Abstract
Dentin matrix protein-1 (DMP1) or phosphate-regulating gene with homologies to endopeptidases on the X chromosome (PHEX) inactivation results in elevation of the phosphaturic hormone fibroblast growth factor (FGF)-23, leading to hypophosphatemia, aberrant vitamin D metabolism, and rickets/osteomalacia. Compound mutant Phex-deficient Hyp and Dmp1ko mice exhibit nonadditive phenotypes, suggesting that DMP1 and PHEX may have interdependent effects to regulate FGF23 and bone mineralization. To determine the relative importance of DMP1 and PHEX in regulating FGF23 and mineralization, we tested whether the transgenic expression of full-length [Dmp1Tg(full-length)] or C-terminal Dmp1 [Dmp1Tg(57kDa)] could rescue the phenotype of Hyp mice. We found that Dmp1ko and Hyp mice have similar phenotypes characterized by decreased cortical bone mineral density (−35% vs. wild type, P < 0.05) and increased serum FGF23 levels (∼12-fold vs. wild type, P < 0.05). This was significantly corrected by the overexpression of either the full-length or the C-terminal transgene in Dmp1ko mice. However, neither of the transgenes rescued the Hyp mice phenotype. Hyp/Dmp1Tg(full-length) and Hyp mice were similar, but Hyp/Dmp1Tg(57 kDa) mice exhibited worsening of osteomalacia (−20% cortical bone mineral density) in association with increased serum FGF23 levels (+2-fold) compared with Hyp mice. Bone FGF23 mRNA expression was decreased and a 2-fold increase in the ratio of the full-length/degraded circulating FGF23 was observed, indicating that degradation of FGF23 was impaired in Hyp/Dmp1Tg(57 kDa) mice. The paradoxical effects of the C-terminal Dmp1 transgene were observed in Hyp/Dmp1Tg(57 kDa) but not in Dmp1Tg(57 kDa) mice expressing a functional PHEX. These findings indicate a functional interaction between PHEX and DMP1 to regulate bone mineralization and circulating FGF23 levels and for the first time demonstrate effects of the C-terminal DMP1 to regulate FGF23 degradation.
Inactivation of the bone extracellular matrix protein dentin matrix protein-1 (DMP1) or the phosphate-regulating gene with homologies to endopeptidases on the X chromosome (PHEX) results in nearly identical elevation in bone production of the phosphaturic hormone fibroblast growth factor (FGF)-23 (1, 2) and leads to similar phenotypes characterized by hypophosphatemia, aberrant vitamin D metabolism, and rickets/osteomalacia, otherwise known as autosomal recessive hypophosphatemic rickets (ARHR) and X-Linked hypophosphatemic rickets, respectively (2–6). The mechanisms whereby DMP1 and PHEX regulate the circulating FGF23 concentration have not been fully elucidated.
DMP1 is 106-kDa extracellular matrix protein expressed in osteoblasts and osteocytes and belongs to the small integrin-binding ligand interacting glycoproteins (SIBLING) protein family. The full-length latent DMP1 protein is cleaved in two fragments: a 37-kDa N-terminus and 57-kDa C-terminus peptides. It has been proposed that the proteolytic processing of DMP1 into its N- and C-terminal fragments is necessary for normal function of DMP1 in bone (7). SIBLING all share a characteristic RGD motif for integrin binding and an acidic serine aspartate rich motif (ASARM). In DMP1, both motifs are located on the highly phosphorylated 57-kDa C-terminus peptide. The 37-kDa N-terminus fragment from DMP1 is a proteoglycan with a chondroitin sulfate chain attached through Ser74 that binds to proMMP-9 and may sequester growth factors (8). Dmp1 knockout (Dmp1ko) mice have a phenotype equivalent to ARHR (9, 10). The phenotype of the Dmp1ko mice is rescued by the overexpression of either the collagen type Ia1 (3.6kb) promoter-driven full-length Dmp1 or the 57-kDa C-terminus Dmp1 transgenes (11). However, it remains unclear whether the rescue was due to a direct effect of DMP1 to correct FGF23 production and hypophosphatemia or due to additional effects of DMP1 to correct the bone mineralization defect.
PHEX is a 105-kDa cell membrane metalloendopeptidase expressed in osteoblastic cells for which no definitive substrate has yet been defined. Indeed, studies have shown that PHEX had a very high affinity for matrix extracellular phosphoglycoprotein (MEPE) and osteopontin, two mineral binding SIBLING proteins but very low proteolytic activity (12, 13). The conditional deletion of Phex in the osteoblast lineage in vivo is sufficient to reproduce the X-Linked hypophosphatemic rickets phenotype in Hyp mice (14). However, the only attempts to rescue the Hyp phenotype by Phex gene restoration in Hyp mice never fully succeeded (15–17), suggesting the presence of a cofactor necessary for PHEX function. One interpretation of the observation of the nonadditive effects on FGF23 expression and defective mineralization in compound mutant Hyp and Dmp1ko is that the similar defects observed in Hyp or Dmp1ko mice are due to a common pathway involving both PHEX and DMP1 (5). Indeed, the inhibition of FGF receptor 1 suppresses increments of FGF23 in either Hyp- or Dmp1ko-derived bone marrow stromal cells, suggesting that this common pathway may involve local activation of FGF receptor signaling in bone (5).
To gain additional insight into the role of DMP1 in the pathology associated with Phex deficiency, we tested whether the transgenic expression of full-length or C-terminal Dmp1 could rescue the phenotype of Hyp mice.
Materials and Methods
Animals and genotyping
The Dmp1ko mice and both types of Dmp1 transgenic (Dmp1Tg) mice were created as described previously (18–20). The Dmp1Tg mice overexpress either the full-length or the C-terminal region of DMP1 [Dmp1Tg(full length) and Dmp1Tg(57 kDa), respectively]. Hyp mice were originally purchased from the Jackson Laboratory (Bar Harbor, ME). All mice were maintained on the C57BL/6J genetic background for more than 10 generations and were fed a standard diet (7912; Harlan Teklad, Madison, WI) containing 0.98% calcium, 0.66% phosphorus, and 2.39 IU of Vitamin D per gram. Animal care and protocols were in accordance with the guidelines established by the University of Tennessee Institutional Animal Care and Use Committee as detailed in the Guide for Care and Use of Laboratory Animals, prepared by the Institute on Laboratory Animal Resources, National Research Council (Department of Health and Human Services Publication NIH 86-23, National Academy Press, 1996).
We first crossed heterozygous Dmp1+/− females to Dmp1Tg males to obtain Dmp1+/−/Dmp1Tg males and then crossed Dmp1+/− females to Dmp1+/−/Dmp1Tg males to obtain the Dmp1−/−/Dmp1Tg mice. We also crossed heterozygous female Hyp (XXHyp) mice to Dmp1Tg males to obtain the Hyp/Dmp1Tg mice. For the entire study, samples were collected from 5-wk-old wild-type (WT), Dmp1Tg(full length), Dmp1Tg(57 kDa), XHypY (Hyp), Hyp/Dmp1Tg(full length), Hyp/Dmp1Tg(57 kDa), Dmp1ko, Dmp1ko/Dmp1Tg(full length), and Dmp1ko/Dmp1Tg(57 kDa) male littermates.
Ear biopsies were collected to genotype the mice. REDExtract-N-Amp tissue PCR kit (Sigma-Aldrich, St. Louis, MO) was used for DNA extraction and PCR amplification. Mice were genotyped for Phex and Dmp1 mutations using previously described primers (3, 18, 20).
Biochemistry
Serum samples were collected by intracardiac exsanguination and urine samples were collected overnight in metabolic cages. Calcium was measured using a calcium CPC Liquicolor kit (Stanbio Laboratories, Boerne, TX), and phosphorus was measured using the phosphomolybdylate-ascorbic acid method, as previously described (3). Creatinine levels were measured using a creatinine kit (Pointe Scientific Inc., Canton, MI). The fractional excretion of phosphate (Pi) was obtained after the equation FE = (urine Pi/serum Pi)/(urine creatinine/serum creatinine) ×100. The fractional excretion of calcium was obtained similarly. Serum PTH levels were measured using the mouse intact PTH ELISA kit (Immutopics, San Clemente, CA). Serum 1,25-(OH)2D levels were measured using the 1,25 dihydroxyvitamin D enzyme immunoassay kit (Immunodiagnostic Systems, Fountain Hills, AZ). Serum intact full-length FGF23 levels were measured using the FGF23 ELISA kit (Kainos Laboratories, Tokyo, Japan). Serum C-terminus FGF23 levels were measured using the FGF23 (C-Term) ELISA kit (Immutopics, Carlsbad, CA). Standards of the C-terminus FGF23 kit were used in both intact and C-terminus FGF23 kits to adjust the C-terminus FGF23 values and compare them with the intact FGF23 values. The degraded FGF23 values were obtained by subtracting the full-length FGF23 values from the C-terminus FGF23 values. The ratio between full-length and degraded FGF23 values was subsequently performed and indicates the level of degradation of FGF23, the higher values corresponding to an increased proportion of the intact FGF23 as compared with the degraded form.
High resolution three-dimensional (3D) microtomography
The femurs were collected, fixed, and dehydrated in 70% ethanol. High-resolution microcomputed tomography (μCT; μCT40; Scanco Medical, Basserdorf, Switzerland) was used to scan and evaluate the metaphyseal trabecular bone microarchitecture and the midshaft cortical bone parameters. The entire femurs were scanned in a 12.3-mm diameter sample holder at 6-μm resolution: energy level of 55 KeV and intensity of 145 μA. Evaluation of the bone growth was obtained by measuring the length of the scanned femur. The trabecular bone volume (BV/TV) was measured within the secondary spongiosa on a set of 50 sections (0.6 mm) underneath the growth plate at a threshold of 200. Trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular separation (Tb.Sp), and structure model index (SMI) were calculated without assuming a constant model, as previously described (21). The cortical bone structure was analyzed from 100 sections chosen at the midshaft of each femur at a threshold of 350.
Bone histology
Femurs were then embedded in methylmetacrylate at low temperature. Animals were labeled by ip injection of 30 μg/g alizarin complexone dehydrate (Sigma-Aldrich) respectively on d 7 and 2 before the animals were killed. Five-micrometer nonserial longitudinal frontal slices were cut from the embedded bones with a microtome (Reichert-Jung Polycut-S, Leica Microsystems Inc., Buffalo Grove, IL) and were either left unstained or were used for a modified Goldner staining.
Bone immunohistochemistry
Tibias were fixed in 4% paraformaldehyde and embedded in paraffin. Five-micrometer-thick sections were cut, dried overnight, deparaffinized, and rehydrated. After antigen retrieval by incubation in citric acid buffer 10 mm (pH 3) for 60 min at 37 C, nonspecific sites were blocked with 1× animal free blocker (Vector Laboratories Inc., Burlingame, CA), and then sections were incubated with a rabbit-raised, antimouse, DMP1-specific polyclonal primary antibody (Abcam Inc., Cambridge, MA) for 1 h. An Immunohistological Vectastain rabbit ABC kit (Vector Laboratories) was used, the endogenous alkaline phosphatase was blocked by levamisol (Vector Laboratories), and the presence of DMP1 was detected using the Vector Red alkaline phosphatase substrate kit (Vector Laboratories). Sections were dehydrated and mounted with Entellan medium. Sections were visualized using bright-field microscopy and fluorescence (IX71; Olympus Latin Inc., Miami, FL).
RT-PCR
RT-PCR analyses were performed on hindlimb bones and kidneys. Total RNA were isolated using TRI-reagent (Molecular Research Center, Cincinnati, OH) according to a previously published method (4). First-strand cDNA was synthesized from RNA using an iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). The 20-μl reverse transcriptase reaction was based on 1 μg total RNA. The iCycler iQ real-time PCR detection system and SsoFast Evagreen Supermix (Bio-Rad Laboratories) were used for real-time quantitative PCR analysis. The expression was normalized by glyceraldehyde-3-phosphate dehydrogenase (Gapdh) in the same sample and expressed as 100% of the control (WT). The sequences of primers used for real-time quantitative RT-PCR are as follows: Dmp1 forward, 5′-AGT GAG GAG GAC AGC CTG AA-3′, reverse, 5′-GAG GCT CTC GTT GGA CTC AC-3′ (85 bp); Fgf23 forward, 5′-CAC TGC TAG AGC CTA TTC-3′, reverse, 5′-CAC TGT AGA TAG TCT GAT GG-3′ (153 bp); sodium-dependent phosphate cotransporter 2 (Npt2)-a forward, 5′-GCC AAT GTC ATC CAG AAG GT-3′, reverse, 5′-ACA GTA GGA TGC CCG AGA TG-3′ (318 bp); Npt2c forward, 5′-CGT GCG GAC TGT TAT CAA TG-3′, reverse, 5′-TAC TGG GCA GTC AGG TTT CC-3′ (374 bp); Cyp27b1 forward, 5′-ACA CTT CGC ACA GTT TAC G-3′, reverse, 5′-TTA GCA ATC CGC AAG CAC-3′ (138 bp).
Statistics
Differences among the groups were tested by one-way ANOVA followed by a Fisher post hoc test using the Statistica software (Statsoft, Tulsa, OK). The differences were considered statistically significant at P < 0.05.
Results
Overexpression of Dmp1 (full length or C terminus) partially rescues the Dmp1ko phenotype
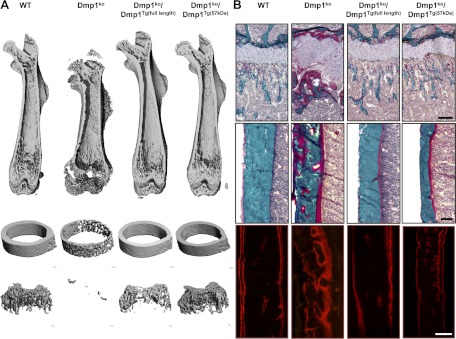
To validate our model, we first tested the effect of the collagen type Ia1 (3.6 kb) promoter-driven overexpression of Dmp1 (full length or C-terminal) on the phenotype of Dmp1ko mice. It was previously reported (11, 20, 22) that the C-terminal peptide of DMP1 is sufficient to correct the Dmp1ko phenotype. Consistent with prior reports (1, 2, 5, 11), Dmp1ko mice display significantly increased serum FGF23 and PTH levels, in addition to hypocalcemia and hypophosphatemia (Table 1). Dmp1ko mice also have pronounced osteomalacia and rickets as evidenced by the disorganized growth plate, shorter bones, increased osteoid volume, and diffused alizarin red S double labeling of the bone (Table 2 and Fig. 1). We verified by RT-PCR that both Dmp1 transgenes were overexpressed in bone in both compound mutants Dmp1ko/Dmp1Tg(full length) (+13-fold, P < 0.05 vs. WT) and Dmp1ko/Dmp1Tg(57 kDa) (+72-fold, P < 0.05 vs. WT). Both Dmp1 transgenes significantly reduced the elevated serum FGF23 levels observed in Dmp1ko mice (1402 to 181 and 140 pg/ml in Dmp1ko/Dmp1Tg(full length) and Dmp1ko/Dmp1Tg(57 kDa), respectively), but serum FGF23 values remained nearly 2-fold greater than WT mice (94 pg/ml), and the hypophosphatemia was only partially corrected, achieving values intermediate by WT and Dmp1ko mice (Table 1). This is in contrast with previous published reports (11) and may be due to the differences in genetic background, the age of the mice, and/or diet between the two studies. This partial rescue is reminiscent of the inability of PHEX overexpression to fully rescue the Hyp phenotype (15–17). The bone phenotype was also partially rescued in Dmp1ko by both transgenes. The growth defect and the growth plate disorders, however were fully corrected (Fig. 1). The 3D-microCT analysis of the femur revealed that the correction of the microarchitecture was partial (Table 2). Indeed, most of the trabecular bone parameters and the cortical bone mineral density (Ct.BMD) were still significantly different from the WT values in both Dmp1ko/Dmp1Tg groups. Of note, the improvement observed in the trabecular compartment due to the C-terminal transgene was greater than the one observed due to the full-length transgene (Table 2 and Fig. 1A).
Table 1.
Serum and urine biochemistry of WT, Dmp1ko, Dmp1ko/Dmp1Tg(full length), and Dmp1ko/Dmp1Tg(57 kDa)
| WT | Dmp1ko | Dmp1ko/Dmp1Tg(full length) | Dmp1ko/Dmp1Tg(57 kDa) | |
|---|---|---|---|---|
| Serum | ||||
| Full-length FGF23 (pg/ml) | 94.1 ± 8.1 | 1402.5 ± 121.0a | 181.1 ± 32.7a, b | 139.5 ± 45.9b |
| 1,25(OH)2D (pg/ml) | 168.6 ± 19.2 | 198.2 ± 37.4 | 132.3 ± 10.2 | 200.2 ± 35.9 |
| PTH (pg/ml) | 44.6 ± 7.9 | 92.4 ± 17.6a | 82.4 ± 40.3 | 70.8 ± 23.0 |
| Pi (mg/dl) | 8.2 ± 0.4 | 4.2 ± 0.2a | 6.4 ± 1.4b | 7.6 ± 0.6b |
| Ca (mg/dl) | 7.8 ± 0.3 | 6.6 ± 0.1a | 7.2 ± 0.5 | 6.9 ± 0.1a, b |
| Urine | ||||
| FE phosphate (%) | 18.9 ± 3.1 | 26.7 ± 5.7 | 11.6 ± 0.2 | 11.8 ± 1.2b |
| FE calcium (%) | 0.8 ± 0.1 | 1.1 ± 0.3 | 1.4 ± 0.5 | 0.4 ± 0.1a |
Values are expressed as mean ± se from at least five mice per group at 5 wk of age. Comparisons were performed using one-way ANOVA and post hoc Fisher test. FE, Fractional excretion.
P < 0.05 vs. WT.
P < 0.05 vs.Dmp1ko.
Table 2.
3D-microCT analysis of femur distal metaphysis trabecular structure and midshaft cortical envelope
| WT | Dmp1ko | Dmp1ko/Dmp1Tg(full length) | Dmp1ko/Dmp1Tg(57 kDa) | |
|---|---|---|---|---|
| Trabecular bone parameters | ||||
| Tb.BMD (mg HA/cm3) | 149.9 ± 11.3 | 2.3 ± 0.8a | 29.8 ± 3.1a,b | 68.4 ± 13.8a,b |
| BV/TV (%) | 21.2 ± 1.2 | 0.7 ± 0.2a | 7.1 ± 3.1a,b | 11.5 ± 2.1a,b |
| Tb.N. (−1) | 5.2 ± 0.2 | 1.8 ± 0.1a | 2.7 ± 0.4a,b | 4.0 ± 0.2a,b |
| Tb.Th (μm) | 56.9 ± 3.1 | 38.1 ± 1.0a | 45.6 ± 4.7b | 49.3 ± 4.9b |
| Tb.Sp (μm) | 188.6 ± 8.4 | 557.6 ± 27.7a | 280.6 ± 51.3a,b | 239.1 ± 17.7a,b |
| Conn.Dens. | 231.8 ± 17.8 | 1.7 ± 1.0a | 74.0 ± 28.5a,b | 116.7 ± 15.3a,b |
| SMI | 1.7 ± 0.1 | 3.5 ± 0.1a | 2.4 ± 0.1a,b | 2.4 ± 0.2a,b |
| DA | 1.89 ± 0.05 | 1.81 ± 0.06 | 1.82 ± 0.07 | 1.85 ± 0.03 |
| Cortical bone parameters | ||||
| Ct.BMD (mg HA/cm3) | 862.0 ± 8.3 | 549.2 ± 16.6a | 809.3 ± 32.5a,b | 797.8 ± 15.5a,b |
| Ct.Th. (μm) | 146.2 ± 7.3 | 50.1 ± 2.3a | 133.9 ± 3.4b | 118.7 ± 4.5a,b |
| CSA (mm2) | 2.31 ± 0.09 | 2.47 ± 0.09 | 2.48 ± 0.01 | 3.03 ± 0.12a,b |
| Ct.Ar. (mm2) | 0.60 ± 0.03 | 0.18 ± 0.02a | 0.59 ± 0.01b | 0.55 ± 0.03b |
| Ma.Ar. (mm2) | 1.73 ± 0.06 | 2.40 ± 0.14a | 1.89 ± 0.02 | 2.33 ± 0.14a |
Values are expressed as mean ± se from five mice per group at 5 wk of age. Comparisons were performed using one-way ANOVA and post hoc Fisher test. Tb.BMD, Trabecular bone mineral density; Conn.Dens, connectivity density; DA, degree of anisotropy; Ct.BMD, cortical bone mineral density; Ct.Th, cortical thickness; CSA, cross sectional area; Ct.Ar, cortical area; Ma.Ar, marrow area.
P < 0.05 vs. WT.
P < 0.05 vs. Dmp1ko.
Fig. 1.
Bone phenotype of 5-wk-old WT, Dmp1ko, Dmp1ko/Dmp1Tg(full length), and Dmp1ko/Dmp1Tg(57 kDa) mutant mice. A, 3D-μCT representation of (from top to bottom) the entire femur, the femoral midshaft, and the femoral distal metaphysis. B, Modified Goldner staining on femur histological section showing (top panels) the distal growth plate and metaphysis and (middle panels) the cortical bone at midshaft; the bottom panels show the fluorescent Alizarine Red S double labeling of the cortical bone. Scale bar represents 100 μm.
The overexpression of Dmp1 (full length or C- terminal) does not rescue the gross phenotype of Hyp mice
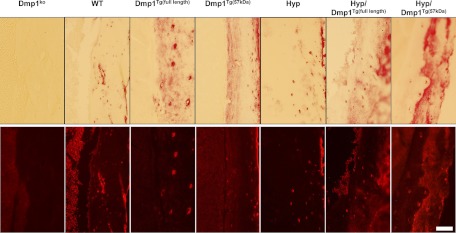
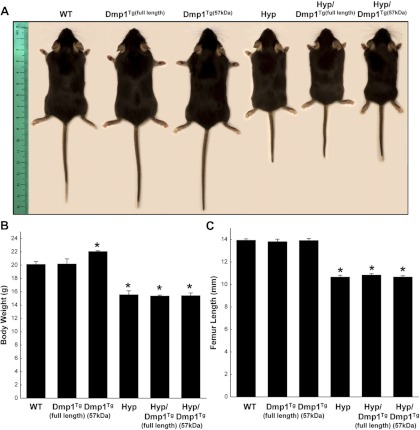
As noted above, Dmp1ko and Phex-deficient (Hyp) mice have nearly identical phenotypes (1–5), possibly due to actions on a common pathway (5). To understand the relative position of DMP1 and PHEX, we tested whether the transgenic expression of Dmp1 (full length or C-terminal) could rescue the phenotype of Hyp mice. We first verified that we achieved overexpression of the transgenes in the bone of Dmp1Tg(full length), Hyp/Dmp1Tg(full length), Dmp1Tg(57 kDa), or Hyp/Dmp1Tg(57 kDa) mice. The expression of Dmp1 was increased by 20-fold in groups expressing the full-length Dmp1 transgene and by 50-fold in groups expressing the C-terminal Dmp1 transgene. We also verified that the expression of the transgenes was detectable in the osteocytes (Fig. 2). We observed that the gross appearance of either Dmp1Tg mice to be indistinguishable from WT controls (Fig. 3). In contrast, Hyp mice exhibited a growth defect characterized by a significantly shorter femur length, short stature, and significantly lower body weight compared with the WT (Fig. 3). This was not corrected by the overexpression of either Dmp1Tg(full length) or Dmp1Tg(57 kDa) in Hyp mice.
Fig. 2.
Cortical bone immunostaining of DMP1 in 5-wk-old WT, Dmp1Tg(full length), Dmp1Tg(57 kDa), Phex-deficient (Hyp), Hyp/Dmp1Tg(full length), Hyp/Dmp1Tg(57 kDa), and Dmp1ko mice. The top panels show the bright fields and bottom panels the corresponding fluorescent fields. The scale bar represents 100 μm.
Fig. 3.
Gross appearance (A), body weight (B), and femur length (C) of 5-wk-old WT, Dmp1Tg(full length), Dmp1Tg(57 kDa), Phex-deficient (Hyp), Hyp/Dmp1Tg(full length), and Hyp/Dmp1Tg(57 kDa) mice. Values are expressed as mean ± se (n ≥ 5 mice/group). *, P < 0.05 vs. WT.
The C-terminal fragment of DMP1 impairs bone mineralization in absence of PHEX
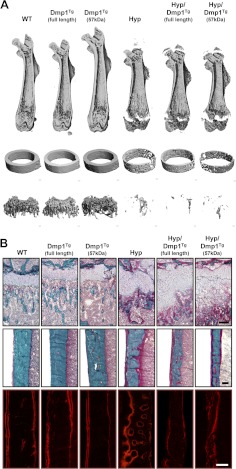
A 3D-microCT analysis of the femur revealed that the overexpression of Dmp1 (full length or C terminal) did not affect the bone microarchitecture when PHEX is functional in Dmp1Tg(full length) and Dmp1Tg(57 kDa) mice (Fig. 4 and Table 3). However, we observed that the overexpression of the C-terminal fragment of DMP1 in Phex-deficient Hyp/Dmp1Tg(57 kDa) mice worsened the mineralization defect observed in Hyp mice, due primarily to a significant reduction of the cortical bone mineralization and thickness compared with Hyp mice (Fig. 4 and Table 3). Interestingly, these effects were not observed with the expression of the full-length transgene in Hyp/Dmp1Tg(full-length) mice, for which the bone phenotype was similar to the phenotype of Hyp mice.
Fig. 4.
Bone phenotype of 5-wk-old WT, Dmp1Tg(full length), Dmp1Tg(57 kDa), Phex-deficient (Hyp), Hyp/Dmp1Tg(full length), and Hyp/Dmp1Tg(57 kDa) mice. A, 3D-μCT representation of (from top to bottom) the entire femur, the femoral midshaft, and the femoral distal metaphysis. B, Modified Goldner staining on femur histological section showing (top panels) the distal growth plate and metaphysis and (middle panels) the cortical bone at midshaft; the bottom panels show the fluorescent Alizarine Red S double labeling of the cortical bone. Scale bar represents 100 μm.
Table 3.
3D-μCT analysis of femur distal metaphysis trabecular structure and midshaft cortical envelope
| WT | Dmp1Tg (full length) | Dmp1Tg (57 kDa) | Hyp | Hyp/Dmp1Tg (full length) | Hyp/Dmp1Tg (57 kDa) | |
|---|---|---|---|---|---|---|
| Trabecular bone parameters | ||||||
| Tb.BMD (mg HA/cm3) | 149.9 ± 11.3 | 121.1 ± 25.0 | 122.5 ± 19.6 | 2.1 ± 0.8a | 1.2 ± 0.6a | 2.8 ± 1.2a |
| BV/TV (%) | 21.2 ± 1.2 | 18.6 ± 3.4 | 18.7 ± 3.1 | 0.7 ± 0.1a | 0.5 ± 0.2a | 0.4 ± 0.1a, b |
| Tb.N. (−1) | 5.2 ± 0.2 | 5.1 ± 0.3 | 4.8 ± 0.2 | 1.8 ± 0.1a | 1.7 ± 0.1a | 1.7 ± 0.1a |
| Tb.Th (μm) | 56.9 ± 3.1 | 46.4 ± 2.7a | 44.9 ± 4.0a | 34.8 ± 1.8a | 35.5 ± 3.2a | 33.8 ± 1.2a |
| Tb.Sp (μm) | 188.6 ± 8.4 | 209.6 ± 17.1 | 208.2 ± 12.0 | 575.1 ± 23.7a | 640.5 ± 39.1a | 610.4 ± 33.7a |
| Conn.Dens. | 231.8 ± 17.8 | 253.3 ± 19.3 | 200.4 ± 15.5 | 1.8 ± 0.7a | 1.4 ± 1.2a | 1.0 ± 0.9a |
| SMI | 1.7 ± 0.1 | 2.1 ± 0.2a | 1.9 ± 0.3 | 3.6 ± 0.1a | 3.8 ± 0.2a | 3.7 ± 0.2a |
| DA | 1.89 ± 0.05 | 1.84 ± 0.02 | 1.76 ± 0.02 | 1.70 ± 0.03a | 1.66 ± 0.07a | 1.65 ± 0.08a |
| Cortical bone parameters | ||||||
| Ct.BMD (mg HA/cm3) | 862.0 ± 8.3 | 819.9 ± 15.0a | 844.4 ± 11.6 | 572.4 ± 16.5a | 580.9 ± 15.8a | 509.7 ± 18.3a, b |
| Ct.Th. (μm) | 146.2 ± 7.3 | 130.1 ± 4.8 | 141.7 ± 8.6 | 54.8 ± 3.2a | 55.7 ± 2.8a | 44.0 ± 2.7a, b |
| CSA (mm2) | 2.31 ± 0.09 | 2.45 ± 0.10 | 2.37 ± 0.05 | 2.76 ± 0.10a | 2.49 ± 0.10b | 2.42 ± 0.05b |
| Ct.Ar. (mm2) | 0.60 ± 0.03 | 0.58 ± 0.04 | 0.57 ± 0.03 | 0.20 ± 0.02a | 0.20 ± 0.02a | 0.14 ± 0.02a, b |
| Ma.Ar. (mm2) | 1.73 ± 0.06 | 1.81 ± 0.08 | 1.81 ± 0.05 | 2.58 ± 0.08a | 2.44 ± 0.06a | 2.26 ± 0.04a, b |
Values are expressed as mean ± se from eight mice per group at 5 wk of age. Comparisons were performed using one-way ANOVA and post hoc Fisher test. Tb.BMD, Trabecular bone mineral density; Conn.Dens, connectivity density; DA, degree of anisotropy; Ct.BMD, cortical bone mineral density; Ct.Th, cortical thickness; CSA, cross sectional area; Ct.Ar, cortical area; Ma.Ar, marrow area.
P < 0.05 vs. WT.
P < 0.05 vs.Hyp.
The effects of the C-terminal fragment of DMP1 on FGF23 regulation are PHEX dependent
The overexpression of full-length Dmp1 in Dmp1Tg(full length) mice induced a mild yet significant hypocalcemia that did not impact on PTH or 1,25-(OH)2D serum levels. This decrease in Ca levels was not observed in Dmp1Tg(57 kDa) mice. Rather, the overexpression of the C-terminal fragment of DMP1 in Dmp1Tg(57 kDa) mice induced a significant increase in serum 1,25-(OH)2D and decrease in PTH levels. However, neither Dmp1Tg groups had alterations in serum FGF23 levels, which were not significantly different from levels observed in WT mice (Table 4).
Table 4.
Serum and urine biochemistry of WT, Dmp1Tg(full length), Dmp1Tg(57 kDa), Phex-deficient (Hyp), Hyp/Dmp1Tg(full length), and Hyp/Dmp1Tg(57 kDa) mice
| WT | Dmp1Tg (full length) | Dmp1Tg (57 kDa) | Hyp | Hyp/Dmp1Tg (full length) | Hyp/Dmp1Tg (57 kDa) | |
|---|---|---|---|---|---|---|
| Serum | ||||||
| Full-length FGF23 (pg/ml) | 94.1 ± 8.1 | 105.9 ± 14.2 | 88.2 ± 11.1 | 1149.8 ± 310.6a | 1330.0 ± 351.3a | 2269.8 ± 373.1a,b |
| Degraded C-terminal FGF23 (pg/ml) | 52.9 ± 6.1 | 61.2 ± 9.2 | 54.0 ± 7.6 | 427.8 ± 6.3a | 373.6 ± 14.9a,b | 232.0 ± 52.4a,b |
| 1,25(OH)2D (pg/ml) | 168.6 ± 19.2 | 142.8 ± 33.1 | 236.1 ± 18.0a | 183.1 ± 22.7 | 198.4 ± 35.5 | 290.2 ± 35.1a,b,c |
| PTH (pg/ml) | 44.6 ± 7.9 | 35.2 ± 10.1 | 28.9 ± 5.0a | 75.4 ± 15.7a | 86.6 ± 18.9a | 87.2 ± 20.9a |
| Pi (mg/dl) | 8.2 ± 0.4 | 9.7 ± 0.5a | 9.0 ± 0.3 | 4.6 ± 0.3a | 5.3 ± 0.5a | 4.5 ± 0.4a |
| Ca (mg/dl) | 7.8 ± 0.3 | 6.8 ± 0.1a | 7.8 ± 0.2 | 7.5 ± 0.3 | 6.9 ± 0.1a | 6.4 ± 0.1a,b,c |
| Urine | ||||||
| FE phosphate (%) | 18.9 ± 3.1 | 11.1 ± 1.8a | 13.6 ± 1.9 | 6.2 ± 1.8a | 24.9 ± 4.7b | 21.2 ± 2.4b |
| FE calcium (%) | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.2 | 0.3 ± 0.1a | 0.8 ± 0.3 | 0.9 ± 0.2b |
Values are expressed as mean ± se from at least five mice per group at 5 wk of age. Comparisons were performed using one-way ANOVA and post hoc Fisher test. FE, Fractional excretion.
P < 0.05 vs. WT.
P < 0.05 vs. Hyp.
P < 0.05 vs. Hyp/Dmp1Tg(full-length).
In the absence of Phex, the overexpression of the C-terminal fragment of DMP1 in Hyp/Dmp1Tg(57 kDa) mice also induced hypocalcemia and increased the serum 1,25-(OH)2D levels. The renal mRNA expression of Cyp27b1, the enzyme hydroxylating 25(OH)D in 1,25-(OH)2D, was increased by 2.3-fold in Hyp/Dmp1Tg(57 kDa) compared with Hyp mice (P < 0.05). However, despite the presence of a significant hypocalcemia in Hyp/Dmp1Tg(57 kDa) compared with Hyp mice, PTH levels were similar in Hyp/Dmp1Tg(57 kDa) and Hyp mice and therefore higher than in WT mice. Interestingly, FGF23 levels were further increased by 2-fold in Hyp/Dmp1Tg(57 kDa) compared with Hyp mice for the same degree of hypophosphatemia (Table 4). This elevation in FGF23 did not affect the mRNA expression of the sodium-phosphate cotransporters in the kidney because it was reduced to the same extend in both Hyp and Hyp/Dmp1Tg(57 kDa) (Npt2a: −25%; Npt2c: −55%; P < 0.05 vs. WT). Such variations in serum FGF23 levels were not observed with the expression of the full-length transgene in Hyp/Dmp1Tg(full-length) mice, for which the only difference with the serum biochemistries of Hyp mice was the presence of mild hypocalcemia comparable with the one observed in Dmp1Tg(full-length) mice.
DMP1 is involved in the posttranscriptional regulation of FGF23
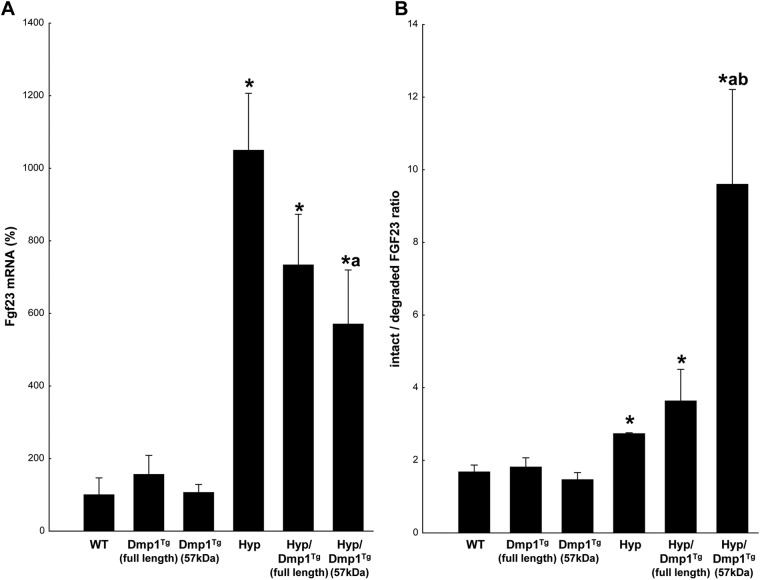
It is known that in Hyp mice, increased serum levels of FGF23 are mainly due to an increase in FGF23 mRNA expression by osteocytes in bone (3, 5). In the present study, we measured FGF23 mRNA expression in hindlimb bones to verify whether the difference observed in serum FGF23 level between Hyp and Hyp/Dmp1Tg(57 kDa) mice reflected similar variations in bone FGF23 expression. We confirmed that FGF23 mRNA expression was significantly increased by approximately 10-fold in Hyp mice compared with the WT (Fig. 5A). An approximately 8-fold increase was similarly observed in Hyp/Dmp1Tg(full-length) bones. However, the elevation of serum FGF23 levels observed in Hyp/Dmp1Tg(57 kDa) mice occurred despite a significant 40% reduction in mRNA expression in the Hyp/Dmp1Tg(57 kDa) compared with Hyp bones (Fig. 5A).
Fig. 5.
A, mRNA expression of Fgf23 measured by RT-PCR in hindlimb bones. Values are expressed as a percentage of the WT value set at 100%. B, Ratio between the nondegraded and degraded forms of FGF23 in the serum: higher values correspond to a decrease in the degradation of FGF23. Values are expressed as mean ± se; n = 5 mice/group; P < 0.05 vs. WT (*), Hyp (a), and Hyp/Dmp1Tg(full-length) (b).
Thus, to understand the reason for increased serum levels of FGF23 in Hyp/Dmp1Tg(57 kDa) vs. Hyp mice, we hypothesized that FGF23 degradation was defective in Hyp/Dmp1Tg(57 kDa) mice and measured the degraded C-terminus form of FGF23 in the serum. We found that the concentration of degraded FGF23 was significantly increased in Hyp mice compared with WT (Table 4). We also found that the concentration of degraded FGF23 is increased in Hyp/Dmp1Tg(57 kDa) compared with Hyp mice. Although the amount of degraded FGF23 seemed to reflect the initial amount of FGF23 produced, measuring the relative proportion of the full-length form over the degraded form of FGF23 gave us interesting insight into the actual level of degradation of the molecule (Fig. 5B). Indeed, this ratio was significantly increased in Hyp mice compared with WT mice, meaning that FGF23 degradation is impaired in absence of PHEX. Additionally, this ratio was further increased in Hyp/Dmp1Tg(57 kDa) compared with Hyp mice, showing that the full-length active form of FGF23 largely prevails on the degraded form in these mice. Therefore, despite a lower mRNA expression of FGF23 in bone, the highly impaired degradation of FGF23 in Hyp/Dmp1Tg(57 kDa) mice leads to the accumulation of the molecule in the serum. Finally, an intermediate increase of the ratio was found in Hyp/Dmp1Tg(full-length) mice. The precise roles of the N- and C-terminus fragments of DMP1 in regulating FGF23 degradation remain to be investigated.
Discussion
Inactivation of osteocyte-derived PHEX and DMP1 leads to elevated circulating FGF23 levels and impaired mineralization of bone (1–4, 6). Mouse genetic studies have revealed nonadditive effects of combined Phex and Dmp1 inactivation (5), suggesting that PHEX and DMP1 are acting through a common pathway to increase serum FGF23 concentrations. In the current study, we created compound mutants Hyp mice overexpressing either the full-length Dmp1 transgene (Hyp/Dmp1Tg(full-length)) or the C-terminal, 57-kDa Dmp1 transgene (Hyp/Dmp1Tg(57 kDa)) to determine whether DMP1 could be a cofactor for PHEX and possibly bypass the effects of Phex deficiency on FGF23 production and bone mineralization. We made three major discoveries. First, we found that the full length Dmp1 transgene did not rescue the phenotype of Hyp mice, suggesting that DMP1 may be upstream of PHEX. Second, and unexpectedly, we found that the function of C-terminal DMP1 on the regulation of bone mineralization and FGF23 expression is influenced by the presence of inactivating Phex mutations. Indeed, the overexpression of the C-terminal Dmp1 transgene in Hyp/Dmp1Tg(57 kDa) mice paradoxically worsened the bone mineralization and increased the serum FGF23 concentrations only in the absence of PHEX. Third, similar to previous reports (11), we found that both the full-length DMP1 and the 57-kDa C-terminal fragment, which retains the RGD and ASARM binding domains for interaction of DMP1 with integrins and PHEX, significantly improved the serum and bone abnormalities of Dmp1ko mice. This indicates that the important functional domains of DMP1 reside in the C-terminal peptide. Collectively, these results indicate that PHEX and DMP1 exhibit a functional interdependence to coordinately regulate FGF23 production and bone mineralization (23).
Prior studies demonstrate that the increase in circulating FGF23 concentrations in Hyp and Dmp1ko mice are primarily due to increased FGF23 gene transcription by osteocytes (3, 4). The mechanisms whereby the C-terminal, but not full-length, DMP1 further increased the serum FGF23 levels in Hyp/Dmp1Tg(57 kDa) mice are not clear. The 1,25-(OH)2D-dependent stimulation of FGF23 does not explain the elevation of FGF23 in Hyp/Dmp1Tg(57 kDa) mice because the serum 1,25-(OH)2D levels were increased in both Dmp1Tg(57 kDa) and Hyp/Dmp1Tg(57 kDa) mice, but FGF23 levels were increased only in Hyp/Dmp1Tg(57 kDa) mice. Additionally, the normal serum FGF23 and Pi-levels in Dmp1Tg(57 kDa) mice makes it unlikely that the transgene itself is regulating FGF23 gene transcription (24). Rather, our data indicate that DMP1 regulates the posttranscriptional degradation of FGF23. In this regard, we report, for the first time, the effects of C-terminal DMP1 to increase serum FGF23 in Hyp/Dmp1Tg(57 kDa) mice by inhibiting FGF23 degradation rather than increasing gene transcription. Indeed, FGF23 mRNA levels were decreased in Hyp/Dmp1Tg(57 kDa) bones compared with Hyp alone, whereas the serum full-length FGF23 levels increased proportionally more than the degraded FGF23 levels in Hyp/Dmp1Tg(57 kDa) compared with Hyp mice. Thus, the presence of C-terminal DMP1 somehow decreases FGF23 degradation leading to the accumulation of the full-length active form of FGF23 in the serum. These effects occurred only in Hyp/Dmp1Tg(57 kDa) mice but not in Dmp1Tg(57 kDa) mice with a functional PHEX, suggesting that Phex deficiency alters the function of the C-terminal DMP1 peptide in some degree that allows it to inhibit both FGF23 degradation and bone mineralization. FGF23 degradation is regulated by actions of an unknown proprotein convertase to cleave FGF23 at a conserved RXXR site, which is protected by the actions of the glycosyltransferase uridine diphosphate-N-acetyl-α-d-galactosamine-polypeptide N-acetylgalactosaminyltransferase 3 to glycosylate the surrounding residues (25). How Dmp1 regulates FGF23 degradation in the absence of PHEX remains to be determined but implicates a role for extracellular matrix, DMP1, and PHEX interactions to regulate protease activity. Hyp mice also may have reduced proteolytic cleavage of intact FGF23 (26) in addition to increased FGF23 gene transcription (5) because expression of candidate proprotein convertases, such as proprotein convertase subtilisin/kexin type 5, is decreased in Hyp bone (27, 28).
In Phex-mutant Hyp mice, the abnormalities of bone mineralization are due not only to the FGF23-induced hypophosphatemia but also to an intrinsic mineralization defect because correction of the hypophosphatemia only partially rescues the bone defects (29). The mechanisms whereby Phex and Dmp1 mutations lead to impaired mineralization are still unclear. Because in ARHR the mutation of Dmp1 occurs at the C-terminus end of the protein and the C-terminal peptide of DMP1 is sufficient to rescue the bone defect of Dmp1ko in the presence of PHEX (11), it has been proposed that the 57-kDa C-terminal DMP1 peptide corresponds to an important functional domain and that cleavage of DMP1 into N- and C-terminal fragments is necessary for mineralization (9, 10). However, the fact that the overexpression of C-terminal Dmp1 paradoxically worsened the bone mineralization defect of Hyp/Dmp1Tg(57 kDa) mice is not consistent with these models. To date, the direct stimulatory effects of DMP1 on mineral deposition have been described only in vitro and do not seem to be restricted to the C-terminal but also the N-terminal DMP1 peptide (10, 30). In addition, the Dmp1Tg(57 kDa) mice do not display a high bone mineralization phenotype (11) and our 3D-μCT analysis rather revealed an approximately 20% reduction in the trabecular bone mineral density and thickness (Table 3), which also fails to support a mineral nucleating role for C-terminal DMP1, at least in the absence of PHEX. Alternatively, the intrinsic mineralization defect associated with PHEX deficiency has been attributed to the ASARM peptides derived from SIBLING proteins (27, 31). Although ASARM binds to and inhibits PHEX activity in vitro (32, 33) and ASARM induces hypophosphatemia in vivo (34), ASARM from the SIBLING protein MEPE is not responsible for the Hyp bone phenotype (35). Indeed, the deletion of Mepe in Hyp/Mepeko mice does not prevent any of the bone or renal phenotypes but leads to increased mineralization of osteoblasts ex vivo only (36), and overexpression of Mepe induces hyperphosphatemia (37). However, we know that peptides containing the ASARM motif accumulate in Hyp bone as a consequence of Phex inactivation (13, 33), and it is possible that these ASARM peptides derive from DMP1 (33, 35).
Interestingly, the overexpression of C-terminal Dmp1 had minimal effects on serum FGF23 levels and bone mineralization in the presence of normal Phex function in Dmp1Tg(57 kDa) mice and had opposite effects in Dmp1ko/Dmp1Tg(57 kDa). This strongly suggests that the effect of the C-terminal DMP1 peptide are dependent on the presence/absence of PHEX and further identifies the C-terminal DMP1 peptide as a factor interacting with PHEX to regulate bone mineralization and FGF23. In this context, the presence of PHEX would protect bone mineralization from the inhibitory effects of C-terminal DMP1, possibly through the formation of a complex between the two proteins on the osteoblast surface. A previous study (38) showing that FGF23-related Pi recovery plays a major role in the recuperation of the Dmp1ko phenotype also supports the possibility that the presence of the C-terminal DMP1 in Dmp1ko/Dmp1Tg(57 kDa) rescues the basal expression of Fgf23 through the restored interaction with PHEX. Further analyses are needed to fully understand this interaction.
Another key findings of our study is the distinct functions of the N- and C-terminal DMP1 fragments. The effects of the overexpression of the full-length Dmp1 transgene in Hyp/Dmp1Tg(full-length) mice were not pronounced and did not induce the same phenotype as the overexpression of C-terminal Dmp1 alone. The bone architecture was similar in Hyp and Hyp/Dmp1Tg(full-length) mice as well as the serum levels of FGF23 and Pi. These results suggest that the N-terminal DMP1 peptide may have opposite effects from the C-terminal DMP1 peptide. Although this is consistent with the function attributed to N-terminal DMP1 on the nucleation of hydroxyapatite crystals in vitro (10, 30), the direct effects of N-terminal DMP1 on bone mineralization remain to be tested in vivo. These results do not entirely exclude the possibility of an altered processing of DMP1 in Hyp bones, which would alternatively explain the absence of effects of the full-length as opposed to the C-terminal Dmp1 transgene on the Hyp phenotype. However, the intermediate values of FGF23 mRNA transcription and degradation ratio obtained in the Hyp/Dmp1Tg(full-length) mice compared with Hyp and Hyp/Dmp1Tg(57 kDa) mice suggest that the N-terminal DMP1 peptide is actively involved at least in the regulation of FGF23.
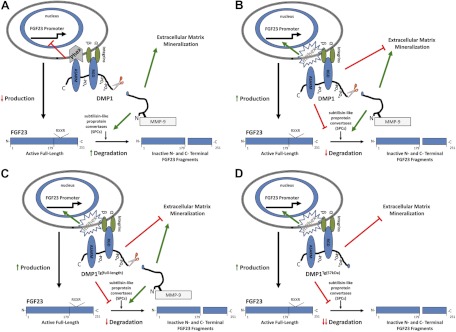
To conclude, the fact that the overexpression of DMP1 did not rescue the hypophosphatemic rickets phenotype of Hyp mice suggests that PHEX plays a role in mediating DMP1 effects on FGF23 gene transcription and bone mineralization. Additionally, for the first time, we show that DMP1 also regulates degradation of FGF23, possibly through functions of both DMP1 fragments. A potential model to show the differential effects of full-length and C-terminal DMP1 to regulate serum FGF23 concentrations is shown in Fig. 6. Although speculative, these findings suggest that serum FGF23 concentrations represent a balance between synthesis and degradation, controlled by DMP1 interaction with PHEX to regulate gene transcription and additional effects of DMP1 to regulate bone mineralization and FGF23 degradation. Under normal conditions, DMP1 binds to PHEX and integrins, leading to the suppression FGF23 gene transcription and increased FGF23 degradation (Fig. 6A). In the setting of inactivating mutations of Phex (Fig. 6B), there is increased production and decreased degradation of FGF23. In this model, the differential effects of the full-length Dmp1 transgene (Fig. 6C) and the 57-kDa C-terminal transgene (Fig. 6D) in Hyp mice is that of the DMP1 N-terminal in full-length DMP1 stimulates subtilisin-like proprotein convertase cleavage protein cleavage of FGF23, whereas the C-terminal fragment does not stimulate subtilisin-like proprotein convertase cleavage leading to a greater imbalance between production and degradation and also worsens the defect in mineralization. Future studies to test the concepts depicted in this model will advance our understanding of how DMP1 and PHEX coordinate bone mineralization with circulating FGF23 concentrations through the regulation of production and degradation of this hormone.
Fig. 6.
Proposed hypothetical model for the function of DMP1 and PHEX interaction in the regulation of FGF23 and bone mineralization in the context of nonmutant WT (A), mutant Phex (B), mutant Phex overexpressing the full-length Dmp1 transgene (C), and mutant Phex overexpressing the C-terminal Dmp1 transgene (D).
Acknowledgments
This work was supported by National Institute of Arthritis and Musculoskeletal Diseases Grant 7R56 AR-045955.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ARHR
- Autosomal recessive hypophosphatemic rickets
- ASARM
- acidic serine aspartate rich motif
- BV/TV
- trabecular bone volume
- μCT
- microcomputed tomography
- Ct.BMD
- cortical bone mineral density
- 3D
- three dimensional
- DMP1
- dentin matrix protein-1
- Dmp1ko
- Dmp1 knockout
- Dmp1Tg
- Dmp1 transgenic
- Dmp1Tg(57 kDa)
- C-terminal region of Dmp1
- Dmp1Tg(full length)
- full-length Dmp1
- FGF
- fibroblast growth factor
- MEPE
- matrix extracellular phosphoglycoprotein
- Npt2
- sodium-dependent phosphatecotransporter 2
- PHEX
- phosphate-regulating gene with homologies to endopeptidases on the X chromosome
- Pi
- phosphate
- SIBLING
- small integrin-binding ligand interacting glycoproteins
- SMI
- structure model index
- Tb.N
- trabecular number
- Tb.Sp
- trabecular separation
- Tb.Th
- trabecular thickness
- WT
- wild type.
References
- 1. Lorenz-Depiereux B, Bastepe M, Benet-Pagès A, Amyere M, Wagenstaller J, Müller-Barth U, Badenhoop K, Kaiser SM, Rittmaster RS, Shlossberg AH, Olivares JL, Loris C, Ramos FJ, Glorieux F, Vikkula M, Jüppner H, Strom TM. 2006. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet 38:1248–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. 2006. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38:1310–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD. 2006. Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab 291:E38–E49 [DOI] [PubMed] [Google Scholar]
- 4. Liu S, Zhou J, Tang W, Menard R, Feng JQ, Quarles LD. 2008. Pathogenic role of Fgf23 in Dmp1-null mice. Am J Physiol Endocrinol Metab 295:E254–E261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martin A, Liu S, David V, Li H, Karydis A, Feng JQ, Quarles LD. 2011. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J 25:2551–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xiao ZS, Crenshaw M, Guo R, Nesbitt T, Drezner MK, Quarles LD. 1998. Intrinsic mineralization defect in Hyp mouse osteoblasts. Am J Physiol 275:E700–E708 [DOI] [PubMed] [Google Scholar]
- 7. Quarles LD. 2008. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest 118:3820–3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ogbureke KU, Fisher LW. 2004. Expression of SIBLINGs and their partner MMPs in salivary glands. J Dent Res 83:664–670 [DOI] [PubMed] [Google Scholar]
- 9. Ling Y, Rios HF, Myers ER, Lu Y, Feng JQ, Boskey AL. 2005. DMP1 depletion decreases bone mineralization in vivo: an FTIR imaging analysis. J Bone Miner Res 20:2169–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. He G, George A. 2004. Dentin matrix protein 1 immobilized on type I collagen fibrils facilitates apatite deposition in vitro. J Biol Chem 279:11649–11656 [DOI] [PubMed] [Google Scholar]
- 11. Lu Y, Yuan B, Qin C, Cao Z, Xie Y, Dallas SL, McKee MD, Drezner MK, Bonewald LF, Feng JQ. 2011. The biological function of DMP-1 in osteocyte maturation is mediated by its 57-kDa C-terminal fragment. J Bone Miner Res 26:331–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Addison WN, Masica DL, Gray JJ, McKee MD. 2010. Phosphorylation-dependent inhibition of mineralization by osteopontin ASARM peptides is regulated by PHEX cleavage. J Bone Miner Res 25:695–705 [DOI] [PubMed] [Google Scholar]
- 13. Addison WN, Nakano Y, Loisel T, Crine P, McKee MD. 2008. MEPE-ASARM peptides control extracellular matrix mineralization by binding to hydroxyapatite: an inhibition regulated by PHEX cleavage of ASARM. J Bone Miner Res 23:1638–1649 [DOI] [PubMed] [Google Scholar]
- 14. Yuan B, Takaiwa M, Clemens TL, Feng JQ, Kumar R, Rowe PS, Xie Y, Drezner MK. 2008. Aberrant Phex function in osteoblasts and osteocytes alone underlies murine X-linked hypophosphatemia. J Clin Invest 118:722–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Erben RG, Mayer D, Weber K, Jonsson K, Jüppner H, Lanske B. 2005. Overexpression of human PHEX under the human beta-actin promoter does not fully rescue the Hyp mouse phenotype. J Bone Miner Res 20:1149–1160 [DOI] [PubMed] [Google Scholar]
- 16. Bai X, Miao D, Panda D, Grady S, McKee MD, Goltzman D, Karaplis AC. 2002. Partial rescue of the Hyp phenotype by osteoblast-targeted PHEX (phosphate-regulating gene with homologies to endopeptidases on the X chromosome) expression. Mol Endocrinol 16:2913–2925 [DOI] [PubMed] [Google Scholar]
- 17. Boskey A, Frank A, Fujimoto Y, Spevak L, Verdelis K, Ellis B, Troiano N, Philbrick W, Carpenter T. 2009. The PHEX transgene corrects mineralization defects in 9-month-old hypophosphatemic mice. Calcif Tissue Int 84:126–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feng JQ, Huang H, Lu Y, Ye L, Xie Y, Tsutsui TW, Kunieda T, Castranio T, Scott G, Bonewald LB, Mishina Y. 2003. The Dentin matrix protein 1 (Dmp1) is specifically expressed in mineralized, but not soft, tissues during development. J Dent Res 82:776–780 [DOI] [PubMed] [Google Scholar]
- 19. Lu Y, Qin C, Xie Y, Bonewald LF, Feng JQ. 2009. Studies of the DMP1 57-kDa functional domain both in vivo and in vitro. Cells Tissues Organs 189:175–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu Y, Ye L, Yu S, Zhang S, Xie Y, McKee MD, Li YC, Kong J, Eick JD, Dallas SL, Feng JQ. 2007. Rescue of odontogenesis in Dmp1-deficient mice by targeted re-expression of DMP1 reveals roles for DMP1 in early odontogenesis and dentin apposition in vivo. Dev Biol 303:191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martin A, David V, Vico L, Thomas T. 2008. Impaired energetic metabolism after central leptin signaling leads to massive appendicular bone loss in hindlimb-suspended rats. J Bone Miner Res 23:2040–2047 [DOI] [PubMed] [Google Scholar]
- 22. Lu Y, Liu S, Xie Y, Yu S, Quarles L, Bonewald LF, Feng JQ. 2007. Use of the transgenic approach to determine the role of DMP1 in phosphate regulation. J Musculoskelet Neuronal Interact 7:309. [PubMed] [Google Scholar]
- 23. Wu H, Teng PN, Jayaraman T, Onishi S, Li J, Bannon L, Huang H, Close J, Sfeir C. 2011. Dentin matrix protein 1 (DMP1) signals via cell surface integrin. J Biol Chem 286:29462–29469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Narayanan K, Ramachandran A, Hao J, He G, Park KW, Cho M, George A. 2003. Dual functional roles of dentin matrix protein 1. Implications in biomineralization and gene transcription by activation of intracellular Ca2+ store. J Biol Chem 278:17500–17508 [DOI] [PubMed] [Google Scholar]
- 25. Chefetz I, Sprecher E. 2009. Familial tumoral calcinosis and the role of O-glycosylation in the maintenance of phosphate homeostasis. Biochim Biophys Acta 1792:847–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ichikawa S, Austin AM, Gray AK, Econs MJ. 2012. A Phex mutation in a murine model of X-linked hypophosphatemia alters phosphate responsiveness of bone cells. J Bone Miner Res 27:453–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rowe PS, Matsumoto N, Jo OD, Shih RN, Oconnor J, Roudier MP, Bain S, Liu S, Harrison J, Yanagawa N. 2006. Correction of the mineralization defect in hyp mice treated with protease inhibitors CA074 and pepstatin. Bone 39:773–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu S, Tang W, Fang J, Ren J, Li H, Xiao Z, Quarles LD. 2009. Novel regulators of Fgf23 expression and mineralization in Hyp bone. Mol Endocrinol 23:1505–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu S, Tang W, Zhou J, Vierthaler L, Quarles LD. 2007. Distinct roles for intrinsic osteocyte abnormalities and systemic factors in regulation of FGF23 and bone mineralization in Hyp mice. Am J Physiol Endocrinol Metab 293:E1636–E1644 [DOI] [PubMed] [Google Scholar]
- 30. Gericke A, Qin C, Sun Y, Redfern R, Redfern D, Fujimoto Y, Taleb H, Butler WT, Boskey AL. 2010. Different forms of DMP1 play distinct roles in mineralization. J Dent Res 89:355–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rowe PS, de Zoysa PA, Dong R, Wang HR, White KE, Econs MJ, Oudet CL. 2000. MEPE, a new gene expressed in bone marrow and tumors causing osteomalacia. Genomics 67:54–68 [DOI] [PubMed] [Google Scholar]
- 32. Liu S, Rowe PS, Vierthaler L, Zhou J, Quarles LD. 2007. Phosphorylated acidic serine-aspartate-rich MEPE-associated motif peptide from matrix extracellular phosphoglycoprotein inhibits phosphate regulating gene with homologies to endopeptidases on the X-chromosome enzyme activity. J Endocrinol 192:261–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martin A, David V, Laurence JS, Schwarz PM, Lafer EM, Hedge AM, Rowe PS. 2008. Degradation of MEPE, DMP1, and release of SIBLING ASARM-peptides (minhibins): ASARM-peptide(s) are directly responsible for defective mineralization in HYP. Endocrinology 149:1757–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. David V, Martin A, Hedge AM, Drezner MK, Rowe PS. 2011. ASARM peptides: PHEX-dependent and -independent regulation of serum phosphate. Am J Physiol Renal Physiol 300:F783–F791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. David V, Quarles LD. 2010. ASARM mineralization hypothesis: a bridge too far? J Bone Miner Res 25:692–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu S, Brown TA, Zhou J, Xiao ZS, Awad H, Guilak F, Quarles LD. 2005. Role of matrix extracellular phosphoglycoprotein in the pathogenesis of X-linked hypophosphatemia. J Am Soc Nephrol 16:1645–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. David V, Martin A, Hedge AM, Rowe PS. 2009. Matrix extracellular phosphoglycoprotein (MEPE) is a new bone renal hormone and vascularization modulator. Endocrinology 150:4012–4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang R, Lu Y, Ye L, Yuan B, Yu S, Qin C, Xie Y, Gao T, Drezner MK, Bonewald LF, Feng JQ. 2011. Unique roles of phosphorus in endochondral bone formation and osteocyte maturation. J Bone Miner Res 26:1047–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]