigid right-angle bend associated with proline causes severe conformational change that is incompatible with its location within the lateral plane of the plasma membrane. Indeed, mutant L117P is not rescuable in cell transfection assays by a compound known to promote correct folding of other misfolded GnRHR mutants (7). Mice heterozygous for L117P were phenotypically normal and fertile; however, homozygotes exhibited severe HH with a complete block in gametogenesis. Although L117P mice were the first mouse model for HH caused by loss of GnRHR function, there is no report of human patients harboring this mutation. Furthermore, the L117P mutant produces much more severe misfolding than that predicted for most known human mutations. For these reasons, we chose to generate a mouse model of a known GNRHR mutation. To this end, we generated GnrhrE90K mice. Amino acid position 90 is located within the second transmembrane helix of GnRHR. Mutant E90K is orthologous to a known mutation found in patients with normosmic idiopathic HH (8).
In cell culture, human GnRHR E90K is retained in the endoplasmic reticulum and not trafficked to the plasma membrane. This mutant acts as a dominant negative to the wild-type receptor (9–11), and signal transduction can be restored by treating cells with compounds that act as molecular chaperones (pharmacological chaperones) that promote correct folding and trafficking to the plasma membrane (10). Furthermore, patients homozygous for E90K present with complete hypogonadism and do not respond to GnRH treatment (8). Men essentially remain in a prepubertal state, lacking sufficient testosterone production necessary to drive development of secondary sexual characteristics. Homozygosity for the E90K mutation has not been reported in women. Therefore, phenotypic analysis of this mutation in mice should provide insight into how protein misfolding mutations affect the hypothalamic-pituitary-gonadal axis in females.
Materials and Methods
Mice
GnrhrE90K mice were generated by introducing a point mutation (G to A) into the first exon of the Gnrhr gene at position 313 by homologous recombination in C57BL/6;129SvEvTac hybrid RJ2.2 mouse embryonic stem (ES) cells (12). The targeting vector was constructed by subcloning of PCR-amplified DNA from a C57BL/6J BAC clone (RPCI-23 library, BacPac Resources at Children's Hospital Oakland Research Institute, Oakland, CA) containing the mouse Gnrhr gene. The G to A substitution producing E90K was introduced into the 5′ arm of homology by site-directed mutagenesis. The targeting strategy is illustrated in Fig. 1A. ES cell clones that survived G418 selection were initially screened by PCR using Phusion polymerase (New England Biolabs, Beverly, MA) and a primer within the neomycin resistance gene expression cassette (neo) and a primer external to the 5′ arm of homology. The primers were 5′-AAG GAT TCT AAG TAG CCT CAA TGT G-3′, 5′-TGG GCT CTA TGG CTT CTG AG-3′ (product size 1.7 kb). Colonies that tested positive in this PCR assay were expanded and then screened again by PCR using a primer within neo and a primer external to the 3′ arm of homology (5′-GTG CCC AGT CAT AGC CGA ATA G-3′ and 5′-TTC TCT CTC AGC GGT TCC TTT G-3′; product size 5.9 kb). The PCR for testing correct 5′ targeting was also repeated. DNA from ES cell clones that tested correct for both 5′ and 3′ targeting was digested with AgeI and analyzed by Southern blot using an internal probe that hybridizes to neo. This Southern blot confirmed the expected size for correct 5′ targeting and showed no off-target integrations. Chimeric mice were generated by ES cell injection into C57BL/6J blastocysts. Germline transmission was achieved by mating chimeras to C57BL/6J females, assaying pups for agouti coat color, and genotyping for the targeted allele. Neo was removed from the locus by breeding to Sox2-cre transgenic females, which express Cre recombinase in the germline (13). All mice were bred on a B6 × 129 mixed genetic background and housed in a barrier facility with a 12-h light, 12-h dark cycle. All mice were fed ad libitum a sterilized standard rodent diet and sterilized water. Because GnrhrE90K homozygous males are fertile, the line was maintained by breeding homozygous males to heterozygous females. All studies involving animals were approved by the Institutional Animal Care and Use Committee at the University of Texas M.D. Anderson Cancer Center.
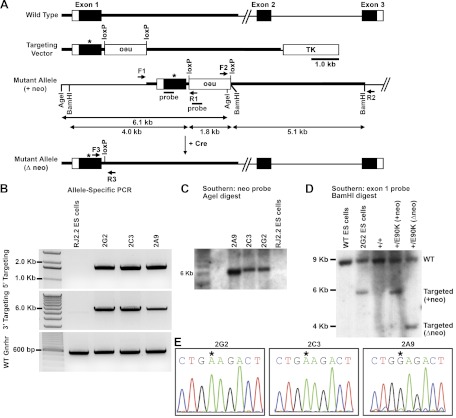
Fig. 1.
Generation of GnrhrE90K mutant mice. A, Illustration of the gene-targeting strategy. The point mutation to generate E90K was introduced into the first exon of the Gnrhr gene. The PGK-neo-bpA (neo) selection cassette was flanked by loxP sites for excision by Cre recombinase. The neo cassette was placed in opposite orientation to the transcriptional direction of the Gnrhr gene. B, Allele-specific PCR using primers within the neo cassette and exterior to the arm of homology. Primers were F1/R1 and F2/R2 (illustrated in A) for testing 5′ and 3′ targeting, respectively. The expected band sizes were 1.7 and 5.9 kb for 5′ and 3′ targeting, respectively. C, Southern blot. ES cell genomic DNA was digested with AgeI and hybridized with a probe to the neo cassette. The expected band size for the targeted allele was 6.1 kb. D, Southern blot. Genomic DNA from ES cells and mice of the indicated genotypes was digested with BamHI and hybridized with a probe to exon 1 of Gnrhr. The predicted band sizes were 9.1 kb for wild type, 5.9 kb for the targeted locus, and 4.0 kb for the targeted locus after the neo cassette was deleted with Cre recombinase. E, Three correctly targeted clones were selected for expansion. Gnrhr exon 1 was sequenced for each clone to determine whether the G to A mutation existed. Clones 2G2 and 2C3 carried the mutation, whereas clone 2A9 did not. Mice generated from clone 2A9 served as a control to test the effect of inserting a loxP into the first intron. WT, Wild type; TK, MC1-thymidine kinase minigene.
GnrhrE90K mice were genotyped using primers F3 and R3 (Fig. 1A), which surround the loxP site and produce band sizes of 169 and 360 bp for wild-type and mutant alleles, respectively. The primer sequences were as follows: F3, 5′-TCA GCA GTA GCC TTT AAC CCT GAC-3′, and R3, 5′-GGG GAA GAG GAT AGA GTC AGT TGT G-3′.
Tissue collection
Tissues were collected at 8 wk of age. Mice were anesthetized with Avertin (240 mg/kg, ip), and blood was drawn by cardiac puncture. The animal was then euthanized by cervical dislocation. The pituitary was dissected and immediately frozen in liquid nitrogen. The gonads were dissected and imaged with a Leica MZ10F stereomicroscope. For males, we weighed both testes after trimming away the epididymis and fat (+/+, n = 26; E90K/+, n = 26; E90K/E90K, n = 21; L117P/L117P, n = 3). For females, we counted the number of corpora lutea on each ovary (E90K/+, n = 12; E90K/E90K, n = 28; L117P/L117P, n = 4). Tissues were then fixed by overnight immersion in Bouin's fixative at room temperature. The next day, tissues were dehydrated with ethanol and embedded in paraffin wax. Hematoxylin and eosin staining was performed using a standard protocol. Images of tissue sections were captured with a Nikon 80i microscope.
Estrous cycle
Stage of the estrous cycle was assayed by daily evaluation of vaginal cytology at 8 wk of age using established methods (14). Mice were monitored for 2–5 d to ensure an accurate assessment of stage.
LH and FSH assays
For genotype comparisons, serum levels of LH and FSH were assayed by immunoradiometric assay and RIA, respectively, by the Ligand Assay and Analysis Core at the University of Virginia. Each sample was run in duplicate. For E90K/+ males and females, n = 11 and 12, respectively. For E90K/E90K males and females, n = 7 and 13, respectively. For L117P/L117P males and females, n = 3 and 4, respectively. As per the Ligand Core, LH and FSH assays with coefficients of variation higher than 20 and 25% were eliminated from the data set, respectively. For the buserelin challenge, serum LH was assayed by RIA using the anti-LH C102 antibody as previously described (15). Only males were used for the buserelin experiment (E90K/+, n = 7; E90K/E90K, n = 9). Statistically significant differences (P < 0.05) between groups were determined by t test.
Real-time PCR
Total RNA was extracted from whole pituitaries using TRIzol reagent according to the manufacturer's protocol (Invitrogen, Carlsbad, CA). Gnrhr mRNA levels were measured using a TaqMan gene expression assay (Mm00439143_m1) from Applied Biosystems (Life Technologies, Carlsbad, CA). The level of Gapdh mRNA was used for normalization. The PCR was run using an ABI Prism 7900HT thermocycler and SDS2.1 software (Applied Biosystems). Data were analyzed by the comparative ΔΔCt method. Statistically significant differences (P < 0.05) between groups were determined by t test. For E90K/+, E90K/E90K, L117P/+, and L117P/L117P, n = 22, 18, 10, and 4, respectively.
Results
Generation of GnrhrE90K mutant mice
To produce mice that express GnrhrE90K, a point mutation (G to A) was introduced into the first exon of the Gnrhr gene at position 313 by homologous recombination in mouse ES cells (Fig. 1A). Correct 5′ and 3′ targeting was confirmed by PCR using primers that extend from outside the arms of homology to inside the neomycin resistance expression cassette (neo) (Fig. 1B). When analyzed by Southern blot using a probe that hybridizes to neo, correctly targeted clones yield a band of 6.1 kb after AgeI digest (Fig. 1C). After germline transmission through mouse chimeras, neo was removed by crossing to a strain that expresses Cre recombinase in the germline (Sox2-cre). Deletion of neo was confirmed by Southern blot using a probe that hybridizes specifically to exon 1 (Fig. 1D). Depending on the location of recombination within the 5′ arm of homology, ES cell clones were derived with or without the G to A substitution. Mice for this study were derived from E90K ES cell clones 2G2 and 2C3 and from wild-type clone 2A9, which served as a loxP site-only control (Fig. 1E). No phenotypic differences were detected between mice derived from clones 2G2 or 2C3; therefore, data obtained from these two lines was combined in the after analyses. Mice homozygous for only the loxP site (clone 2A9) were fertile and phenotypically indistinguishable from wild-type mice (data not shown).
GnrhrE90K affects circulating concentrations of gonadotropins
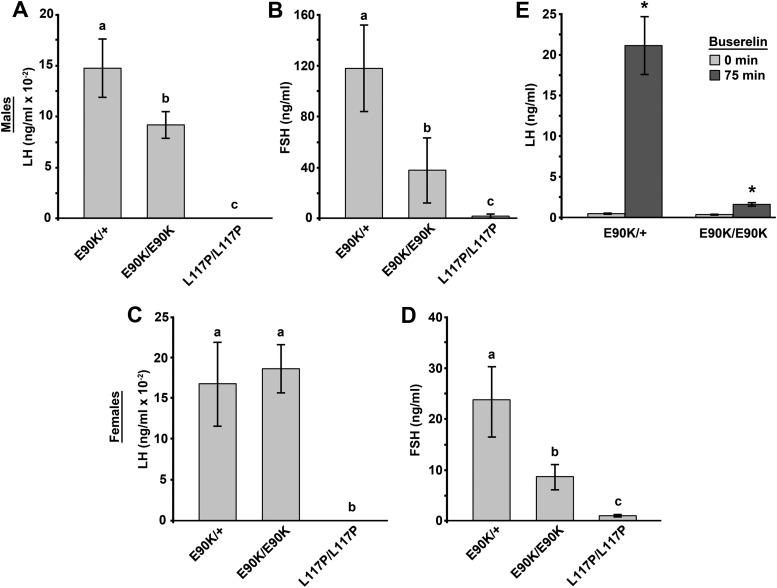
The concentrations of LH and FSH in circulation were assayed from blood collected when the mice were killed. Male mice homozygous for E90K exhibited a significant reduction in blood levels of both LH and FSH. Compared with E90K heterozygotes, serum LH and FSH were 30 and 62% lower, respectively. This reduction was modest compared with mice homozygous for L117P, for which blood levels of LH and FSH are, for the most part, below the detectable range (Fig. 2, A and B).
Fig. 2.
GnrhrE90K affects circulating concentrations of gonadotropins. Blood levels of LH and FSH were measured by RIA. A and B, Males. Serum levels for both LH (A) and FSH (B) were reduced in E90K homozygotes as compared with E90K heterozygotes, which are phenotypically identical to wild type. Serum levels of FSH and LH were mostly below the lower limit of detection for L117P homozygotes. C and D, Females. The serum level of LH (C) was not different between E90K heterozygotes and homozygotes. The serum level of FSH (D) was reduced in E90K homozygotes as compared with E90K heterozygotes, which are phenotypically identical to wild type. Serum levels of FSH and LH were mostly below the lower limit of detection for L117P homozygotes. E, Buserelin challenge. The serum level of LH was determined before and 75 min after buserelin injection. Buserelin increased serum LH levels for both E90K/+ and E90K/E90K mice; however, LH release was much greater for E90K/+. Significant differences are denoted by the lowercase letters above each bar (A–D) or by an asterisk (E). Equivalent means have the same letter; different letters indicate statistically significant differences (P < 0.05). Error bars represent sem.
Randomly cycling females were used for these assays because the goal was solely to determine whether E90K was causing HH. Although the females were randomly cycling, E90K homozygotes are persistently in estrus and L117P homozygotes are anestrus. It is only E90K heterozygotes that were consistently cycling in this study. We did not detect a difference in circulating LH in female E90K homozygotes (Fig. 2C). As compared with heterozygotes, blood levels of FSH were 36% lower in female mice homozygous for E90K (Fig. 2D). Both gonadotropin levels were significantly higher for E90K homozygotes than L117P homozygotes, which exhibit arrested follicular development at the primary follicle stage.
GnrhrE90K homozygotes exhibit reduced sensitivity to a GnRHR agonist
Based on in vitro observations, mutant E90K produces a misfolded GPCR that is trapped by the QCS, resulting in reduced plasma membrane expression (7, 16, 17). Reduced GnRHR plasma membrane expression should manifest in reduced responsiveness to a GnRHR agonist. To this end, we compared the effect of buserelin, a GnRHR agonist, on LH release in E90K heterozygotes and homozygotes. Serum levels of LH were measured before and 75 min after buserelin injection (ip, 3 ng/g body weight). LH levels were increased after buserelin treatment for both heterozygotes and homozygotes; however, a much larger increase in serum LH was observed for E90K heterozygotes (48- vs. 4.6-fold) (Fig. 2E).
Gonadal function is correlated with Gnrhr gene expression
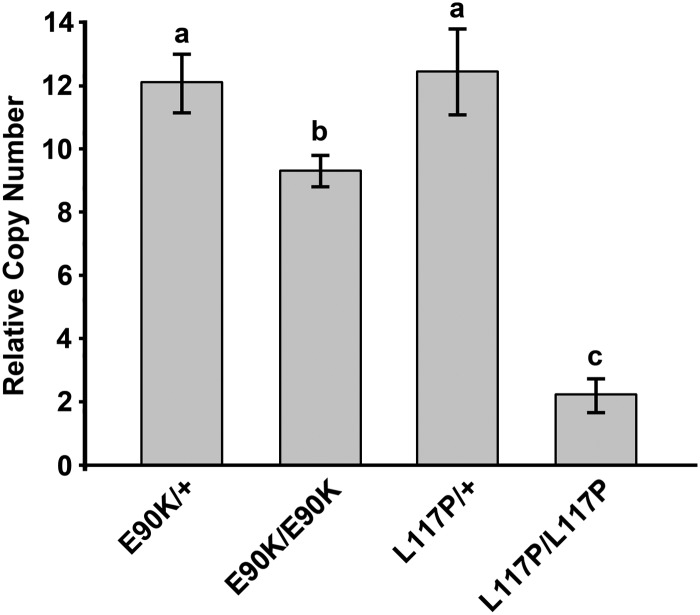
We next determined whether E90K affected expression of the Gnrhr gene. We assayed the relative abundance of Gnrhr mRNA in pituitary extracts from E90K/+, E90K/E90K, L117P/+, and L117P/L117P mice. Males and females of each genotype were grouped together. E90K/+ and L117P/+ mice had similar levels of Gnrhr mRNA expression. Compared with E90K heterozygotes, Gnrhr mRNA levels were 0.2-fold lower in E90K homozygous mice and 0.8-fold lower in L117P homozygous mice (Fig. 3).
Fig. 3.
Gonadal function is correlated with Gnrhr gene expression. Real-time PCR was performed using total RNA extracted from the pituitary. Data were analyzed by the ΔΔCt method and reported as copy number relative to that of Gapdh. E90K and L117P heterozygotes, which are both phenotypically normal, exhibited similar levels of Gnrhr mRNA. E90K homozygotes, which exhibit mild HH, had reduced Gnrhr mRNA. Very little Gnrhr mRNA was detected for L117P homozygotes, which exhibit severe HH. Significant differences are denoted by the lowercase letters above each bar. Equivalent means have the same letter; different letters indicate statistically significant differences (P < 0.05). Error bars represent sem.
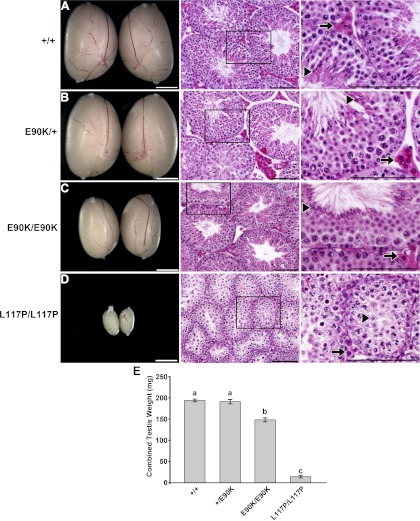
GnrhrE90K homozygous males exhibit normal testis histology despite reduced testis size
Male mice were tested for alterations in testis size and morphology. These effects were compared with that of mice homozygous for L117P, a Gnrhr mutation that we previously showed resulted in GnRHR misfolding and hypogonadism. Upon gross examination of the testes, GnrhrE90K/+ mice were morphologically similar to their wild-type littermates (Fig. 4, A and B). The testes of GnrhrE90K/E90K mice were smaller than that of wild-type or heterozygous mice (Fig. 4C) but larger than the testes of mice homozygous for L117P (Fig. 4D). Despite a reduction in overall size, hematoxylin- and eosin-stained sections revealed comparably normal testis morphology for wild-type, E90K heterozygotes, and E90K homozygotes, including complete spermatogenesis and Leydig cells with abundant eosin staining (Fig. 4, A–C). This is in contrast to the histology of mice homozygous for L117P, which exhibit a block in spermatogenesis and Leydig cell defects (Fig. 4D).
Fig. 4.
GnrhrE90K homozygous males exhibit normal testis histology despite reduced testis size. A and B, Wild-type (A) and E90K/+ (B) testes were indistinguishable in size, abundance of eosin-stained Leydig cells (arrows), and presence of spermatozoa in the seminiferous tubules (arrowheads). C, Testes from E90K homozygotes were smaller but still displayed abundant Leydig cells (arrow) and spermatozoa (arrowhead), indicating normal function. D, For comparison, L117P homozygotes exhibit extremely small testes, lack of eosin-stained Leydig cells (arrow), and spermatogenic arrest at the round spermatid stage (arrowhead). On the left are stereomicroscope images. Scale bar, 2 mm. Center and right columns show hematoxylin- and eosin-stained sections. Scale bar, 0.1 mm. E, Differences in testis weight between genotypes were quantitated by weighing them as a pair. No difference was found between wild-type and E90K heterozygotes, whereas the testes of E90K homozygotes were smaller. L117P homozygotes, which cannot complete spermatogenesis, exhibited the lowest testis weight of any genotype. Significant differences are denoted by the lowercase letters above each bar. Equivalent means have the same letter; different letters indicate statistically significant differences (P < 0.05). Error bars represent sem.
Because the testes of E90K homozygotes appeared histologically normal, we reasoned that the males were fertile. Indeed, mating between homozygous males and heterozygous females consistently yielded litters. For this reason, we maintained the strain in this manner. In contrast, all male mice homozygous for L117P are infertile (7).
To quantify differences in testis size, testes from each genotype were dissected and weighed as a pair. Testis weight was not different between wild-type and E90K heterozygotes. Testes from E90K homozygotes weighted 22% less than that of wild-type or E90K heterozygotes. This effect was mild compared with that of mice homozygous for L117P, which exhibited a 94% reduction in testis weight (Fig. 4E).
GnrhrE90K homozygous females do not ovulate
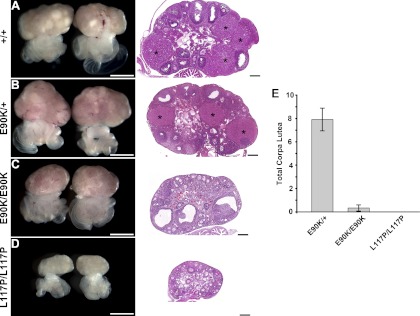
Female mice were histologically examined for alterations in folliculogenesis and luteinization. The ovaries of E90K heterozygous females were indistinguishable from their wild-type littermates, including comparable size, presence of follicles at all stages of development, and presence of corpora lutea (Fig. 5, A and B). In contrast, the ovaries of GnrhrE90K/E90K mice consistently had large numbers of antral follicles, and corpora lutea were extremely rare (Fig. 5C). Mice homozygous for L117P, which produces severe HH, exhibited very small ovaries and no follicles past the primary stage (Fig. 5D).
Fig. 5.
GnrhrE90K prevents ovulation and luteinization. A and B, Ovaries from both wild-type (A) and E90K/+ (B) mice exhibited normal histology, including multiple corpora lutea (asterisks) and follicles in all stages of growth. C, In contrast, almost all ovaries from E90K homozygotes displayed multiple antral follicles and no corpora lutea. D, No follicular development past the primary stage was observed for L117P homozygotes. On the left are stereomicroscope images. Scale bar, 1 mm. On the right are hematoxylin- and eosin-stained sections. Scale bar, 0.2 mm. E, The total number of corpora lutea on both ovaries was counted. The data are plotted as the mean number of corpora lutea present on both ovaries. Error bars represent sem.
We quantified the ovulation/luteinization defect by counting the number of corpora lutea on both ovaries. The average number of corpora lutea for E90K heterozygotes, E90K homozygotes, and L117P homozygotes was 7.9, 0.2, and 0, respectively (Fig. 5E). Specifically, only two of the 28 homozygous females examined had ovaries with corpora lutea. One had two corpora lutea on each ovary. The other had one corpus luteum on one ovary.
Effects on the estrous cycle
We assayed the stage of the estrous cycle in homozygous and heterozygous E90K females by evaluation of vaginal cytology at 8 wk of age. E90K homozygotes exhibited a strong propensity to remain in estrus. Eight of nine females did not cycle and repeatedly presented in estrus. In contrast, 11 of 12 E90K heterozygotes cycled normally. These data are consistent with the ovarian histology of E90K homozygotes, which exhibit multiple antral follicles and no corpora lutea.
Discussion
We know from in vitro experiments that E90K has little ability to initiate signal transduction. Its lack of activity is inferred to be the result of misfolding and trapping by the QCS, because its activity can be restored upon treatment with compounds that mimic molecular chaperones. In contrast, biological activity cannot be restored in the same manner for L117P (7). The multitudes of missense mutations and species-specific amino acid differences that affect GnRHR folding and routing to the plasma membrane have been studied in detail. Thus, we are confident that the phenotype observed in GnrhrE90K mice is due to reduced plasma membrane expression of GnRHR. That said, it is not feasible to directly measure GnRHR plasma membrane expression in these mice. This is due to lack of a high-quality antibody that cross-reacts with mouse GnRHR. To circumvent this problem, we compared GnRHR activity in E90K heterozygotes and homozygotes by assaying LH release in response to the Gnrhr agonist buserelin. LH release was much greater for E90K heterozygotes than homozygotes. These data support the conclusion that the phenotype of mice homozygous for GnrhrE90K is due to reduced GnRHR plasma membrane expression.
The hypothalamus produces and secretes the neuropeptide GnRH, which travels through the hypothalamic-hypophyseal portal veins to reach the anterior pituitary. GnRH binds to its receptor on the plasma membrane of gonadotropes to stimulate the synthesis and secretion of the gonadotropins LH and FSH. Whether LH or FSH is released in response to GnRH is dependent on both the frequency and amplitude of GnRH release. High-frequency GnRH pulses primarily stimulate expression of the gene encoding the LHβ-subunit, whereas the gene encoding FSHβ is primarily expressed during periods of low GnRH pulse frequency (18–22). Gonadotropes sense GnRH solely through the GnRHR. Thus, it is conceivable that a certain number of receptors are required for this sensitivity. Indeed, GnRHR plasma membrane expression varies throughout the estrous cycle, with highest expression during proestrus (which coincides with the LH surge) (23). Because plasma membrane expression is reduced for E90K (7, 9), it is likely that the lack of ovulation and luteinization is due to insufficient numbers of GnRHR on the plasma membrane. These data support the hypothesis that a threshold level of GnRHR must be expressed on the plasma membrane for ovulation to occur. In other words, a critical amount of GnRHR plasma membrane expression is required to decode GnRH pulse frequency and amplitude and produce the desired response in terms of LH and/or FSH release.
Spermatogenesis is primarily controlled by the actions of FSH and testosterone in Sertoli cells. Here we found that spermatogenesis was intact in GnrhrE90K homozygous males, but testis size was reduced. Because FSH is known to regulate Sertoli cell proliferation and thus overall testis size and spermatogenic capacity, the reduced testis size seen in E90K homozygotes may be a direct effect of reduced FSH secretion from the anterior pituitary.
Species-specific differences in plasma membrane expression of GnRHR may account for the hypomorphic phenotype observed for homozygous E90K mice. Mice homozygous for E90K exhibited a much milder phenotype than that reported for humans with the same mutation. Male patients homozygous for E90K present with complete hypogonadism and are nonresponsive to GnRH treatment (8). In cell culture, human GnRHR E90K is nonfunctional and acts as a dominant negative to the wild-type receptor (9, 10). In contrast, male mice were fertile despite reduced testis size. Female mice underwent folliculogenesis but failed to ovulate or form corpora lutea.
Due to specific amino acid differences between human and mouse, human GnRHR is less efficiently trafficked to the plasma membrane and thus is susceptible to retention by the QCS. The net result is that only about half of human GnRHR is expressed on the plasma membrane (6). In contrast, nearly 100% of mouse/rat GnRHR is routed to the plasma membrane. This species effect is primarily due to the steric effect of a lysine at position 191 in humans, which decreases the probability of the formation of the C14-C200 bond required for the correct folding of the human sequence, whereas this amino acid is absent from rodent GnRHR sequences, which also do not require the Cys bond formation for correct folding (24).
For human GnRHR, the E90K mutant can be functionally restored by deleting K191 (16, 25). However, in cell culture, mouse E90K is trapped by the QCS even though mouse GnRHR lacks K191. Due to the hypomorphic phenotype of mouse E90K vs. that of L117P, it appears that E90K does not completely ablate GnRHR function in vivo. Thus, the lack of K191 in the mouse protein may indeed support correct routing of the E90K mutant to the plasma membrane.
Because mouse GnRHR is more efficiently routed to the plasma membrane than human GnRHR, it may be less susceptible to QCS trapping of the E90K mutant. One might argue that lack of QCS activity in mouse gonadotropes could lead to plasma membrane expression of E90K. This is unlikely because mice homozygous for the L117P mutant, which also causes receptor misfolding, exhibit severe HH, including very small gonads and extremely low levels of serum gonadotropins (7). Thus, the QCS actively captures mutant L117P. In summary, it seems that mouse E90K, to some degree, escapes the QCS and produces a functional receptor on the plasma membrane.
The degree of gonadal function is correlated with Gnrhr gene expression. Mice homozygous for E90K and L117P exhibited a 20 and 80% reduction in pituitary Gnrhr mRNA expression, respectively. This could be due to reduced GnRHR signaling in these mutants (as a result of reduced plasma membrane expression of GnRHR). Because L117P homozygotes exhibit more severe HH than E90K homozygotes (presumably due to less plasma membrane GnRHR expression), it follows that Gnrhr mRNA levels were lowest for L117P homozygotes. These data support the conclusion of several in vitro studies, in which GnRH was shown to positively regulate Gnrhr transcript levels. In cultured gonadotropes, GnRH treatment increased Gnrhr mRNA levels in a dose-dependent manner (26). In αT3-1 cells, GnRH positively regulated the Gnrhr 5′ promoter region through pathways involving activator protein-1 and protein kinase C (27, 28). Activin synergized with GnRH to promote Gnrhr gene expression in this same cell type (29).
In conclusion, because receptor expression levels can alter sensitivity to ligand and because the preovulatory LH surge correlates with elevated GnRHR expression (23), it is likely that gonadotrope sensitivity to GnRH is regulated by the plasma membrane expression level of GnRHR. In this study, mice homozygous for GnrhrE90K, a mutation that inhibits proper folding of the GnRHR and thus reduces plasma membrane expression, exhibited reduced testis size in males and ovulation failure in females. Our studies suggest that the plasma membrane expression level of GnRHR regulates the interpretation of the GnRH signal by the gonadotropes.
Acknowledgments
We thank Dr. Randy Johnson for the RJ2.2 mouse ES cell line.
This work was supported by the National Institutes of Health (NIH) (HD30284 to R.R.B. and DK85040, RR030229, and RR000163 to P.M.C.), the Ben F. Love Chair, the Kleberg Foundation (to R.R.B), and the American Heart Association (10BGIA4250008 to M.D.S.). Serum LH and FSH were assayed by the Ligand Assay and Analysis Core at the University of Virginia, which is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NIH) (U54-HD28934). DNA sequencing and veterinary services at the University of Texas M.D. Anderson Cancer Center are supported by the NIH (CA16672).
Disclosure Summary: The authors have no conflicts of interest to disclose.
Footnotes
- ES
- Embryonic stem
- GnRHR
- GnRH receptor
- HH
- hypogonadotropic hypogonadism
- GPCR
- G protein-coupled receptor
- QCS
- quality control system.
References
- 1. Filippi G. 1986. Klinefelter's syndrome in Sardinia. Clinical report of 265 hypogonadic males detected at the time of military check-up. Clin Genet 30:276–284 [PubMed] [Google Scholar]
- 2. Fromantin M, Gineste J, Didier A, Rouvier J. 1973. [Impuberism and hypogonadism at induction into military service. Statistical study]. Probl Actuels Endocrinol Nutr 16:179–199 (French) [PubMed] [Google Scholar]
- 3. Beranova M, Oliveira LM, Bédécarrats GY, Schipani E, Vallejo M, Ammini AC, Quintos JB, Hall JE, Martin KA, Hayes FJ, Pitteloud N, Kaiser UB, Crowley WF, Jr, Seminara SB. 2001. Prevalence, phenotypic spectrum, and modes of inheritance of gonadotropin-releasing hormone receptor mutations in idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 86:1580–1588 [DOI] [PubMed] [Google Scholar]
- 4. Karges B, Karges W, de Roux N. 2003. Clinical and molecular genetics of the human GnRH receptor. Hum Reprod Update 9:523–530 [DOI] [PubMed] [Google Scholar]
- 5. Conn PM, Ulloa-Aguirre A. 2010. Trafficking of G-protein-coupled receptors to the plasma membrane: insights for pharmacoperone drugs. Trends Endocrinol Metab 21:190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Janovick JA, Ulloa-Aguirre A, Conn PM. 2003. Evolved regulation of gonadotropin-releasing hormone receptor cell surface expression. Endocrine 22:317–327 [DOI] [PubMed] [Google Scholar]
- 7. Pask AJ, Kanasaki H, Kaiser UB, Conn PM, Janovick JA, Stockton DW, Hess DL, Justice MJ, Behringer RR. 2005. A novel mouse model of hypogonadotrophic hypogonadism: N-ethyl-N-nitrosourea-induced gonadotropin-releasing hormone receptor gene mutation. Mol Endocrinol 19:972–981 [DOI] [PubMed] [Google Scholar]
- 8. Soderlund D, Canto P, de la Chesnaye E, Ulloa-Aguirre A, Mendez JP. 2001. A novel homozygous mutation in the second transmembrane domain of the gonadotrophin releasing hormone receptor gene. Clin Endocrinol (Oxf) 54:493–498 [DOI] [PubMed] [Google Scholar]
- 9. Knollman PE, Janovick JA, Brothers SP, Conn PM. 2005. Parallel regulation of membrane trafficking and dominant-negative effects by misrouted gonadotropin-releasing hormone receptor mutants. J Biol Chem 280:24506–24514 [DOI] [PubMed] [Google Scholar]
- 10. Brothers SP, Cornea A, Janovick JA, Conn PM. 2004. Human loss-of-function gonadotropin-releasing hormone receptor mutants retain wild-type receptors in the endoplasmic reticulum: molecular basis of the dominant-negative effect. Mol Endocrinol 18:1787–1797 [DOI] [PubMed] [Google Scholar]
- 11. Leaños-Miranda A, Ulloa-Aguirre A, Ji TH, Janovick JA, Conn PM. 2003. Dominant-negative action of disease-causing gonadotropin-releasing hormone receptor (GnRHR) mutants: a trait that potentially coevolved with decreased plasma membrane expression of GnRHR in humans. J Clin Endocrinol Metab 88:3360–3367 [DOI] [PubMed] [Google Scholar]
- 12. Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu Q, Wang Y, Halder G, Finegold MJ, Lee JS, Johnson RL. 2010. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci USA 107:1437–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayashi S, Tenzen T, McMahon AP. 2003. Maternal inheritance of Cre activity in a Sox2Cre deleter strain. Genesis 37:51–53 [DOI] [PubMed] [Google Scholar]
- 14. Byers SL, Wiles MV, Dunn SL, Taft RA. 2012. Mouse estrous cycle identification tool and images. PLoS One 7:e35538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stanislaus D, Janovick JA, Ji T, Wilkie TM, Offermanns S, Conn PM. 1998. Gonadotropin and gonadal steroid release in response to a gonadotropin-releasing hormone agonist in Gqα and G11α knockout mice. Endocrinology 139:2710–2717 [DOI] [PubMed] [Google Scholar]
- 16. Maya-Núñez G, Janovick JA, Ulloa-Aguirre A, Söderlund D, Conn PM, Méndez JP. 2002. Molecular basis of hypogonadotropic hypogonadism: restoration of mutant (E(90)K) GnRH receptor function by a deletion at a distant site. J Clin Endocrinol Metab 87:2144–2149 [DOI] [PubMed] [Google Scholar]
- 17. Janovick JA, Maya-Nunez G, Conn PM. 2002. Rescue of hypogonadotropic hypogonadism-causing and manufactured GnRH receptor mutants by a specific protein-folding template: misrouted proteins as a novel disease etiology and therapeutic target. J Clin Endocrinol Metab 87:3255–3262 [DOI] [PubMed] [Google Scholar]
- 18. Ciccone NA, Kaiser UB. 2009. The biology of gonadotroph regulation. Curr Opin Endocrinol Diabetes Obes 16:321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haisenleder DJ, Dalkin AC, Ortolano GA, Marshall JC, Shupnik MA. 1991. A pulsatile gonadotropin-releasing hormone stimulus is required to increase transcription of the gonadotropin subunit genes: evidence for differential regulation of transcription by pulse frequency in vivo. Endocrinology 128:509–517 [DOI] [PubMed] [Google Scholar]
- 20. Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. 1997. Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology 138:1224–1231 [DOI] [PubMed] [Google Scholar]
- 21. Kaiser UB, Sabbagh E, Katzenellenbogen RA, Conn PM, Chin WW. 1995. A mechanism for the differential regulation of gonadotropin subunit gene expression by gonadotropin-releasing hormone. Proc Natl Acad Sci USA 92:12280–12284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reame N, Sauder SE, Kelch RP, Marshall JC. 1984. Pulsatile gonadotropin secretion during the human menstrual cycle: evidence for altered frequency of gonadotropin-releasing hormone secretion. J Clin Endocrinol Metab 59:328–337 [DOI] [PubMed] [Google Scholar]
- 23. Marian J, Cooper RL, Conn PM. 1981. Regulation of the rat pituitary gonadotropin-releasing hormone receptor. Mol Pharmacol 19:399–405 [PubMed] [Google Scholar]
- 24. Janovick JA, Knollman PE, Brothers SP, Ayala-Yáñez R, Aziz AS, Conn PM. 2006. Regulation of G protein-coupled receptor trafficking by inefficient plasma membrane expression: molecular basis of an evolved strategy. J Biol Chem 281:8417–8425 [DOI] [PubMed] [Google Scholar]
- 25. Janovick JA, Patny A, Mosley R, Goulet MT, Altman MD, Rush TS, 3rd, Cornea A, Conn PM. 2009. Molecular mechanism of action of pharmacoperone rescue of misrouted GPCR mutants: the GnRH receptor. Mol Endocrinol 23:157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheon M, Park D, Kim K, Park SD, Ryu K. 1999. Homologous upregulation of GnRH receptor mRNA by continuous GnRH in cultured rat pituitary cells. Endocrine 11:49–55 [DOI] [PubMed] [Google Scholar]
- 27. Albarracin CT, Kaiser UB, Chin WW. 1994. Isolation and characterization of the 5′-flanking region of the mouse gonadotropin-releasing hormone receptor gene. Endocrinology 135:2300–2306 [DOI] [PubMed] [Google Scholar]
- 28. Norwitz ER, Cardona GR, Jeong KH, Chin WW. 1999. Identification and characterization of the gonadotropin-releasing hormone response elements in the mouse gonadotropin-releasing hormone receptor gene. J Biol Chem 274:867–880 [DOI] [PubMed] [Google Scholar]
- 29. Norwitz ER, Xu S, Jeong KH, Bédécarrats GY, Winebrenner LD, Chin WW, Kaiser UB. 2002. Activin A augments GnRH-mediated transcriptional activation of the mouse GnRH receptor gene. Endocrinology 143:985–997 [DOI] [PubMed] [Google Scholar]