Abstract
Relaxin and insulin-like peptide 3 (INSL3) are peptide hormones with a number of important physiological roles in reproduction, regulation of extracellular matrix turnover, and cardiovascular function. Relaxin and INSL3 mediate their actions through the closely related G-protein coupled receptors, relaxin family peptide receptors 1 and 2 (RXFP1 and RXFP2), respectively. These receptors have large extracellular domains (ECD) that contain high-affinity ligand-binding sites within their 10 leucine-rich repeat (LRR)-containing modules. Although relaxin can bind and activate both RXFP1 and RXFP2, INSL3 can only bind and activate RXFP2. To investigate whether this difference is related to the nature of the high-affinity ECD binding site or to differences in secondary binding sites involving the receptor transmembrane (TM) domain, we created a suite of constructs with RXFP1/2 chimeric ECD attached to single TM helices. We show that by changing as little as one LRR, representing four amino acid substitutions, we were able to engineer a high-affinity INSL3-binding site into the ECD of RXFP1. Molecular modeling of the INSL3-RXFP2 interaction based on extensive experimental data highlights the differences in the binding mechanisms of relaxin and INSL3 to the ECD of their cognate receptors. Interestingly, when the engineered RXFP1/2 ECD were introduced into full-length RXFP1 constructs, INSL3 exhibited only low affinity and efficacy on these receptors. These results highlight critical differences both in the ECD binding and in the coordination of the ECD-binding site with the TM domain, and provide new mechanistic insights into the binding and activation events of RXFP1 and RXFP2 by their native hormone ligands.
The relaxin peptides are a family of hormones structurally related to insulin, comprising two peptide chains cross-linked by three disulfide bonds (1). They have numerous physiological functions, including in reproduction, cardiovascular regulation, and neuronal signaling (reviewed in Ref. 1), and mediate their actions through interactions with different classes of G protein-coupled receptors referred to as relaxin family peptide (RXFP) receptors (2). The receptors for relaxin (H2 relaxin in human) and insulin-like peptide 3 (INSL3), RXFP1 and RXFP2, respectively, are characterized by large extracellular domains (ECD), which contain 10 leucine-rich repeats (LRR) and single low-density lipoprotein class-A modules (LDLa) at their N termini (3). The role of the LDLa modules in these receptors has been demonstrated to be crucial for receptor activation, but not ligand binding (4–6). High-affinity ligand binding, on the other hand, is mediated by the concave surfaces of the LRR of RXFP1 and RXFP2 through interactions with the B-chains of their cognate hormones (7, 8). In the case of relaxin binding to RXFP1, three residues in the B-chain α-helix, ArgB13, ArgB17, IleB20, are thought to interact with several pockets of surface-exposed amino acids located in LRR 4–8 of RXFP1 (8). Relaxin ArgB13 is proposed to interact with Glu277 and Asp279 in LRR 8 of RXFP1, whereas relaxin ArgB17 is predicted to bind to Asp231 and Glu233 in LRR 6 of RXFP1. Relaxin IleB20 is thought to interact with a cluster of hydrophobic residues (Trp180, Ile182, and Leu204) in LRR 4 and 5 of RXFP1 (8). All of these RXFP1 residues, except Leu204, are highly conserved in RXFP2, potentially explaining why relaxin can bind to RXFP2 with high affinity (9). In contrast, INSL3 binds with very low affinity to RXFP1 (10); thus the binding mode of INSL3 is distinctly different.
We recently reported several interactions that occur between INSL3 and RXFP2, which were mapped using mutant receptors partnered with mutant INSL3 peptides. The most prominent interactions identified included RXFP2 Asp227 binding INSL3 ArgB16 and RXFP2 Phe131 and Gln133 interacting with INSL3 TrpB27 (7). Hence INSL3 appears to bind to RXFP2 in a different manner to relaxin on RXFP1, using residues across at least LRR 2–7. Interestingly, all of the key RXFP2 LRR residues are conserved in RXFP1 except for RXFP2 Phe131, which corresponds to Tyr134 in human RXFP1 or Cys134 in dog, horse, panda, mouse, and rat RXFP1 (Fig. 1A). Considering that INSL3 binds to RXFP1 with a very low affinity (1, 10, 11), there must be additional factors that account for the specificity of INSL3 for RXFP2. The inability of INSL3 to bind to RXFP1 may be related, in part, to the overall mechanism of receptor activation, where ligand binding to both RXFP1 and RXFP2 is proposed to involve both a high-affinity interaction with the LRR mediated by the B-chain and a low-affinity interaction with the transmembrane domain (TMD) extracellular loops, suggested to be mediated by the A-chain (12, 13). Indeed, studies using the ECD of RXFP1 (ECD-1) and RXFP2 (ECD-2), fused to the single trans-membrane-spanning domain of CD8, previously termed 7BP and 8BP, have shown that the RXFP1 LRR can bind INSL3 with higher affinity than full-length RXFP1, although not as high as RXFP2 (11). This suggests that the inability of INSL3 to bind to RXFP1 is not simply due to the lack of an INSL3 binding site.
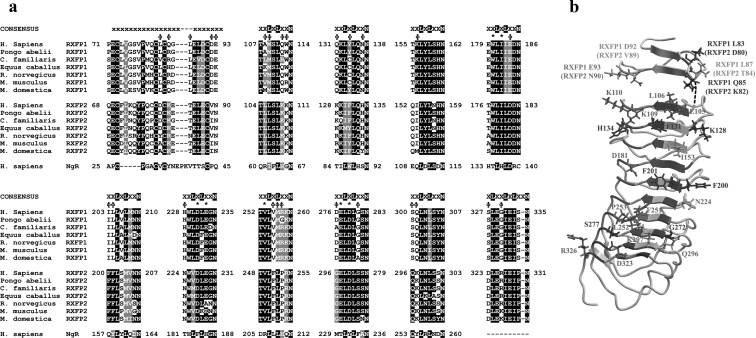
Fig. 1.
A, Multiple sequence alignment of the inner faces of the LRR of RXFP1 and RXFP2 from human (H. sapiens), orangutan (P. abelii), dog (C. familiaris), horse (E. caballus), rat (R. norvegicus), mouse (M. musculus), and opossum (M. domestica) and compared with the human Nogo receptor (NgR), which was used for the homology models. Residues involved with INSL3 binding to RXFP2 and conserved in RXFP1 are indicated (*), whereas RXFP2-specific residues that were investigated experimentally are labeled with φ. B, Homology model of the LRR of RXFP2 with receptor-specific residues identified in the multiple sequence alignment highlighted as previously described (7).
In this study we modified the LRR of RXFP1 in the ECD-1 construct to fully reconstruct a high-affinity INSL3 binding site. Highly conserved RXFP2 unique residues from the inner surface of each LRR were identified and subsequently inserted into their corresponding position in ECD-1, producing chimeric LRR. These receptor chimeras were used to determine the requirements for high-affinity INSL3 binding to RXFP2 and elucidate the differences between hormone-mediated activation of RXFP1 and RXFP2.
Materials and Methods
Peptides
H2 relaxin was kindly provided by Corthera (San Mateo, CA), and INSL3 was synthesized as previously described (14). Mono-Eu-labeled H2 relaxin and INSL3, which maintained high affinity for receptor binding studies, have been described previously (15).
Receptor sequence analyses
The amino acid sequences of LGR7 and LGR8 from the human (Homo sapiens) (Q9HBX9 and Q8WXD0), mouse (Mus musculus) (AAR97515 and Q91ZZ5), and rat (Rattus norvegicus) (AAR97516 and AAW84088) were retrieved from the GenBank database at National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov). Predicted amino acid sequences of RXFP1 and RXFP2 from the orangutan (Pongo abelii), dog (Canis familiaris), horse (Equus caballus), and opossum (Monodelphis domestica) were retrieved from available genomes at Ensembl (http://www.ensembl.org) and NCBI. Sequences were aligned using ClustalW with default parameters and shaded using Boxshade. All LGR7 and LGR8 protein sequences are numbered from the predicted signal peptide cleavage sites. Homology models of the LRR of RXFP1 and RXFP2 were generated with Swiss-Model using the Nogo receptor as structural template, as described previously (7).
Receptor constructs and nomenclature
In this study native RXFP1 and RXFP2 receptors were used in parallel with chimeric and ECD-only receptors. A previously described chimeric, full-length receptor construct was used called RXFP12TMD [previously called LGR7/8 (10)] in which the ECD of RXFP1 is linked to the TMD of RXFP2. The ECD-only receptor constructs ECD-1 and ECD-2, previously described as 7BP and 8BP (9), contain the ECD of RXFP1 and RXFP2, respectively, fused to the single TMD of CD8. The inner LRR β-strands are named based on the repeat they are derived from, e.g. the β-strand of LRR 1 is termed β1. The LRR domain contains an N-terminal cap, which is composed of two antiparallel β-strands with the N-terminal referred to as βN1 and the strand toward the C terminus as βN2 (7). Chimeric constructs are named based on their template, with inserted sequences written in superscript. For example, ECD-1 containing the RXFP2-specific residues from the inner β-strand of LRR 1 is named ECD-12β1. All the constructs were cloned in pcDNA3.1/Zeo (Invitrogen, Carlsbad, CA) plasmids and contained an N-terminal FLAG tag together with the bovine prolactin signal peptide as described previously (7). Mutagenesis was performed using the QuikChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA). Mutagenic primer pairs were designed following the protocol described in Ref. 16. Plasmid DNA was extracted from selected clones, and the full coding region was sequenced to confirm mutagenesis and ensure no other deleterious mutations were present.
Ligand-binding and cell surface-expression assays
Human embryonic kidney (HEK)-293T cells were transfected in 96-well optiplates with plasmids encoding the constructs of interest and Eu-INSL3 and Eu-H2 relaxin binding assays conducted as described previously (15, 17). Competition binding assays were performed using increasing concentrations of unlabeled H2 relaxin or INSL3, and nonspecific binding was determined using 1 μm unlabeled INSL3 or H2 relaxin for Eu-INSL3 and Eu-H2 relaxin binding assays, respectively. Data are expressed as mean ± sem of % specific binding of triplicate measurements pooled from at least three independent experiments. Data were analyzed using Graphpad PRISM (GraphPad, Software, Inc., San Diego, CA), and a nonlinear regression one-site binding model was used to plot curves and calculate pIC50 values. Final pooled pIC50 data were analyzed using one-way ANOVA coupled to Newman-Keuls multiple comparison test for multiple group comparison.
All the RXFP1 and RXFP2 constructs used in this study contained an N-terminal FLAG epitope, which, as we have shown previously, does not affect ligand binding or signaling of RXFP1 or RXFP2 (10, 11). The FLAG epitope was detected using cell surface expression assays that were performed in 48-well plates as previously described (18).
cAMP activity assays
The ability of the receptor chimeras to signal in response to ligand stimulation was assessed using a cAMP reporter gene assay (19) as previously described (4). Briefly, HEK-293T cells were cotransfected with chimeric receptors and a pCRE-β-galactosidase reporter plasmid (19) in 96-well plates. Cotransfected cells were incubated with increasing concentrations of H2 relaxin or INSL3 for 6 h, after which the media were aspirated and the cells frozen at −80 C overnight. The amount of cAMP-driven β-galactosidase expression was then determined in each well as described (4). The cAMP activity responses to ligand stimulation were normalized to the cAMP activity response to 5 μm forskolin. Data points were performed in triplicate, and each experiment was repeated three times. Concentration-response curves were analyzed using GraphPad PRISM (Graphpad Software, Inc.), and a sigmoidal dose-response curve with variable slope model was used to plot curves and calculate pEC50 values. Final pooled pEC50 data were analyzed using one-way ANOVA coupled to Newman-Keuls multiple comparison test for multiple group comparison.
Ligand docking
The structure of the INSL3-RXFP2 complex was generated by a docking protocol within the program HADDOCK (20). As starting structures the homology model of the LRR of RXFP2 and the nuclear magnetic resonance structure (NMR) solution of INSL3 (14) were used. Importantly, as the conformation of the B chain C terminus with the key TrpB27 residue is considered to be in an inactive state in the absence of the receptor, the tail structure was altered manually and energy minimized in a more extended conformation and kept fully flexible during the docking to allow better sampling of space to find favorable interactions.
The HADDOCK docking was driven by ambiguous restraints based on previously published mutational data on INSL3-RXFP2. This allowed us to assign Phe131, Gln133, Trp177, Ile179, Asp227, Glu229, Glu273, and Asp275 in RXFP2 (7) and HisB12, ArgB16, ValB19, and TrpB27 in INSL3 (14, 21) as “active” residues significantly contributing to binding, with the residues surrounding these and having considerable surface exposed residues being defined as “passive” residues, i.e. potential contributors to the interaction but for which there is no experimental evidence confirming significant contributions. The docking protocol in HADDOCK involves three steps 1) initial rigid body docking followed by 2) restrained molecular dynamics simulation and 3) refinement in explicit water. The regions of INSL3 known to be flexible from the NMR studies, residues A1–A6, A25–A26, B1–B5 and B21–B29 were defined as fully flexible, and their side-chain and backbone atoms allowed to move during all steps of the molecular dynamics. The best 200 of 1000 models generated by rigid-body docking were further refined by molecular dynamics and energy minimization. The 200 resultant structures were clustered by HADDOCK using a 5-Å cutoff, and the individual clusters were analyzed for energies and agreement of the experimental data. The cluster chosen to represent the complex of INSL3-RXFP2 comprised 63 of the 200 final structures, all of which were in good agreement with the data and showed good properties in terms of energies and geometry of the interaction.
Results
Identification of conserved RXFP2 unique LRR residues
The LRR of RXFP1 and RXFP2 were modeled on the crystal structure of their closest structural homolog, the Nogo receptor (22), and used to predict the amino acids of the human RXFP1 and RXFP2 ECD corresponding to the inner face of the LRR superstructure, including the N-terminal LRR cap (4, 7). These sequences were then aligned with those of RXFP1 and RXFP2 from other mammalian species (Fig. 1A). The inner face of the LRR domain is a parallel β-sheet composed of β-strands from each LRR unit. These β-strands follow a consensus sequence of xxLxLxxN, where the x residues are variable and generally displayed on the surface of the domain. The N-terminal LRR cap also contributes two β-strands to the inner LRR face. It has been previously demonstrated that particular RXFP1 and RXFP2 unique x residues are the mediators of INSL3 and H2 relaxin binding on RXFP2 (7) and RXFP1 (8), respectively. The residues involved in INSL3 binding to RXFP2 are indicated on Fig. 1A and are conserved in RXFP1 except for RXFP2 Phe131, which corresponds to Tyr134 in human RXFP1 or Cys134 in dog, horse, panda, mouse, and rat RXFP1. Because INSL3 binds poorly to human, mouse, and rat RXFP1, we postulated that other RXFP2-specific residues would be necessary for INSL3 binding and hence set out to perform gain of function studies in RXFP1 to determine the reason for the lack of INSL3 binding.
The sequence alignment highlighted numerous RXFP2 specific x residues that were selected for further investigation, which are presented in a structural model in Fig. 1B. Specifically, the residues thought to warrant further investigation were; Asp80 and Lys82 from the first β-strand of the N-terminal LRR cap (βN1) and Thr84, Val89, and Asn90 from the second β-strand of the N-terminal LRR cap (βN2) Leu105, Leu106, Lys109, and Lys110 from LRR 1(β1), Lys128, Phe131, and His134 from LRR 2 (β2), Ile153 from LRR 3 (β3), Asp181 from LRR 4 (β4), Phe200 and Phe201 from LRR 5 (β5), Asn224 from LRR VI (β6), Phe251, Leu252, and Pro253 from LRR VII (β7), Gly272 and Ser277 from LRR 8 (β8), Gln296 and Lys297 from LRR 9 (β9), and Asp323 and Arg326 from LRR 10 (β10). Note that these residues comprise the major differences between the inner LRR faces of RXFP1 and RXFP2, and thus this study was an extensive investigation into the LRR of both receptors.
Screening for gain of INSL3 binding in RXFP1/2-BP chimeras
Codons encoding the RXFP2 unique residues from each LRR (Fig. 1A) were introduced into their corresponding positions into a plasmid encoding the LRR of human RXFP1 linked to the single TM spanning domain of CD8 (ECD-1), via mutagenic PCR. For example, ECD-12β2 encodes ECD-1 with all the RXFP2 unique residues from the inner face of LRR 2 inserted into the native RXFP1 LRR 2 sequence.
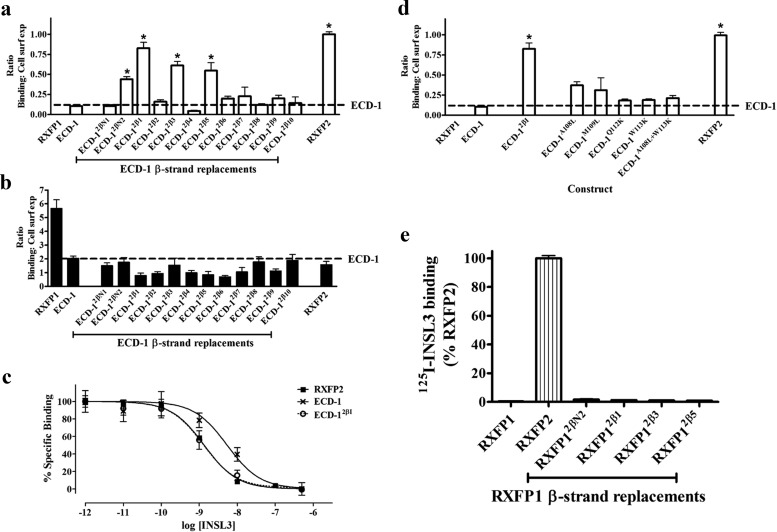
These chimeric single TM constructs were transfected into HEK-293T cells and screened for specific INSL3 binding and cell surface expression (Fig. 2A and Supplemental Fig. 1A published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). Interestingly, four of these chimeric constructs, ECD-12βN2, ECD-12β1, ECD-12β3, and ECD-12β5 exhibited significantly increased INSL3 binding. All of these constructs were also tested for their ability to bind to H2 relaxin to ensure that the LRR structure was correctly folded (Fig. 2B and Supplemental Fig. 1B). No major changes to H2 relaxin binding were detected for any of the constructs, indicating that the LRR were correctly folded.
Fig. 2.
A, INSL3 binding to chimeric ECD-only receptors expressed on the surface of cells. Data are expressed as a ratio of specific Eu-INSL3 binding to the cell surface expression of each construct and are normalized to RXFP2. *, P < 0.01 vs. ECD-1. B, Eu-H2 relaxin binding to chimeric ECD constructs. C, Eu-INSL3 competition binding curves on RXFP2 (solid squares), ECD-1 (black crosses), and ECD-12β1 (open circles). D, INSL3 binding to ECD-1 mutants containing the individual substitutions that constitute ECD-12β1. *, P < 0.01 vs. ECD-1. E, Specific [125I]INSL3 binding to full-length chimeric receptors (with TMD).
Of these mutants, ECD-12β1 exhibited the most impressive gain of INSL3 binding. It was therefore tested in competition binding studies in comparison to RXFP2 and ECD-1 (Fig. 2C). The insertion of RXFP2 βI into ECD-1 resulted in a significant gain in INSL3 binding affinity to a level that was not significantly different to RXFP2 (Table 1). ECD-12β1 contained four substitutions (A108L, M109L, Q112K, and W113K) when compared with ECD-1. To identify whether any single substitution was able to mediate this gain of INSL3 binding, each of these mutations was individually introduced into ECD-1, and the resultant INSL3 binding and cell surface expression of these mutants were tested (Fig. 2D and Supplemental Fig. 1C). Of these single mutants, ECD-1A108L and ECD-1M109L exhibited slightly increased INSL3 binding whereas the other substitutions gave no gain in INSL3 binding. It would therefore appear that it was a combination of all these mutations that caused the gain of INSL3 binding in ECD-12β1.
Table 1.
Summary of binding data and cAMP activity results for RXFP1/2 chimeric constructs
| Receptor construct | pIC50 |
n | pEC50 |
n | ||
|---|---|---|---|---|---|---|
| H2 relaxin | INSL3 | H2 relaxin | INSL3 | |||
| RXFP1 | 8.85 ± 0.05 | 5.55 ± 0.27 | 3 | 10.7 ± 0.04 | No activity | 3 |
| RXFP12TMD | ND | ND | 10.2 ± 0.08 | No activity | 3 | |
| RXFP12&β1 | 8.82 ± 0.34 | 6.44 ± 0.12 | 3 | 10.7 ± 0.17 | <6 | 3 |
| RXFP12&β1&TMD | 8.96 ± 0.13 | 6.53 ± 0.17 | 3 | 10.2 ± 0.03 | No activity | 3 |
| RXFP2&βN2 | 8.54 ± 0.29 | 6.26 ± 0.19 | 4 | 10.6 ± 0.14 | <6 | 3 |
| RXFP12&βN2&TMD | 8.31 ± 0.19 | 6.19 ± 0.09 | 4 | 9.59 ± 0.20a | No activity | 3 |
| RXFP12&β3 | 8.42 ± 0.25 | 6.57 ± 0.27 | 4 | 9.63 ± 0.05a | 7.52 ± 0.06 | 3 |
| RXFP12&β5 | 8.45 ± 0.30 | 7.09 ± 0.63b | 4 | 10.56 ± 0.12 | 6.72 ± 0.05 | 3 |
| Eu-INSL3 binding | ||||||
| RXFP12(N-LRR6) | 7.58 ± 0.79 | 9.14 ± 0.08 | 3 | 7.17 ± 0.07c | No activity | 3 |
| RXFP2 | 7.73 ± 0.06 | 9.14 ± 0.11 | 3 | 9.13 ± 0.06 | 10.2 ± 0.12 | 3 |
| ECD-12&β1 | ND | 8.94 ± 0.19 | ||||
| ECD-1 | ND | 8.19 ± 0.20d,e | ||||
ND, not determined
P < 0.01 vs. RXFP1;
, P < 0.05 vs. RXFP1;
, P < 0.001 vs. all;
, P < 0.01 vs. RXFP2;
, P < 0.01 vs. RXFP12&β1.
INSL3 binding to full length in RXFP1/2 chimeras
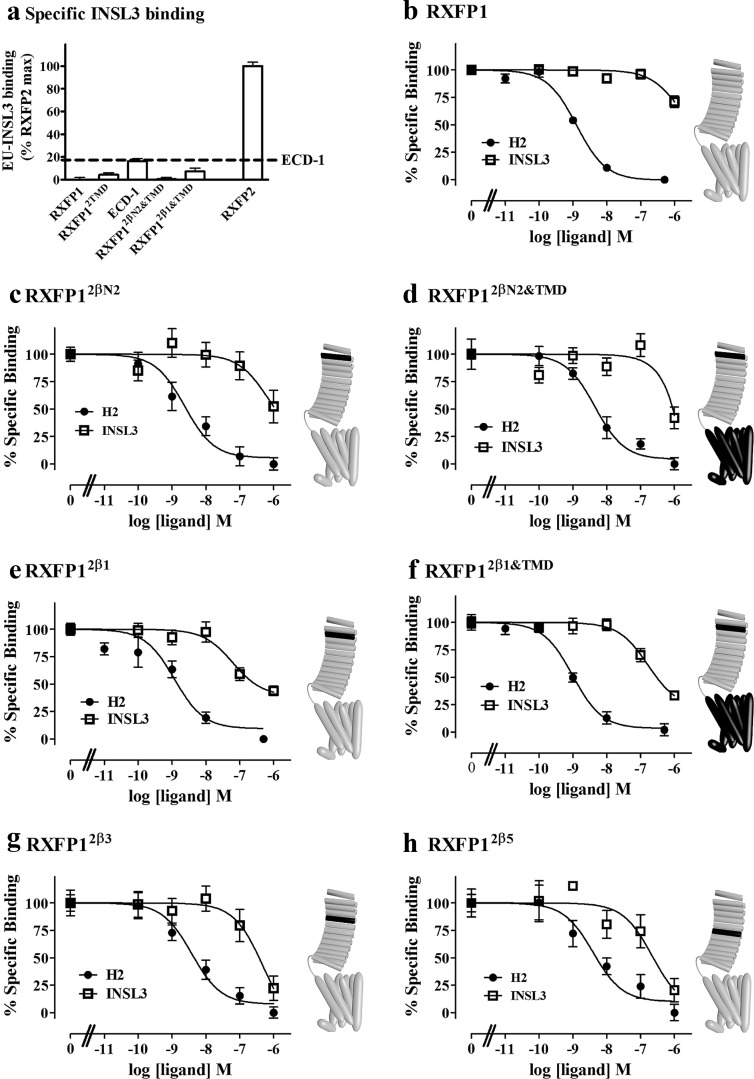
The above results demonstrated that inserting a small set of conserved residues from the LRR of RXFP2 into their corresponding positions in the LRR of RXFP1 leads to an increase in INSL3 affinity to the same level as RXFP2. To complement these findings, these mutations were placed into the LRR of full-length RXFP1. These receptors were expressed in HEK 293T cells, and their ability to bind INSL3 was determined. Interestingly, no significant binding of EU-INSL3 was detected in these full-length chimeric receptors (Fig. 2E), suggesting that the RXFP1 TMD was interfering with INSL3 binding. It is now generally accepted that the A chain of relaxin is involved in secondary interactions with the TMD of RXFP1 to stimulate receptor signaling. It is possible that the role of the A chain in INSL3 is different and that the inability of the INSL3 A chain to bind to the TMD of RXFP1 may be inhibiting binding of INSL3 to these chimeric receptors. Thus we inserted the RXFP2 TMD into the full-length RXFP1 chimeras producing RXFP12β1&TMD and RXFP12βN2&TMD. These receptors also exhibited no significant gain of INSL3 binding over RXFP1, even though they contain both a high-affinity INSL3 binding site and the TMD of RXFP2, which should be compatible with INSL3 binding (Fig. 3A).
Fig. 3.
A, Eu-INSL3 binding to full-length chimeric receptors, normalized to that of RXFP2. Eu-H2 relaxin competition binding curves on (B) RXFP1, (C) RXFP12βN2, (D) RXFP12βN2&TMD, (E) RXFP12β1, (F) RXFP12β1&TMD, (G) RXFP12β3, and (H) RXFP12β5 expressed on the surface of cells. Eu-H2 relaxin was competed with either H2 relaxin (solid circles) or INSL3 (open squares). Schematic representations of the receptor chimeras are displayed to the right of relevant graphs. Gray regions indicate RXFP1 sequence with black regions representing the insertion of RXFP2 sequences.
For a more sensitive measure of INSL3 binding, competition binding assays using Eu-H2 relaxin were used. As can be seen in Fig. 3, C–H and Table 1, Eu-H2 relaxin was able to bind to all of the chimeric receptors with similar affinity, indicating that the LRR were correctly folded and that all of the chimeric receptors were expressed on the cell surface. Although INSL3 competed poorly with Eu-H2 on RXFP1, binding of INSL3 could be detected with each of the full-length chimeric constructs. RXFP12βN2 and RXFP12βN2&TMD showed slight improvements in INSL3 binding, RXFP12β1 and RXFP12β1&TMD showed slightly more again, with RXFP12β3 and RXFP12β5 showing the greatest improvements in binding. However the INSL3 binding affinity was still much lower on the chimeric receptor when compared with the affinity of INSL3 to RXFP2 or ECD-1.
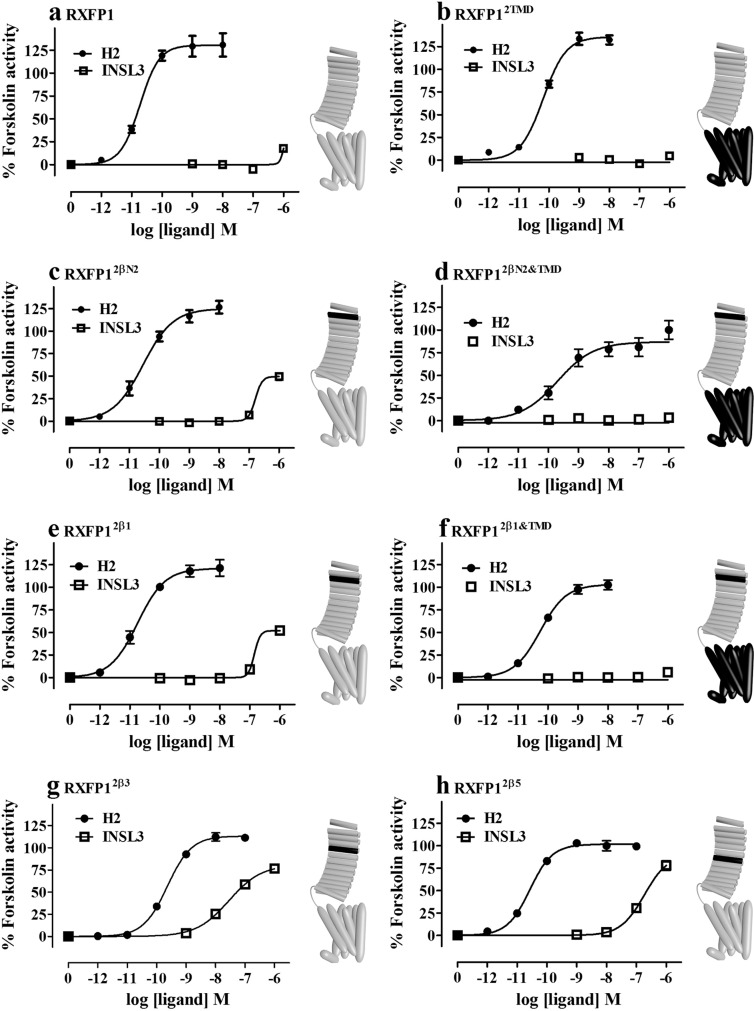
We then tested the ability of the peptides to stimulate cAMP in these chimeric receptors in comparison with RXFP1 and the RXFP12TMD chimera (Fig. 4). There were gains in INSL3-mediated activation using the chimeric receptors, which matched the Eu-H2 competition binding results. Although RXFP12β1 and RXFP12βN2 showed modest increases in activity (Fig. 4, C and E), this gain of function was not seen with the chimeras containing the TMD of RXFP2 (Fig. 4, D and F). Importantly, the potency of INSL3 was dramatically improved for RXFP12β3 and RXFP12β5 (Fig. 4, G and H). Thus, when RXFP1 is endowed with LRR that can bind to INSL3 with near-native RXFP2 affinity, some gain of INSL3 binding is conferred, but not to the same degree as seen in the ECD constructs, and these chimeras are largely irresponsive to INSL3.
Fig. 4.
Dose-response cAMP activity curves were measured from cells expressing (A) RXFP1, (B) RXFP2TMD, (C) RXFP12βN2, (D) RXFP12βN2&TMD, (E) RXFP12β1, (F) RXFP12β1&TMD, (G) RXFP12β3, and (H) RXFP12β5. Cells were stimulated with increasing concentrations of either H2 relaxin (solid circles) or INSL3 (open squares). Schematic representations of the receptor chimeras are displayed to the right of relevant graphs. Gray regions indicate RXFP1 sequence with black regions representing the insertion of RXFP2 sequences.
Structural model of the RXFP2-INSL3 complex
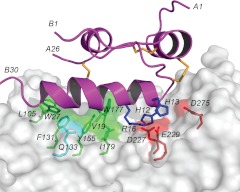
To provide a structural explanation for these mutational data, a model of the INSL3-RXFP2 ECD complex was created through molecular docking using the program HADDOCK (20). The solution NMR structure of INSL3 and the homology model of the LRR domain of the RXFP2 were used as starting structures. The docking process was driven by ambiguous restraints derived from mutational data on both the receptor and the peptide ligand. The ambiguous restraints were created in such a way that they place residues that are known to be important for binding in proximity to the binding partner protein, but with no bias as to which specific residues on the partner protein they interact with. Key residues on INSL3 identified as critical for receptor binding included HisB12, ArgB16, ValB19, and TrpB27 (14, 23), and key residues on RXFP2 identified as critical for ligand binding included Phe131, Gln133, Trp177, Ile179, Asp227, Glu229, Glu273, and Asp275 (7). The advantage of HADDOCK over other docking programs is that the docking involves both a rigid body docking and a refinement phase with molecular dynamics simulations, in which residues involved in binding and other regions defined by the user are allowed to move to optimize binding. This is particular useful for INSL3 in which the C-terminal tail, including the critical TrpB27, is flexible and only adopts correct conformation upon binding to the receptor. The final model of the complex is shown in Fig. 5, and from here it is clear that INSL3 orients in a way in which the B-chain helix is stretched in a straight line along the LRR repeats, forming contacts with a large portion of the surface.
Fig. 5.
Haddock model of INSL3 binding to RXFP2 ECD. RXFP2 ECD is shown as a transparent surface with side chains from key residues involved in the interaction shown as sticks. INSL3 is shown in ribbon representation, highlighting the positioning of the B-chain helix along the concave surface of the ECD. Interacting residues are labeled with single-letter amino acid codes and residue numbers, and the N- and C-termini of the INSL3 A and B chain are labeled with residue numbers for clarity. The figure was generated in PyMol.
Analysis of the models reveals a number of key interactions. Electrostatic attractions include HisB12-Asp227, HisB13-Glu229/Asp275, and the guanidinium group of ArgB16 is being chelated between the carboxyl groups of Asp227 and Glu229. Additional favorable interactions include packing of ValB19 against Tyr155, Trp177, and Ile179. The key side chain of TrpB27 of INSL3 interacts with Phe131 and Gln133 as previously proposed (7), but it also forms direct contacts with residues in LRR 1, including Leu105 (Ala108 in RXFP1), consistent with the mutational data identifying this region as critical for INSL3 binding. An increase in binding to the constructs ECD-12β3 and ECD-12β5 was also seen during the mutational studies. ECD-12β3 contains a single amino acid substitution, K156I, which is located on the leading edge of the β-strand, whereas the two adjacent substitutions found in ECD-12β5, I203F and L204F, are located on the edge of LRR5, at the start of the B-strand. Our model suggests potential interactions between Phe201 (Leu204 in RXFP1) and INSL3 ValB15, which may contribute to this effect. Ile153(Lys156 in RXFP1) is not in direct contact with the peptide, but its clustering together with other residues of the ligand-binding site, including Tyr155 and Trp177, may help in coordinating a correct conformation of the LRR surface. It is also possible that a longer Lys side chain in this position, as in native RXFP1, would provide steric hindrance for INSL3 binding.
Discussion
INSL3 and H2 relaxin are structurally related peptide hormones that mediate their actions through activation of the closely related relaxin family peptide receptors RXFP2 and RXFP1, respectively. Although H2 relaxin is also a high-affinity agonist for RXFP2, INSL3 has poor affinity for RXFP1. In this study we have described the differences in the primary ligand-binding site of the LRR responsible for this pharmacological difference. In addition we have uncovered a fundamental difference between these two receptors that is independent of the primary ligand-binding site. Our data indicate that it is likely that the LRR of RXFP1 are coordinated with the TMD of the receptor in a manner that precludes INSL3 binding, even if a high-affinity INSL3 binding site is present in the LRR. Overall, these two findings provide new mechanistic insights into the events leading to the activation of these complex G protein-coupled receptors.
INSL3 and H2 relaxin binding to the RXFP LRR differs
That the activation of RXFP1 and RXFP2 requires multiple interaction sites both in the LRR and the TMD is well established (10, 11). The fact that INSL3 binds with higher affinity to ECD-1 than full-length RXFP1, but nonetheless still with lower affinity than to RXFP2 or ECD-2 (11), suggests that a suboptimal primary binding site in the LRR of RXFP1 is not the only factor inhibiting INSL3 binding. Nonetheless, to identify the differences in the binding to the LRR, we created a suite of chimeric ECD constructs. Strikingly, three individual RXFP2 LRR repeats were identified that, when spliced into ECD-1, significantly improved INSL3 binding. By far, the most impressive gain in binding was achieved when the β-strand of LRR 1 from RXFP2 was introduced into ECD-1, with the INSL3 binding restored to the same level as RXFP2, suggesting that this region is critical for INSL3 binding. The four single amino acid substitutions making up RXFP12β1 were tested individually, but none were found to independently mediate high-affinity INSL3 binding, suggesting a coordination effect of multiple sites rather than a distinct pairwise interaction.
All ECD-1 chimeras were tested for H2 relaxin binding, and no significant drop in binding was seen for any construct, suggesting that the overall structure was retained and that the relaxin binding site was not altered, which is consistent with previous mutation studies on RXFP1 that identified binding residues that are highly conserved also in RXFP2.
A model of the INSL3-RXFP2 LRR was created and revealed an arrangement in which INSL3 lies with the B chain helix along the face of the domain, thereby interacting with a large portion of the LRR. Charged residues of INSL3 HisB12, HisB13, and ArgB16 interact with a cluster of negatively charged residues, Asp227, Glu229, and Asp275 located in LRR 6 and LRR 8 whereas the hydrophobic ValB19 interacts with a cluster of hydrophobic residues, Tyr155, Trp177 and Ile179, in LRR 3 and LRR 4. TrpB27 packs closely against the side chains Phe131 and Gln133 of LRR 2 as well as Leu108 and Ser110 of LRR 1. This overall arrangement is distinctly different from the model of H2 relaxin binding to RXFP1 presented by Büllesbach and Schwabe (8) in which the B chain helix of H2 relaxin lies on an angle of about 45° across the face, limiting the interactions to involve only LRR 5–8. This difference is consistent with mutational data on both RXFP2 and RXFP1 from this and previous studies (7, 13) and fits well with the fact that H2 relaxin has a smaller receptor binding site, only including an Arg-Arg-Ile binding cassette presented on the B chain helix. In contrast, INSL3 uses residues on the B chain helix as well as the key contributor to binding, TrpB27, which is located further away. These distinct differences in binding mode nicely explain all observations of binding affinities to chimeric ECD constructs.
Optimizing the RXFP1-ECD is not enough to allow INSL3 binding and activation
Having identified the RXFP1 LRR mutations required for INSL3 binding, these changes were introduced into full-length RXFP1. Strikingly, despite ECD-12β1 having indistinguishable affinity for INSL3 compared with RXFP2, when these mutations were placed into full-length RXFP1, no gain of INSL3 binding was observed. This was a rather unexpected finding indicating that secondary factors other than the primary binding site played a large role in disallowing INSL3 binding.
To probe whether the proposed secondary hormone interaction site within the extracellular loops of the TMD was playing a role in the lack of INSL3 binding, we replaced the RXFP1 TMD with that of RXFP2 in these chimeras. Thus these receptors contained a high-affinity INSL3 binding site as well as the RXFP2 TMD containing any potential secondary INSL3 binding site. However, no dramatic restoration of INSL3 binding was observed, with only a slight increase in affinity for INSL3 measurable, indicating that the chimeric full-length receptors were not missing a secondary INSL3 interaction site that could be reconstituted with the RXFP2 TMD.
Thus it seemed likely that the structural arrangement of the LRR in relation to the TMD of RXFP1 and RXFP2 was incompatible with INSL3 binding. Because of the large conformational differences between the primary binding conformations of relaxin on RXFP1 and INSL3 on RXFP2, the juxtaposition of the RXFP1 LRR in relation to the TMD may result in steric hindrances to the INSL3 mode of binding. Interactions between the LRR and the TMD are likely to be mediated by residues on the side or back of the LRR superhelix, not in the concave binding surface that we have mutated to achieve high-affinity INSL3 binding.
From a number of studies it is clear that the interaction between relaxin and RXFP1 is distinct from the interaction between INSL3 and RXFP2, despite the close homology between both the receptors and the peptides. Truncation of the A chain of H2 relaxin results in the progressive loss of both binding affinity and efficacy on RXFP1 and RXFP2 (24), whereas the removal (25, 26) or complete replacement (27) of the A chain of INSL3 results in the retention of high-affinity binding to RXFP2, but a complete loss of efficacy. This highlights the synergy between the B and A chains of H2 relaxin in mediating receptor binding to RXFP1 and RXFP2.
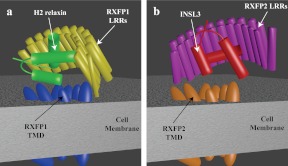
These facts, along with the results presented here, enable us to postulate models for the molecular function of RXFP1 and RXFP2. RXFP1 requires strict coordination of the ECD with the TMD to allow sequential A chain- and B chain-mediated interactions and subsequent receptor activation (Fig. 6A). It is likely that interactions between the TMD and the N-terminal LRR maintain the ECD in the correct juxtaposition with the TMD. Because the B chain helix of H2 relaxin binds across the LRR at an angle of 45°, the A chain helices lie across the top of the TMD, where secondary hormone binding occurs (Fig. 6A). The B chain helix of INSL3, on the other hand, binds perpendicular to the LRR β-strands of RXFP2 (Fig. 5), resulting in projection of the A-chain helices downward, parallel to the LRR β strands. This binding mechanism is incompatible with the coordination of the ECD of RXFP1, possibly because the A chain cannot bind the TMD in a H2 relaxin-like manner, which is absolutely essential for H2 relaxin-like binding. It is likely that the interactions controlling the coordination of the ECD of RXFP1 would need to be modified to allow the LRR to move into a conformation that allows binding of INSL3, reliant solely on B chain-mediated interactions (Fig. 6B). Further, in-depth analysis of the overall conformations would require elucidation of the receptor structures in complex with H2 relaxin or INSL3; however, this study has clearly demonstrated that there is a complex interplay between the ECD and TMD of RXFP1 and RXFP2 that enables these ancient and complex receptors to respond to the different binding mechanisms of their native hormone ligands. As with the related glycoprotein hormone receptors (GPHR), RXFP1 and RXFP2 are unusual members of G protein-coupled receptor family A in that they have large ECD that are intricately involved in receptor activation. It is clear that RXFP1 and RXFP2 function in a completely unique manner to the GPHR, with activation being dependent on the LDLa module, which is absent in all GPHR (4, 9). However, similar to the GPHR (28, 29), it is unclear exactly how the ECD contribute to RXFP1 and RXFP2 activation, and studies like ours are essential for elucidating the molecular mechanisms of these clinically relevant receptors so that they can be more effectively targeted for drug discovery.
Fig. 6.
Schematic representation of how RXFP1 and RXFP2 may bind H2 relaxin and INSL3. A, H2 relaxin binds to RXFP1 with the relaxin B chain α-helix binding across the face of the LRR at an angle of 45°. This positions the A chain N-terminal α-helix across the extracellular loops of the TMD of RXFP1. Interactions between the LRR and the TMD of RXFP1 hold the receptor in the conformation needed to coordinate the sites and facilitate H2 relaxin binding. These interactions, however, hinder the binding mode of INSL3 to the ECD, which requires a 90° binding angle between the LRR β-strands and the B chain α-helix (B).
Supplementary Material
Acknowledgments
We thank Tracey Wilkinson (Florey Neuroscience Institutes) for assistance with assembly of RXFP1 and RXFP2 sequences; Tania Ferraro (Florey Neuroscience Institutes) and Sharon Layfield (Florey Neuroscience Institutes) for binding and signaling assays; Dr. Quentin Kaas (University of Queensland) for assistance with modeling; Dr. Satoko Sudo and Professor Aaron Hsueh (both at Stanford University) for provision of ECD1, ECD2, and RXFP12TMD constructs; Professor John Wade (Florey Neuroscience Institutes) for provision of synthetic INSL3; and Professor Geoff Tregear (Florey Neuroscience Institutes) for support.
Studies at the Florey Neuroscience Institutes were supported by National Health and Medical Research Council (NHMRC) grants 454375 and 628427 and by the Victorian Government's Operational Infrastructure Support Program. D.J.S. and R.A.D.B. are recipients of Australian NHMRC and C. J. Martin and Senior Research Fellowships, respectively. K.J.R. is a recipient of an NHMRC Career Development Award.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ECD
- Extracellular domain
- GPHR
- glycoprotein hormone receptors
- HEK
- human embryonic kidney
- INSL3
- insulin-like peptide 3
- LDLa
- lipoprotein class A
- LRR
- leucine-rich repeat
- NMR
- nuclear magnetic resonance
- RXFP
- relaxin family peptide receptor
- TM
- transmembrane
- TMD
- TM domain.
References
- 1. Bathgate RAD, Hsueh AJ, Sherwood OD. 2006. Physiology and molecular biology of the relaxin peptide family. In: (Secondary Bathgate RAD, Hsueh AJ, Sherwood OD.) (Neill JD, ed. Physiology of reproduction. 3rd ed San Diego: Elsevier; 679–770 [Google Scholar]
- 2. Bathgate RA, Ivell R, Sanborn BM, Sherwood OD, Summers RJ. 2006. International Union of Pharmacology: recommendations for the nomenclature of receptors for relaxin family peptides. Pharmacol Rev 58:7–31 [DOI] [PubMed] [Google Scholar]
- 3. Hsu SY, Liang SG, Hsueh AJ. 1998. Characterization of two LGR genes homologous to gonadotropin and thyrotropin receptors with extracellular leucine-rich repeats and a G protein-coupled, seven-transmembrane region. Mol Endocrinol 12:1830–1845 [DOI] [PubMed] [Google Scholar]
- 4. Scott DJ, Layfield S, Yan Y, Sudo S, Hsueh AJ, Tregear GW, Bathgate RA. 2006. Characterization of novel splice variants of LGR7 and LGR8 reveals that receptor signaling is mediated by their unique LDLa modules. J Biol Chem 281:34942–34954 [DOI] [PubMed] [Google Scholar]
- 5. Hopkins EJ, Layfield S, Ferraro T, Bathgate RA, Gooley PR. 2007. The NMR solution structure of the relaxin (RXFP1) receptor lipoprotein receptor class A module and identification of key residues in the N-terminal region of the module that mediate receptor activation. J Biol Chem 282:4172–4184 [DOI] [PubMed] [Google Scholar]
- 6. Kern A, Agoulnik AI, Bryant-Greenwood GD. 2007. The low-density lipoprotein class A module of the relaxin receptor (leucine-rich repeat containing G-protein coupled receptor 7): its role in signaling and trafficking to the cell membrane. Endocrinology 148:1181–1194 [DOI] [PubMed] [Google Scholar]
- 7. Scott DJ, Wilkinson TN, Zhang S, Ferraro T, Wade JD, Tregear GW, Bathgate RA. 2007. Defining the LGR8 residues involved in binding insulin-like peptide 3. Mol Endocrinol 21:1699–1712 [DOI] [PubMed] [Google Scholar]
- 8. Büllesbach EE, Schwabe C. 2005. The trap-like relaxin-binding site of the leucine-rich G-protein-coupled receptor 7. J Biol Chem 280:14051–14056 [DOI] [PubMed] [Google Scholar]
- 9. Hsu SY, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Sherwood OD, Hsueh AJ. 2002. Activation of orphan receptors by the hormone relaxin. Science 295:671–674 [DOI] [PubMed] [Google Scholar]
- 10. Sudo S, Kumagai J, Nishi S, Layfield S, Ferraro T, Bathgate RA, Hsueh AJ. 2003. H3 relaxin is a specific ligand for LGR7 and activates the receptor by interacting with both the ectodomain and the exoloop 2. J Biol Chem 278:7855–7862 [DOI] [PubMed] [Google Scholar]
- 11. Halls ML, Bond CP, Sudo S, Kumagai J, Ferraro T, Layfield S, Bathgate RA, Summers RJ. 2005. Multiple binding sites revealed by interaction of relaxin family peptides with native and chimeric relaxin family peptide receptors 1 and 2 (LGR7 and LGR8). J Pharmacol Exp Ther 313:677–687 [DOI] [PubMed] [Google Scholar]
- 12. Hartley BJ, Scott DJ, Callander GE, Wilkinson TN, Ganella DE, Kong CK, Layfield S, Ferraro T, Petrie EJ, Bathgate RAD. 2009. Resolving the unconventional mechanisms underlying RXFP1 and RXFP2 receptor function. Ann NY Acad Sci 1160:67–73 [DOI] [PubMed] [Google Scholar]
- 13. Scott DJ, Tregear GW, Bathgate RAD. 2009. Modelling the primary binding site of RXFP1 and RXFP2. Ann NY Acad Sci 1160:74–77 [DOI] [PubMed] [Google Scholar]
- 14. Rosengren KJ, Zhang S, Lin F, Daly NL, Scott DJ, Hughes RA, Bathgate RA, Craik DJ, Wade JD. 2006. Solution structure and characterization of the receptor binding surface of insulin-like peptide 3. J Biol Chem 281:28287–28295 [DOI] [PubMed] [Google Scholar]
- 15. Shabanpoor F, Hughes RA, Bathgate RA, Zhang S, Scanlon DB, Lin F, Hossain MA, Separovic F, Wade JD. 2008. Solid-phase synthesis of europium-labeled human INSL3 as a novel probe for the study of ligand-receptor interactions. Bioconjug Chem 19:1456–1463 [DOI] [PubMed] [Google Scholar]
- 16. Zheng L, Baumann U, Reymond JL. 2004. An efficient one-step site-directed and site-saturation mutagenesis protocol. Nucleic Acids Res 32:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hossain MA, Rosengren KJ, Zhang S, Bathgate RA, Tregear GW, van Lierop BJ, Robinson AJ, Wade JD. 2009. Solid phase synthesis and structural analysis of novel A-chain dicarba analogs of human relaxin-3 (INSL7) that exhibit full biological activity. Org Biomol Chem 7:1547–1553 [DOI] [PubMed] [Google Scholar]
- 18. Yan Y, Scott DJ, Wilkinson TN, Ji J, Tregear GW, Bathgate RA. 2008. Identification of the N-linked glycosylation sites of the human relaxin receptor and effect of glycosylation on receptor function. Biochemistry 47:6953–6968 [DOI] [PubMed] [Google Scholar]
- 19. Chen W, Shields TS, Stork PJ, Cone RD. 1995. A colorimetric assay for measuring activation of Gs- and Gq-coupled signaling pathways. Anal Biochem 226:349–354 [DOI] [PubMed] [Google Scholar]
- 20. Dominguez C, Boelens R, Bonvin AM. 2003. HADDOCK: a protein-protein docking approach based on biochemical or biophysical information. J Am Chem Soc 125:1731–1737 [DOI] [PubMed] [Google Scholar]
- 21. Büllesbach EE, Schwabe C. 2006. The mode of interaction of the relaxin-like factor (RLF) with the leucine-rich repeat G protein-activated receptor 8. J Biol Chem 281:26136–26143 [DOI] [PubMed] [Google Scholar]
- 22. He XL, Bazan JF, McDermott G, Park JB, Wang K, Tessier-Lavigne M, He Z, Garcia KC. 2003. Structure of the Nogo receptor ectodomain: a recognition module implicated in myelin inhibition. Neuron 38:177–185 [DOI] [PubMed] [Google Scholar]
- 23. Büllesbach EE, Schwabe C. 2005. LGR8 signal activation by the relaxin-like factor. J Biol Chem 280:14586–14590 [DOI] [PubMed] [Google Scholar]
- 24. Hossain MA, Rosengren KJ, Haugaard-Jönsson LM, Zhang S, Layfield S, Ferraro T, Daly NL, Tregear GW, Wade JD, Bathgate RA. 2008. The A-chain of human relaxin family peptides has distinct roles in the binding and activation of the different relaxin family peptide receptors. J Biol Chem 283:17287–17297 [DOI] [PubMed] [Google Scholar]
- 25. Del Borgo MP, Hughes RA, Bathgate RA, Lin F, Kawamura K, Wade JD. 2006. Analogs of insulin-like peptide 3 (INSL3) B-chain are LGR8 antagonists in vitro and in vivo. J Biol Chem 281:13068–13074 [DOI] [PubMed] [Google Scholar]
- 26. Shabanpoor F, Bathgate RA, Hossain MA, Giannakis E, Wade JD, Hughes RA. 2007. Design, synthesis and pharmacological evaluation of cyclic mimetics of the insulin-like peptide 3 (INSL3) B-chain. J Pept Sci 13:113–120 [DOI] [PubMed] [Google Scholar]
- 27. Shabanpoor F, Zhang S, Hughes RA, Hossain MA, Layfield S, Ferraro T, Bathgate RA, Separovic F, Wade JD. 2011. Design and development of analogues of dimers of insulin-like peptide 3 B-chain as high-affinity antagonists of the RXFP2 receptor. Biopolymers 96:81–87 [DOI] [PubMed] [Google Scholar]
- 28. Fan QR, Hendrickson WA. 2005. Structure of human follicle-stimulating hormone in complex with its receptor. Nature 433:269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jiang X, Liu H, Chen X, Chen PH, Fischer D, Sriraman V, Yu HN, Arkinstall S, He X. 2012. Structure of follicle-stimulating hormone in complex with the entire ectodomain of its receptor. Proc Natl Acad Sci USA 109:13491–12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.