Abstract
Progesterone (P4) and estradiol-17β (E2) play critical and opposing roles in regulating myometrial quiescence and contractility during pregnancy and labor. Although these contrasting hormonal effects are likely mediated via differential regulation of inflammatory and contractile genes, the underlying mechanisms remain incompletely understood. Recently we discovered that targets of the microRNA (miR)-200 family, transcription factors zinc finger E-box binding homeobox (ZEB)-1 and ZEB2, serve as P4/progesterone receptor-mediated regulators of uterine quiescence during pregnancy. In the present study, we found that levels of the clustered miRNAs, miR-199a-3p and miR-214, were significantly decreased in laboring myometrium of pregnant mice and humans and in an inflammatory mouse model of preterm labor, whereas the miR-199a-3p/miR-214 target, cyclooxygenase-2, a critical enzyme in synthesis of proinflammatory prostaglandins, was coordinately increased. Overexpression of miR-199a-3p and miR-214 in cultured human myometrial cells inhibited cyclooxygenase-2 protein and blocked TNF-α-induced myometrial cell contractility, suggesting their physiological relevance. Notably, E2 treatment of ovariectomized mice suppressed, whereas P4 enhanced uterine miR-199a-3p/214 expression. Intriguingly, these opposing hormonal effects were mediated by ZEB1, which is induced by P4, inhibited by E2 and activates miR199a/214 transcription. Together, these findings identify miR-199a-3p/miR-214 as important regulators of myometrial contractility and provide new insight into strategies to prevent preterm birth.
Preterm birth (birth before 37 wk gestation) is a major cause of neonatal morbidity and mortality in developed countries. The incidence of premature birth in the United States has steadily increased over the past 2 decades and accounts for more than half a million preterm births per year (1). Unfortunately, the safety and efficacy of current therapies to prevent premature birth are inadequate (2). This is due, in part, to our incomplete understanding of the complex molecular events that underlie the maintenance of uterine quiescence during pregnancy and result in increased myometrial contractility leading to term and preterm labor (2).
Myometrial quiescence is sustained throughout most of pregnancy by increased circulating levels of progesterone (P4) and increased progesterone receptor (PR) activity (2). P4/PR promotes uterine quiescence, in part, by directly interacting with the inflammatory transcription factor, nuclear factor-κB (NF-κB) to suppress NF-κB activation of contraction-associated genes, such as cyclooxygenase-2 (COX-2) (3) and prostaglandin F2α receptor (4), and by inducing expression of the NF-κB inhibitor, inhibitor of κBα (3). On the other hand, the progression to labor, which is associated with an increased inflammatory response to mechanical and hormonal signals from mother (5) and fetus (6–8), involves a concerted series of molecular events that impair PR function. The decline in myometrial PR function near term is caused by a decrease in PR coactivators (9), increased expression of inhibitory PR isoforms (10, 11), enhanced local metabolism of P4 in the cervix (12–14) and uterus (15), and PR antagonism by NF-κB (3, 16, 17), which is activated in the myometrium near term (6, 10).
Notably, increased circulating estradiol-17β (E2) levels (18, 19) and enhanced estrogen receptor (ER)-α activity (11, 20) also are involved in the proinflammatory cascade leading to parturition. Estrogens induce the influx of macrophages and neutrophils into the uterus and antagonize antiinflammatory actions of P4/PR (11, 21). Furthermore, ERα activation facilitates labor by enhancing transcription of genes encoding contraction-associated proteins (CAP), including oxytocin receptor (OXTR) (22), connexin-43 (CX43) (23), and COX-2 (11). COX-2 is a highly inducible gene that is expressed at low to undetectable levels in the uterus throughout most of pregnancy but is highly up-regulated by proinflammatory cytokines and by estrogen at term (24, 25). COX-2 catalyzes the production of prostaglandins, which play a crucial physiological role in the initiation of labor by acting as potent uterine contractility agents (26).
Recently we uncovered novel roles for microRNAs (miRNA, miR) as hormonally regulated modulators of uterine contractility in the maintenance of pregnancy and initiation of labor (27). miRNAs are 22-nucleotide molecules that serve particularly important roles in female reproductive physiology (28–31) and have been identified as promising potential drug targets for a variety of pathological conditions (32). These small noncoding RNA predominantly regulate gene expression by targeting the 3′untranslated region (UTR) of mRNAs, resulting in either degradation of the mRNA transcript or translational repression. Recently we discovered that members of the miR-200 family, which increase in the pregnant myometrium toward term and their targets, zinc finger E-box binding homeobox proteins zinc finger E-box binding homeobox (ZEB)-1 and ZEB2, which coordinately decline, serve as novel P4/PR-mediated regulators of genes encoding the CAPs, OXTR, and CX43 (27). We further observed that increased expression of miR-200a in the pregnant myometrium near term targets signal transducer and activator of transcription-5b (STAT5b), resulting in enhanced myometrial expression of the P4-metabolizing enzyme, 20α-hydroxysteroid dehydrogenase, causing a local decline in PR function (15).
Herein we show that levels of the clustered miRNAs, miR-199a-3p and miR-214, were significantly decreased in laboring myometrium of pregnant mice and humans and in an inflammatory mouse model of preterm labor, whereas the miR-199a-3p/miR-214 target, COX-2, a critical enzyme in synthesis of proinflammatory prostaglandins, was coordinately increased. Overexpression of miR-199a-3p and miR-214 in cultured human myometrial cells blocked TNF-α-induced myometrial cell contractility, suggesting their physiological relevance. Notably, E2 treatment of ovariectomized mice suppressed, whereas P4 enhanced uterine miR-199a-3p/214 expression; these effects were mediated by opposing actions of E2 and P4 on ZEB1.
Materials and Methods
Mouse and human tissue preparations
All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center. Eight-week-old timed pregnant ICR/CD1 mice were obtained from Harlan Laboratories (Houston, TX). Myometrial tissues were isolated at 15.5 and 18.5 d postconception (dpc) and after the delivery of the first pup (in labor) as previously described (27).
All human studies and consent forms were approved by the Institutional Review Board of University of Texas Southwestern Medical Center in accordance with the Donors Anatomical Gift Act of the State of Texas. Informed consent was obtained in writing from each woman before surgery. Myometrial tissues were acquired from the lower uterine segment of pregnant women undergoing cesarean section before and after the onset of active labor. After the tissues were collected, they were flash frozen and stored at −80 C for subsequent protein and mRNA analysis.
Mouse model of lipopolysaccaride (LPS)/inflammation-induced preterm labor
Induction of preterm labor via LPS treatment has been previously described (27, 33). Briefly, 15.5 dpc pregnant ICR mice underwent laparotomy to expose the uterus after anesthetization. LPS (Sigma, St. Louis, MO) (1.5 μg in 50 μl) or sterile PBS (vehicle) were injected into each amniotic sac; the uterus was carefully reinserted into the abdominal cavity, the abdominal muscle wall and skin were sutured, and the mouse was allowed to recuperate. There was a high rate of preterm delivery in this mouse model of preterm labor; none of the vehicle-injected mice manifested preterm labor (27). The LPS-injected mice were killed upon the preterm birth of one pup; gestation-matched vehicle-injected controls were killed immediately afterward.
Progesterone and estrogen treatment studies
Six- to 8-wk-old ovariectomized female mice were obtained from Harlan Laboratories. Two weeks after ovariectomy, the mice were sc injected with E2 (Sigma) (1 μg in 0.3 ml sesame oil vehicle), P4 (Sigma) (1 mg in 0.25 mL sesame oil), or vehicle alone. The uterus was harvested 24 h after the injection and flash frozen for subsequent protein and mRNA analysis.
Studies also were conducted to assess the effects of P4 on expression of miR-199a/214 in myometrium of timed-pregnant mice. For these studies, timed-pregnant mice were injected sc with P4 (1.0 mg) or vehicle each day from 15.5 to 18.5 dpc. None of the P4-injected mice manifested spontaneous labor at term. At the time that a vehicle-injected mouse delivered the first pup, a P4 treated mouse also was killed.
Transfection and transduction
hTERT-HM cells (34) were cultured in DMEM-F12 medium (GIBCO, Grand Island, NY) with 10% fetal bovine serum (vol/vol). For miRNA mimic studies, the cells were transfected with 20 nm scramble mimic, miR-199a-3p or miR-214 mimics (QIAGEN, Valencia, CA) using HiPerFect transfection reagent (QIAGEN), as described previously (27). The cells were then treated with IL-1β or vehicle 4 h before collection. The cells were harvested for mRNA and protein analysis 24 h after transfection with miRNA mimics.
For ZEB1 overexpression experiments, hTERT-HM cells were transduced with recombinant adenoviruses expressing β-galactosidase (β-gal) or ZEB1 (27) at a multiplicity of infection of 500 pfu/cell and processed 48 h after infection. To assess transduction efficiency (which was ∼100%), β-gal staining (β-gal staining kit; Invitrogen, Carlsbad, CA) was performed on those cells transduced with the β-gal virus. The hTERT cells were at approximately 80% confluence for these experiments. In the ZEB1 knockdown studies, hTERT cells were either transfected with 20 nm control small interfering RNA (siRNA) or ZEB1 siRNA and harvested 72 h later. These concentrations of miR mimics and siRNA had previously been shown to achieve profound miR overexpression and ZEB1 knockdown, respectively, without manifesting toxic effects to the cells (27).
Collagen matrix contraction assay
The collagen matrix contraction assay was carried out as described in detail previously (27). Briefly, hTERT-HM cells were transfected with miRNA mimics, as described above, for 24 h. The cells were then harvested, embedded into collagen matrices, and cultured in DMEM-F12 medium with or without TNF-α (Cell Signaling, Danvers, MA) (10 ng/ml) for 24 h. As a positive control, untransfected hTERT-HM cells cultured ± TNF-α were treated with 5 μm indomethacin (Sigma). Contractility of the collagen gels was assessed by analyzing the total area of the gel (square millimeters) using Carestream Molecular Imaging Software (Carestream, Rochester, NY).
Quantitative RT-PCR (qRT-PCR)
For gene expression analysis, total RNA was reverse transcribed using a SuperScript III-RT kit (Invitrogen) and subsequently amplified using a SYBR Green universal PCR master mix (Applied Biosystems, Carlsbad, CA), as described previously (27). The primer sequences for analysis were as follows: mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH; forward: 5′-AGG TCG GTG TGA ACG GAT TTG-′3; reverse: 5′-TGT AGA CCA TGT AGT TGA GGT CA-′3), mouse COX-2 (forward: 5′-CAG CCA GGC AGC AAA TCC-′3; reverse: 5′-ACA TTC CCC ACG GTT TTG AC-′3), mouse ZEB1 (forward: 5′-GCT GGC AAG ACA ACG TGA AAG-′3; reverse: 5′-GCC TCA GGA TAA ATG ACG GC-′3), human 36B4 (forward: 5′-TGC ATC AGT ACC CCA TTC TAT CA-′3; reverse: 5′-AAG GTG TAA TCC GTC TCC ACA GA-′3), human COX-2 (forward: 5′-TTC CAG ATC CAG AGC TCA TTA AA-′3; reverse: 5′-CCG GAG CGG GAA GAA CT-′3), and human ZEB1 (forward: 5′-GATGATGAATGCGAGTCAGATGC-′3; reverse: 5′-CTGGTCCTCTTCAGGTGCC-′3).
MiRNA qRT-PCR was carried out as described previously (27). Briefly, a TaqMan reverse transcription kit (Applied Biosystems) was used to reverse transcribe total RNA; a TaqMan universal PCR master mix (Applied Biosystems) was then used for miRNA analysis. All the RNA samples were treated with deoxyribonuclease (Invitrogen) before analysis. The comparative cycle threshold was used to determine relative gene and miRNA expression.
Immunoblotting
Immunoblotting was conducted as previously described (27). Briefly, cytoplasmic and nuclear protein extracts were isolated from cellular and tissue samples using NE-PER extraction reagent kit (Pierce, Rockford, IL). Expression levels of proteins of interest were analyzed using primary polyclonal antibodies against COX-2 (Abcam, Cambridge, MA) and ZEB1 (graciously provided by Douglas Darling, Ph.D., University of Louisville School of Dentistry, Louisville, KY) at 1:500 and 1:1000 dilutions, respectively. Horseradish peroxidase-conjugated goat antirabbit secondary antibody (Invitrogen) was used at a 1:7,000 dilution. The blots were stripped and reprobed with antibodies to β-actin (Abcam) as a loading control.
Luciferase reporter assays
Identification of the miR-214 binding element in the COX-2 3′UTR
TargetScan prediction software (http://www.targetscan.org) was used to identify the putative miR-214 binding site in the mouse and human COX-2 3′UTR. The 3′UTR of COX-2 containing the potential miR-214 binding site was amplified from human genomic DNA using the following primers: forward: 5′-GGGACTAGTCACAAAGAATATTGTCTCATTAGCCTGAAT-3′, reverse: 5′-GGGAAGCTTTCAGAAAAGATCTGTCAATTTTTAAATAGT-3′. The 530-bp fragment was then cloned into the 3′UTR region of pMIR-REPORT (Invitrogen); the sequence of the recombinant plasmid was confirmed before transfection. For mutation analysis, the QuikChange II site-directed mutagenesis kit (Stratagene, Santa Clara, CA) was used to mutate three nucleotides in the putative miR-214 binding site (GATCTGCTGACA to GATCTGACAACA). COS7 cells were cotransfected with these reporter plasmids and 20 nm miR-214 or miR-1 (control) and then assayed for relative luciferase activity 48 h later. The cells were cotransfected with a cytomegalovirus expression vector containing β-gal to normalize for efficiency of transfection.
Regulation of Dnm3os promoter activity by ZEB1
A portion of the Dnm3os promoter (−640 to 0 bp) and an E-box-deleted promoter region of Dnm3os (−640 to −357) were amplified from mouse genomic DNA using previously published primers (35). These PCR fragments were then cloned upstream of luciferase in PGL4.23; the sequence of the recombinant plasmid DNA was confirmed before transfection. COS-7 cells were transfected with a recombinant plasmid expressing CMV-ZEB1 or with an empty vector for 24 h before transfection with the luciferase reporters. Relative luciferase activity was assayed 24 h after luciferase reporter transfection. To normalize for efficiency of transfection, the cells were cotransfected with a CMV expression vector containing β-gal.
Chromatin immunoprecipitation (ChIP) studies
ChIP was performed using a ChIP assay kit (catalog no. 17-295; Millipore, Billerica, MA) to assess the binding of endogenous ZEB1 to the Dnm3os promoter in myometrial tissues from timed-pregnant mice at 15.5 and 18.5 dpc. Briefly, mouse myometrial tissues were isolated, homogenized, cross-linked with formaldehyde (1%) and sonicated to produce sheared soluble chromatin, as described in detail previously (27). Precleared chromatin was incubated with ZEB1 antibody (provided by Douglas Darling, Ph.D.) or nonimmune IgG, as control, at 4 C overnight. Immune complexes were collected on Protein A agarose beads (ChIP assay kit; Millipore). Chromatin complexes were eluted from the beads and cross-linking was reversed. DNA purified from the samples and input controls was analyzed for Dnm3os promoter sequences containing putative ZEB1 response elements using quantitative PCR and the following primers: forward: 5′-GAC AGG CTC TCC CCA GCC CC-3′; reverse: 5′-CAG CCG TCC ATG GCG TTG CT-3′.
Statistical analyses
Excel (Microsoft, Richmond, CA) as used to perform statistical analyses of the data. Statistical significance was determined using the two-tailed Student's t test; P > 0.05 was considered to be significant.
Results
miR-199a-3p/miR-214 and COX-2 are reciprocally regulated during late gestation and labor
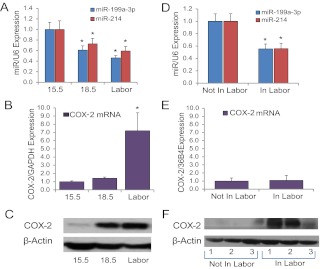
In a previous miRNA array analysis of myometrial tissues from pregnant mice at 15.5 dpc vs. 18.5 dpc (19.0 dpc = term) to discover miRNAs that are differentially expressed during the transition of the pregnant myometrium from a quiescent state to a highly contractile unit, we identified a conserved cluster of miRNAs, miR-199a-3p and miR-214, that were significantly down-regulated near term (27). miR-199a-3p and miR-214 are synthesized as part of a 6-kb antisense transcript (Dnm3os), which is highly expressed in the pregnant uterus (36). Interestingly, miR-199a-3p and miR-214 are known (31) and predicted, respectively, to target the mRNA encoding COX-2, a key regulatory enzyme in synthesis of prostaglandins, which are potent stimulators of uterine contractility (26, 37, 38). qRT-PCR of RNA isolated from myometrial tissues of pregnant mice at 15.5 dpc, 18.5 dpc, and in active labor confirmed that both miR-199a-3p and miR-214 were significantly down-regulated at 18.5 dpc and during labor as compared with 15.5 dpc (Fig. 1A). Notably, COX-2 mRNA remained low in the pregnant mouse myometrium until active labor, when its expression was markedly induced (Fig. 1B). On the other hand, a pronounced increase in COX-2 protein expression was evident by 18.5 dpc and remained elevated in laboring myometrium (Fig. 1C).
Fig. 1.
miR-199a–3p/miR-214 and their target COX-2 are reciprocally regulated during pregnancy and labor in mouse and human myometrium. A, miR-199a–3p and miR-214 expression declined significantly in pregnant mouse myometrium from 15.5 dpc to labor/term (term = 19.0 dpc) (n = 5 mice at each time point). B and C, COX-2 protein expression (C) was coordinately increased in pregnant mouse myometrium from 15.5 dpc to labor, but COX-2 mRNA (B) was induced only in labor. D, miR-199a–3p and miR-214 expression was significantly decreased in myometrium from women undergoing cesarean section at term who were in labor (n = 9) when compared with gestationally matched women not in labor (n = 11). E and F, COX-2 mRNA (E) levels were similar in myometrium of women in labor (n = 8), compared with those not in labor (n = 8), whereas COX-2 protein (F) levels in myometrium from women in labor were markedly increased compared with gestationally matched women not in labor (n = 3 per group). Levels of each miRNA/mRNA were determined by qRT-PCR, normalized to U6/GAPDH and expressed relative to 15.5 dpc (A and B) or not in labor (D and E) samples, which were assigned a value of 1. Data shown are the mean ± sem for the number of samples noted above. *, P < 0.05.
In studies of myometrium from women in labor at term, miR-199a-3p and miR-214 were decreased, as compared with myometrial samples from gestationally matched nonlaboring women (Fig. 1D). Interestingly, although we did not observe a significant change in COX-2 mRNA (Fig. 1E), there was marked induction of COX-2 protein in laboring, compared with the nonlaboring human myometrium (Fig. 1F), further suggesting potential translational regulation.
COX-2 is a direct target of miR-199a-3p and miR-214 in the myometrium
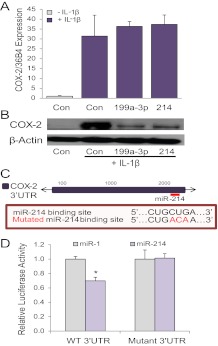
To determine whether miR-199a-3p and miR-214 directly regulate COX-2 in myometrium, we conducted studies using immortalized human myometrial cells (hTERT-HM) (34) transfected with mimics for miR-199a-3p and miR-214. Because COX-2 mRNA and protein are undetectable in hTERT-HM under resting conditions, the cells were cultured with or without the proinflammatory cytokine IL-1β to induce COX-2 expression (38–40). Overexpression of miR-199a-3p or miR-214 in the hTERT-HM cells did not significantly affect COX-2 mRNA (Fig. 2A) but caused a marked decline in COX-2 protein levels, as compared with cells transfected with scramble (control) mimic (Fig. 2B). These findings suggest that miR-199a-3p and miR-214 promote COX-2 translational repression.
Fig. 2.
miR-199a–3p and miR-214 target COX-2 expression at the level of protein translation, rather than mRNA stability. Like miR-199a–3p, miR-214 targets COX-2. A and B, Overexpression of miR-199a–3p or miR-214 in human immortalized myometrial cells (hTERT-HM) cultured ± IL-1β for 24 h had no effect on COX-2 mRNA expression (A) but markedly decreased COX-2 protein levels (B). COX-2 mRNA expression was analyzed by qRT-PCR and normalized to h36B4. Data shown in A are expressed relative to hTERT-HM cells transfected with a scrambled control miR (Con) and cultured in the absence of IL-1β, which were assigned a value of 1. C, Schematic of the location of the putative miR-214 binding site in the 3′UTR of COX-2. Sequence of the putative miR-214 binding element and of the mutated binding element also are shown. D, Luciferase activity of COS-7 cells cotransfected with the recombinant pMIR-REPORT plasmid containing the WT COX-2 3′UTR subcloned downstream of luciferase was decreased when cotransfected with mimics for miR-214, compared with cells cotransfected with miR-1 (used as a negative control and assigned a value of 1). This repression was lost when the putative miR-214 binding site was mutated (n = 3 per group). Values shown in all bar graphs are the mean ± sem. *, P < 0.05. Immunoblots are representative of findings from three independent experiments.
Although previous studies confirmed that miR-199a-3p directly targets COX-2 in the uterus (31), this had not been demonstrated for miR-214. To determine whether COX-2 is a direct target of miR-214, we conducted luciferase reporter assays in COS-7 cells using COX-2–3′-UTR-luciferase reporters in which a portion of the COX-2 3′UTR containing the putative miR-214 binding site was subcloned downstream of luciferase (Fig. 2C). These studies revealed that miR-214 overexpression caused significant down-regulation of luciferase via the COX-2 3′-UTR. Furthermore, this repression was lost when the miR-214 binding site in the COX-2 3′-UTR was mutated (Fig. 2D). Taken together, these findings suggest that both miR-199a-3p and miR-214 directly target COX-2 in the myometrium and that regulation is at the level of translational repression, rather than mRNA stability.
Proinflammatory factors promote miR-199a-3p/miR-214 expression in the uterus
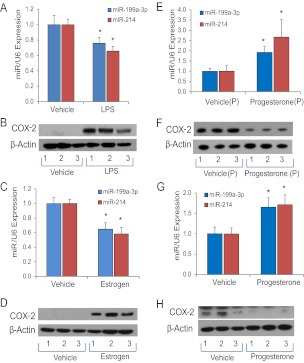
The initiation of myometrial contractility leading to term and preterm labor is mediated by an increased inflammatory response (3). In fact, 30% or more of all preterm births are associated with bacterial infection (41). To assess changes in miR-199a-3p/214 and COX-2 expression in mouse myometrium using an infection/inflammation-induced preterm labor model, we analyzed effects of intraamniotic injection of the Gram-negative bacterial endotoxin, LPS, at 15.5 dpc (27). Approximately 70% of all LPS injected mice delivered within 12–18 h of treatment. In those LPS-treated mice that delivered preterm, myometrial miR-199a-3p and miR-214 expression were significantly reduced, as compared with gestation-matched vehicle injected controls (Fig. 3A), whereas COX-2 protein levels were markedly up-regulated (Fig. 3B).
Fig. 3.
miR-199a–3p/miR-214 and COX-2 are reciprocally regulated in mouse uterine tissues by pro- and antiinflammatory hormones and factors. A and B, Pregnant mice were injected intraamniotically at 15.5 dpc with vehicle or with LPS to induce preterm labor. Myometrial tissues were harvested from each LPS-injected mouse upon preterm birth of the first pup and from a gestation-matched, vehicle-injected control at the same time. LPS treatment decreased myometrial miR-199a–3p and miR-214 expression and induced COX-2 protein expression (n = 5 mice per group). C and D, Ovariectomized mice were treated with a single sc injection of E2 (1 μg). E2 treatment significantly inhibited expression of miR-199a–3p and miR-214 (C), whereas COX-2 protein levels (D) were reciprocally induced in uterine tissues after 24 h. E and F, Timed-pregnant mice were injected with P4 (1 mg) or vehicle daily from 15.5 to 18.5 dpc. Myometrial tissues were harvested from each vehicle-injected mother at the time of delivery of the first pup and from a P4-injected mother at the same time. miR-199a–3p and miR-214 were significantly increased (E) and COX-2 protein was decreased (F) in myometrial tissues from P4 treated pregnant mice (n = 5 mice per group). G and H, Ovariectomized mice were given a single injection of P4 or vehicle; miR-199a–3p and miR-214 were significantly increased (G) and COX-2 protein levels were decreased after 48 h in the uterine tissues (n = 5 mice per group). Values shown in all bar graphs are the mean ± sem. *, P < 0.05. Levels of each miRNA shown in A, C, E, and G were determined by qRT-PCR, normalized to U6 and expressed relative to data from vehicle-injected mice, which were assigned a value of 1.
Estrogens promote an inflammatory response in the uterus and antagonize antiinflammatory actions of P4/PR (11, 21). Moreover, circulating E2 levels increase markedly near term in a number of species (18, 19, 42) with the decline in uterine PR function (2, 3, 5). To assess the effects of estrogen on miR-199a-3p and miR-214 expression in uterus, ovariectomized mice were given a single sc injection of E2 (1 μg) and uterine tissues were collected 24 h later. Notably, E2 treatment of ovariectomized mice significantly inhibited uterine miR-199a/214 expression (Fig. 3C) and caused an associated induction of COX-2 protein (Fig. 3D).
By contrast, P4 maintains uterine quiescence, in part, by its antiinflammatory actions (3, 43). To investigate effects of P4 on expression of miR-199a/214 in myometrium, pregnant mice were injected sc with P4 (1.0 mg) or vehicle each day from 15.5 to 18.5 dpc. P4 treatment caused a delay in labor that was associated with a significant induction of miR-199a/214 expression and repression of COX-2 protein in the pregnant myometrium (Fig. 3, E and F). We observed a similar up-regulation of miR-199a/214 (Fig. 3G) and inhibition of COX-2 protein expression (Fig. 3H) in uterine tissues from ovariectomized mice 48 h after P4 treatment.
ZEB1 up-regulates transcription of miR-199a-3p/miR-214 in the myometrium
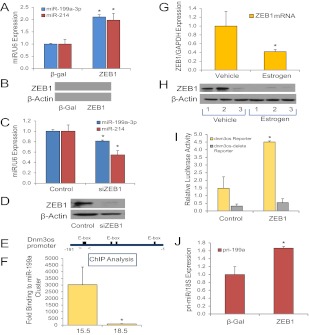
To begin to define mechanisms for the opposing effects of P4 and E2 on miR-199a-3p/214 expression, we considered the possible roles of ZEB1 and ZEB2, which decline in the pregnant uterus near term (27) in a temporal pattern highly similar to miR-199a/214. We previously observed that P4 specifically induced expression of ZEB1, which represses miR-200 family expression, allowing for up-regulation of ZEB2 (27). Importantly, ZEBs are structurally related to Twist1, a transcription factor reported to increase miR-199a/214 cluster expression (35, 44). Twist1 and ZEB both bind to E-boxes and have several overlapping functions (35, 45–49). However, unlike ZEB1/2, we observed that myometrial Twist1 mRNA and protein were not altered during the transition to labor (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). We therefore postulated that ZEB1 might coordinately induce miR-199a-3p/214 expression and inhibit miR-200 family expression in the pregnant uterus. This hypothesis was supported by observations that ZEB1 overexpression in cultured human myometrial hTERT-HM cells markedly induced miR-199a-3p/214 (Fig. 4, A and B). Conversely, siRNA-mediated knockdown ZEB1 caused a significant down-regulation of miR-199a-3p/214 levels (Fig. 4, C and D).
Fig. 4.
ZEB1, which is induced by P4 (27, 56) and inhibited by E2 treatment, binds to the Dnm3os promoter and stimulates expression of the miR-199a–3p/miR-214 cluster at the transcriptional level. A and B, hTERT-HM human myometrial cells were infected with recombinant adenoviruses expressing either ZEB1 or β-gal, as a control. Overexpression of ZEB1 significantly increased miR-199a–3p and miR-214 expression after 48 h (n = 3). Data shown in A were normalized to U6 and expressed relative to those of cells infected with the β-gal adenovirus, which were assigned a value of 1. C and D, hTERT-HM cells were transfected with either ZEB1 siRNA or nontargeting control siRNA. Knockdown of ZEB1 decreased expression of miR-199a and miR-214 after 72 h (n = 3). Data shown in C were normalized to U6 and expressed relative to those of cells transfected with a nontargeting siRNA, which were assigned a value of 1. E and F, ChIP and quantitative PCR were used to quantify relative binding activity of endogenous ZEB1 to the Dnm3os promoter in pregnant mouse myometrium at 15.5 dpc vs. labor (19.0 dpc). E, Schematic of the Dnm3os promoter containing E-boxes previously shown to bind Twist1 (35). Primers used to amplify genomic DNA containing the putative ZEB1 binding site(s) are indicated by the arrows. F, Binding of endogenous ZEB1 was determined by quantitative PCR, normalized to input, and expressed as fold-increase over binding using nonimmune IgG in place of antibodies to ZEB1. In myometrial tissues from pregnant mice, endogenous ZEB1 binding to the Dnm3os promoter was significantly decreased at 18.5 dpc, when compared with 15.5 dpc. Data shown are the mean ± sem from three independent experiments, each conducted in triplicate. G and H, Ovariectomized, nonpregnant mice were injected with E2 or vehicle. ZEB1 mRNA (G) and protein (H) expression were decreased in uterine tissues after 24 h estrogen treatment. Data shown in bar graph (G) are the mean ± sem (n = 5 mice per treatment group). ZEB1 mRNA levels were analyzed by qRT-PCR, normalized to GAPDH, and expressed relative to the vehicle-injected control values, which were assigned a value of 1. I, Luciferase activity of COS-7 cells infected with recombinant adenoviruses expressing ZEB1 or β-gal (Control) and transfected with luciferase reporter constructs containing the Dnm3os promoter (−640 to 0 bp) or a truncated Dnm3os promoter (−640 to −357 bp) lacking critical E-boxes shown in E. Enhancing ZEB1 expression induced expression of the luciferase reporter containing the intact Dnm3os promoter but had no effect on the reporter lacking the putative ZEB1 binding sites. Data are the mean ± sem of values from three independent experiments, conducted in triplicate and expressed relative to cells transfected with an empty luciferase vector. J, Overexpression of ZEB1 in hTERT-HM cells induced expression of the primary transcript of miR-199a/miR214, pri-miR-199a, when compared with cells treated with the control adenovirus expressing β-gal (n = 3). *, P < 0.05. Pri-miR-199a values were normalized to 18S RNA and expressed relative to those of cells infected with the β-gal adenovirus, which were assigned a value of 1.
To determine whether myometrial ZEB1 binds to E-boxes in the region upstream of the miR-199a/214 cluster previously reported to bind Twist1 and to assess the gestational changes in binding activity, ChIP analysis of the E-box-containing region of the Dnm3os promoter was used (Fig. 4E). Endogenous ZEB1 bound specifically to the E-box-containing region in pregnant mouse myometrium at 15.5 dpc, whereas binding was dramatically reduced at 18.5 dpc (Fig. 4F). These findings suggest that P4 stimulation of miR-199a-3p/214 expression in the pregnant mouse uterus may be mediated by the P4/PR induction of ZEB1 (27).
In light of the opposing effects of E2 on uterine miR-199a-3p/214 levels, we investigated effects of E2 treatment of ovariectomized mice on ZEB1 expression. Notably, E2 treatment of ovariectomized mice significantly inhibited expression of uterine ZEB1 mRNA (Fig. 4G) and protein (Fig. 4H) within 24 h. Thus, P4 and E2 exert opposing effects on miR-199a-3p/214 transcription in myometrium via ZEB1. To determine whether this action of ZEB1 is mediated through its interaction with the E-box-containing region of the Dnm3os promoter, COS-7 cells were cotransfected with a luciferase reporter controlled by the Dnm3os promoter region previously observed to drive miR-199a/214 expression and be regulated by Twist1 (35). Cotransfection of ZEB1 increased Dnm3os promoter activity; induction was lost when the genomic region containing the ZEB1 binding sites was deleted (Fig. 4I). Moreover, ZEB1 overexpression in hTERT-HM cells up-regulated expression of the primary transcript of miR-199a/214, pri-miR-199a (Fig. 4J), further suggesting that ZEB1 enhances miR-199a/214 expression at the transcriptional level.
Manipulation of miR-199a-3p/miR-214 affects contraction of myometrial cells
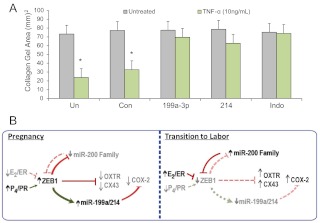
Finally, to determine whether the miR-199a-3p- and miR-214-mediated decline in COX-2 protein expression alters myometrial contractility, we used collagen gel contraction assays (27), in which the cultured hTERT-HM cells transfected with miR-199a-3p or miR-214 mimics or with scramble miRNA, were embedded into three-dimensional collagen gel matrices. Contraction of the human myocytes within the matrix in response to treatment with TNF-α, a proinflammatory cytokine that enhances uterine contractility (40), was assessed by a reduction in the diameter of the collagen disc. As a positive control, untransfected hTERT-HM cells cultured with or without TNF-α were treated with the cyclooxygenase inhibitor, indomethacin, a tocolytic agent for preterm labor (50). TNF-α significantly induced contraction of collagen gel matrices containing hTERT-HM cells that were untransfected or transfected with the control/scrambled miRNA. By contrast, the contractile effect of TNF-α was blocked in cells overexpressing miR-199a-3p or miR-214, in a manner comparable with the action of indomethacin (Fig. 5A).
Fig. 5.
miR-199a–3p/miR-214 repression of COX-2 inhibits myometrial contractility to the same extent as indomethacin. A, hTERT-HM human myometrial cells were either untransfected (Un), transfected with a scrambled miRNA mimic (Con), or with mimic for miR-199a–3p or miR-214, embedded in collagen gels and cultured ± TNF-α. A parallel set of untransfected cells embedded in collagen was cultured ± indomethacin, as a positive control for COX-2 inhibition. Contraction of the collagen gels was significantly inhibited by miR-199a–3p and miR-214 transfection after 24 h, when compared with gels containing cells that were either untransfected or transfected with scrambled-control mimics. Data are the mean ± sem of collagen gel area values from three replicate experiments, each conducted in duplicate; *, P < 0.05. B, ZEB1 serves a pivotal role in mediating opposing effects of P4 and E2 on myometrial contractility during pregnancy and labor. Based on the findings presented herein and in our previous work (27), the following mechanism is proposed. During pregnancy, increased circulating levels of P4 and of PR function and decreased levels of E2/ERα activity mediate up-regulation of ZEB1 in myometrium. This causes suppression of the miR-200 family and the CAP genes, OXTR and CX43. The increased ZEB1 also binds to response elements upstream of the miR-199/miR-214 cluster to enhance its expression, resulting in suppression of COX-2 and prostaglandin synthesis. During the transition to labor, the decline in PR function and increase in ERα activity in myometrium result in a down-regulation of ZEB1. This permits up-regulation of the miR-200 family, which further suppresses ZEB1 and inhibits ZEB2. This, in turn, allows de-repression of OXTR and CX43. The decline in ZEB1 also results in decreased expression of the miR-199a/miR-214 cluster, which permits up-regulation of their target, COX-2, and the synthesis of contractile prostaglandins. Collectively these molecular events contribute to the induction of uterine contractility, leading to labor.
Discussion
The molecular mechanisms that maintain quiescence of the myometrium throughout most of pregnancy and mediate its conversion into a synchronously contractile unit culminating in parturition remain incompletely understood. To obtain a deeper understanding of the molecular events that underlie the transition of the pregnant myometrium from a refractory to a contractile state leading to labor and the effects of P4 and E2 in this regulation, we have further investigated the roles of miRNAs and their transcriptional and hormonal regulation.
miRNAs are evolutionarily conserved, potent regulators of gene expression that have been shown to play important roles in immune (51) and reproductive (28, 29, 31) physiology. Because a single miRNA can have a considerable number of mRNA targets, they have the capacity to regulate functionally linked gene networks, such as those that control pregnancy and labor. The coordinated regulation of different miRNAs can allow either fine-tuning or profound changes in expression of related set of genes (52). Although the involvement of different clusters and families of miRNAs has been evaluated in a number of female reproductive processes, including implantation, menstruation, endometriosis, and leiomyomas (28), the roles of miRNAs have not been explored in pregnancy and labor until recently (27).
In previous studies, using a microarray based approach and a number of mouse models, we uncovered a role for the miR-200 family and its targets, transcription factors ZEB1 and ZEB2, as mediators of P4 suppression of CAP gene expression in the pregnant myometrium. We observed that P4/PR maintains uterine quiescence during pregnancy, in part, via the up-regulation of the ZEB1, which inhibits expression of the contraction-associated genes, OXTR and CX43 and suppresses the miR-200 family, which promotes further up-regulation of ZEB1 and increases ZEB2 (27). Toward term, the decline in PR function results in a decrease in ZEB1/2 and de-repression of the miR-200 family and CAP genes. Similar findings were obtained in two different mouse models of preterm labor and in myometrium from women in labor at term, as compared with gestation matched myometrium from women not in labor (27).
Using miRNA array analysis of myometrial tissues from pregnant mice at 15.5 dpc vs. 18.5 dpc (19.0 dpc = term) to discover additional miRNAs that are differentially expressed during the transition of the pregnant myometrium from a quiescent state to a highly contractile unit (27), we identified a conserved cluster of miRNAs, the miR-199a cluster, which was down-regulated near term (27). The miR-199a cluster, comprised of miR-199a-5p, miR-199a-3p, and miR-214, are synthesized as part of a 6-kb antisense transcript of the Dynamin3 gene (Dnm3os), which is highly expressed in pregnant uterus (36). The seed sequences for miR-199a-3p, miR-199a-5p, and miR-214 are highly conserved from mouse to human. Whereas seed sequences of miR-199a-3p and miR-214 are highly similar, that of miR-199a-5p differs significantly from the other two miRNAs in the cluster, suggesting that miR-199a-5p targets a different set of mRNAs. In fact, miR-199a-3p and miR-214 were known (31) and predicted, respectively, to target the mRNA encoding COX-2, the key regulatory enzyme in the synthesis of prostaglandins, which are potent stimulators of uterine contractility (26, 53). On the other hand, miR-199a-5p has been shown to target inhibitor of κB kinase-β, thereby suppressing NF-κB pathway activation (44). The qRT-PCR of RNA isolated from the myometrial tissues of pregnant mice at 15.5 dpc, 18.5 dpc, and in active labor confirmed that miR-199a-3p and miR-214 were significantly down-regulated in pregnant mouse myometrium at 18.5 dpc and during labor, as compared with 15.5 dpc. However, we did not observe a significant change in the expression of miR-199a-5p (Supplemental Fig. 2). In the present study, we, therefore focused on the roles of miR-199a-3p and miR-214 in the regulation of COX-2.
In the present study, we demonstrated that COX-2 is an authentic target of miR-214 (Fig. 2). We observed that the levels of miR-199a-3p and miR-214 were significantly decreased in laboring myometrium of pregnant mice and humans (Fig. 1) and in an inflammatory mouse model of preterm labor (Fig. 3), whereas COX-2 protein was coordinately increased (Figs. 1 and 3). In pregnant mouse myometrium, miR-199a-3p/miR-214 expression was significantly decreased at 18.5 dpc and during labor, as compared with 15.5 dpc, whereas COX-2 mRNA levels increased only during labor. On the other hand, COX-2 protein levels were significantly increased in the myometrium by 18.5 dpc in association with the decline in miR-199a-3p/miR-214 expression (Fig. 1). The relevance of the gestational increase in COX-2 protein is supported by the observation that prostaglandin F2α levels were significantly increased in the pregnant mouse uterus between 16 and 18 dpc (54). We therefore suggest that miR-199a-3p/miR-214 may exert a direct effect on COX-2 mRNA translation, rather than on COX-2 mRNA stability. This conjecture was supported by the finding that the overexpression of miR-199a-3p and miR-214 in cultured human myometrial cells inhibited COX-2 protein but had no effect on COX-2 mRNA levels (Fig. 2). Moreover, in myometrial samples from women in labor vs. those not in labor at term in the absence of underlying infection, COX-2 mRNA was unchanged, whereas COX-2 protein levels were markedly increased during labor (Fig. 1). Thus, our collective findings suggest that COX-2 expression in the pregnant myometrium is regulated at the level of mRNA translation and that the miR-199a/214 cluster may serve an important role in this regulation.
We view these findings to be of great import because the significance of myometrial COX-2 up-regulation in the initiation of normal labor at term has been questioned (55). This query was based on the observation that COX-2 mRNA levels were not found to be increased in myometrial tissues from women in labor, as compared with not in labor, unless there was underlying chorioamnionitis (55). However, in those studies, expression of COX-2 protein was not analyzed. Moreover, the physiological relevance of the miR-199a/miR-214-COX-2 relationship in the regulation of myometrial contractility is further supported by the finding that miR-199a/miR-214 overexpression blocked TNF-α-induced myometrial cell contractility to the same extent as the cyclooxygenase inhibitor, indomethacin (Fig. 5).
As mentioned previously, P4 and E2 are known to exert opposing effects on myometrial quiescence/contractility. In the present study, we observed that E2 treatment of ovariectomized mice suppressed, whereas P4 enhanced uterine miR-199a-3p/miR-214 expression. Interestingly, these opposing hormonal effects were mediated by ZEB1, which is induced by P4 (27, 56), inhibited by E2 and activates miR-199a/miR-214 transcription. Thus, in the course of these studies, we have uncovered an intriguing pivotal role of ZEB1 as a negative regulator of the miR-200b/miR-200a/miR-429 cluster and a positive regulator of the miR-199a cluster that is under opposing control by P4 and E2. 17α-Hydroxyprogesterone caproate has been found to be efficacious in reducing the incidence of preterm labor in women with a history of spontaneous preterm delivery; however, its mechanism of action is not clearly understood (57). In this regard, it would be of interest to analyze the expression of miR-199a-3p/miR-214, miR-200 and ZEB in the myometrial biopsies of women treated with this compound, compared with gestation-matched controls.
In conclusion, this study provides compelling evidence that miR-199a-3p and miR-214 play crucial roles in the maintenance of uterine quiescence by suppressing COX-2 and production of contractile prostaglandins (Fig. 5B). Our findings also point to the key pivotal role of myometrial ZEB1, which is up-regulated by P4/PR throughout most of pregnancy (27) and declines with the decrease in P4/PR function and the increase in E2/ERα activity during the progression to labor. ZEB maintains myometrial quiescence by suppressing the miR-200 family (58) and the expression of CAP genes, such as the OXTR and CX43 (27), on the one hand, and by enhancing miR-199a-3p/miR-214 expression and causing the suppression of COX-2, on the other hand. Near term, enhanced ERα activity and the increased inflammatory response in the myometrium promote a decline in PR function causing decreased expression of ZEB1. This results in de-repression of the CAP genes and the down-regulation of miR-199a/miR-214 expression with an associated increase in COX-2, causing further up-regulation of the inflammatory response and active labor (Fig. 5B). The combinatorial role of miR-199a-3p and miR-214 in the selective suppression of myometrial COX-2 may have important therapeutic implications because the overexpression of these miRNAs could attenuate premature uterine contractions and prevent preterm labor.
Supplementary Material
Acknowledgments
We thank Dr. Y. Higashi (Osaka University, Osaka, Japan) for ZEB1 and ZEB2 expression plasmids that we used to make recombinant adenoviruses and Dr. Douglas Darling (University of Louisville School of Dentistry, Louisville, KY) for the antibody to ZEB1.
This research was supported by the March of Dimes Foundation (Prematurity Research Initiative Grant 21-FY11-30; to C.R.M.) and by the National Institutes of Health (Grant 5-P01-HD11149; to C.R.M.). K.C.W. was supported, in part, by the Pharmacological Sciences Training Grant GM007062.
Disclosure Summary: The authors have nothing to disclose.
NURSA Molecule Pages†:
Nuclear Receptors: Ligands: 17β-estradiol | Progesterone.
Annotations provided by Nuclear Receptor Signaling Atlas (NURSA) Bioinformatics Resource. Molecule Pages can be accessed on the NURSA website at www.nursa.org.
- CAP
- Contraction-associated protein
- ChIP
- chromatin immunoprecipitation
- COX-2
- cyclooxygenase-2
- CX43
- connexin-43
- dpc
- d postconception
- E2
- estradiol-17β
- ER
- estrogen receptor
- β-gal
- β-galactosidase
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- LPS
- lipopolysaccaride
- miRNA
- microRNA
- NF-κB
- nuclear factor-κB
- OXTR
- oxytocin receptor
- P4
- progesterone
- PR
- progesterone receptor
- qRT-PCR
- quantitative RT-PCR
- siRNA
- small interfering RNA
- UTR
- untranslated region
- ZEB
- zinc finger E-box binding homeobox.
References
- 1. Osterman MJ, Martin JA, Mathews TJ, Hamilton BE. 2011. Expanded data from the new birth certificate, 2008. Natl Vital Stat Rep 59:1–28 [PubMed] [Google Scholar]
- 2. Mendelson CR. 2009. Minireview: fetal-maternal hormonal signaling in pregnancy and labor. Mol Endocrinol 23:947–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hardy DB, Janowski BA, Corey DR, Mendelson CR. 2006. Progesterone receptor (PR) plays a major anti-inflammatory role in human myometrial cells by antagonism of NF-κB activation of cyclooxygenase 2 (COX-2) expression. Mol Endocrinol 20:2724–2733 [DOI] [PubMed] [Google Scholar]
- 4. Olson DM. 2003. The role of prostaglandins in the initiation of parturition. Best Pract Res Clin Obstet Gynaecol 17:717–730 [DOI] [PubMed] [Google Scholar]
- 5. Shynlova O, Tsui P, Jaffer S, Lye SJ. 2009. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur J Obstet Gynecol Reprod Biol 144(Suppl 1):S2–S10 [DOI] [PubMed] [Google Scholar]
- 6. Condon JC, Jeyasuria P, Faust JM, Mendelson CR. 2004. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci USA 101:4978–4983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Toyoshima K, Narahara H, Furukawa M, Frenkel RA, Johnston JM. 1995. Platelet-activating factor. Role in fetal lung development and relationship to normal and premature labor. Clin Perinatol 22:263–280 [PubMed] [Google Scholar]
- 8. López BA, Newman GE, Phizackerley PJ, Turnbull AC. 1988. Surfactant stimulates prostaglandin E production in human amnion. Br J Obstet Gynaecol 95:1013–1017 [DOI] [PubMed] [Google Scholar]
- 9. Condon JC, Jeyasuria P, Faust JM, Wilson JW, Mendelson CR. 2003. A decline in progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of labor. Proc Natl Acad Sci USA 100:9518–9523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Condon JC, Hardy DB, Kovaric K, Mendelson CR. 2006. Upregulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of NF-κB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol 20:764–775 [DOI] [PubMed] [Google Scholar]
- 11. Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. 2002. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab 87:2924–2930 [DOI] [PubMed] [Google Scholar]
- 12. Mahendroo MS, Cala KM, Russell DW. 1996. 5α-Reduced androgens play a key role in murine parturition. Mol Endocrinol 10:380–392 [DOI] [PubMed] [Google Scholar]
- 13. Mahendroo MS, Porter A, Russell DW, Word RA. 1999. The parturition defect in steroid 5α-reductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol 13:981–992 [DOI] [PubMed] [Google Scholar]
- 14. Andersson S, Minjarez D, Yost NP, Word RA. 2008. Estrogen and progesterone metabolism in the cervix during pregnancy and parturition. J Clin Endocrinol Metab 93:2366–2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williams KC, Renthal NE, Condon JC, Gerard RD, Mendelson CR. 2012. MicroRNA-200a serves a key role in the decline of progesterone receptor function leading to term and preterm labor. Proc Natl Acad Sci USA 109:7529–7534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kalkhoven E, Wissink S, van der Saag PT, van der Burg B. 1996. Negative interaction between the RelA(p65) subunit of NF-κB and the progesterone receptor. J Biol Chem 271:6217–6224 [DOI] [PubMed] [Google Scholar]
- 17. Hardy DB, Janowski BA, Chen CC, Mendelson CR. 2008. Progesterone receptor inhibits aromatase and inflammatory response pathways in breast cancer cells via ligand-dependent and ligand-independent mechanisms. Mol Endocrinol 22:1812–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Challis JR. 1971. Sharp increase in free circulating oestrogens immediately before parturition in sheep. Nature 229:208. [DOI] [PubMed] [Google Scholar]
- 19. Buster JE, Chang RJ, Preston DL, Elashoff RM, Cousins LM, Abraham GE, Hobel CJ, Marshall JR. 1979. Interrelationships of circulating maternal steroid concentrations in third trimester pregnancies. II. C18 and C19 steroids: estradiol, estriol, dehydroepiandrosterone, dehydroepiandrosterone sulfate, Δ5-androstenediol, Δ4-androstenedione, testosterone, and dihydrotestosterone. J Clin Endocrinol Metab 48:139–142 [DOI] [PubMed] [Google Scholar]
- 20. Wu WX, Myers DA, Nathanielsz PW. 1995. Changes in estrogen receptor messenger ribonucleic acid in sheep fetal and maternal tissues during late gestation and labor. Am J Obstet Gynecol 172:844–850 [DOI] [PubMed] [Google Scholar]
- 21. Tibbetts TA, Conneely OM, O'Malley BW. 1999. Progesterone via its receptor antagonizes the pro-inflammatory activity of estrogen in the mouse uterus. Biol Reprod 60:1158–1165 [DOI] [PubMed] [Google Scholar]
- 22. Murata T, Narita K, Honda K, Matsukawa S, Higuchi T. 2003. Differential regulation of estrogen receptor α and β mRNAs in the rat uterus during pregnancy and labor: possible involvement of estrogen receptors in oxytocin receptor regulation. Endocr J 50:579–587 [DOI] [PubMed] [Google Scholar]
- 23. Piersanti M, Lye SJ. 1995. Increase in messenger ribonucleic acid encoding the myometrial gap junction protein, connexin-43, requires protein synthesis and is associated with increased expression of the activator protein-1, c-fos. Endocrinology 136:3571–3578 [DOI] [PubMed] [Google Scholar]
- 24. Tsuboi K, Sugimoto Y, Iwane A, Yamamoto K, Yamamoto S, Ichikawa A. 2000. Uterine expression of prostaglandin H2 synthase in late pregnancy and during parturition in prostaglandin F receptor-deficient mice. Endocrinology 141:315–324 [DOI] [PubMed] [Google Scholar]
- 25. Engstrom T. 2001. The regulation by ovarian steroids of prostaglandin synthesis and prostaglandin-induced contractility in non-pregnant rat myometrium. Modulating effects of isoproterenol. J Endocrinol 169:33–41 [DOI] [PubMed] [Google Scholar]
- 26. Gibb W. 1998. The role of prostaglandins in human parturition. Ann Med 30:235–241 [DOI] [PubMed] [Google Scholar]
- 27. Renthal NE, Chen CC, Williams KC, Gerard RD, Prange-Kiel J, Mendelson CR. 2010. miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proc Natl Acad Sci USA 107:20828–20833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hawkins SM, Buchold GM, Matzuk MM. 2011. Minireview: the roles of small RNA pathways in reproductive medicine. Mol Endocrinol 25:1257–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Montenegro D, Romero R, Pineles BL, Tarca AL, Kim YM, Draghici S, Kusanovic JP, Kim JS, Erez O, Mazaki-Tovi S, Hassan S, Espinoza J, Kim CJ. 2007. Differential expression of microRNAs with progression of gestation and inflammation in the human chorioamniotic membranes. Am J Obstet Gynecol 197:289.e1–289.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luense LJ, Carletti MZ, Christenson LK. 2009. Role of Dicer in female fertility. Trends Endocrinol Metab 20:265–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chakrabarty A, Tranguch S, Daikoku T, Jensen K, Furneaux H, Dey SK. 2007. MicroRNA regulation of cyclooxygenase-2 during embryo implantation. Proc Natl Acad Sci USA 104:15144–15149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McDermott AM, Heneghan HM, Miller N, Kerin MJ. 2011. The Therapeutic potential of microRNAs: disease modulators and drug targets. Pharm Res 28:3029. [DOI] [PubMed] [Google Scholar]
- 33. Dudley DJ, Branch DW, Edwin SS, Mitchell MD. 1996. Induction of preterm birth in mice by RU486. Biol Reprod 55:992–995 [DOI] [PubMed] [Google Scholar]
- 34. Condon J, Yin S, Mayhew B, Word RA, Wright WE, Shay JW, Rainey WE. 2002. Telomerase immortalization of human myometrial cells. Biol Reprod 67:506–514 [DOI] [PubMed] [Google Scholar]
- 35. Lee YB, Bantounas I, Lee DY, Phylactou L, Caldwell MA, Uney JB. 2009. Twist-1 regulates the miR-199a/214 cluster during development. Nucleic Acids Res 37:123–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Loebel DA, Tsoi B, Wong N, Tam PP. 2005. A conserved noncoding intronic transcript at the mouse Dnm3 locus. Genomics 85:782–789 [DOI] [PubMed] [Google Scholar]
- 37. Olson DM, Ammann C. 2007. Role of the prostaglandins in labour and prostaglandin receptor inhibitors in the prevention of preterm labour. Front Biosci 12:1329–1343 [DOI] [PubMed] [Google Scholar]
- 38. Rauk PN, Chiao JP. 2000. Interleukin-1 stimulates human uterine prostaglandin production through induction of cyclooxygenase-2 expression. Am J Reprod Immunol 43:152–159 [DOI] [PubMed] [Google Scholar]
- 39. Soloff MS, Cook DL, Jr, Jeng YJ, Anderson GD. 2004. In situ analysis of interleukin-1-induced transcription of cox-2 and IL-8 in cultured human myometrial cells. Endocrinology 145:1248–1254 [DOI] [PubMed] [Google Scholar]
- 40. Fitzgibbon J, Morrison JJ, Smith TJ, O'Brien M. 2009. Modulation of human uterine smooth muscle cell collagen contractility by thrombin, Y-27632, TNF-α and indomethacin. Reprod Biol Endocrinol 7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel L, Hassan S. 2007. The role of inflammation and infection in preterm birth. Semin Reprod Med 25:21–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kamel RM. 2010. The onset of human parturition. Arch Gynecol Obstet 281:975–982 [DOI] [PubMed] [Google Scholar]
- 43. Loudon JA, Elliott CL, Hills F, Bennett PR. 2003. Progesterone represses interleukin-8 and cyclo-oxygenase-2 in human lower segment fibroblast cells and amnion epithelial cells. Biol Reprod 69:331–337 [DOI] [PubMed] [Google Scholar]
- 44. Yin G, Chen R, Alvero AB, Fu HH, Holmberg J, Glackin C, Rutherford T, Mor G. 2010. TWISTing stemness, inflammation and proliferation of epithelial ovarian cancer cells through MIR199A2/214. Oncogene 29:3545–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Postigo AA, Dean DC. 1997. ZEB, a vertebrate homolog of Drosophila Zfh-1, is a negative regulator of muscle differentiation. EMBO J 16:3935–3943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Peinado H, Olmeda D, Cano A. 2007. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 7:415–428 [DOI] [PubMed] [Google Scholar]
- 47. Yang J, Weinberg RA. 2008. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 14:818–829 [DOI] [PubMed] [Google Scholar]
- 48. Hebrok M, Wertz K, Füchtbauer EM. 1994. M-twist is an inhibitor of muscle differentiation. Dev Biol 165:537–544 [DOI] [PubMed] [Google Scholar]
- 49. Hjiantoniou E, Anayasa M, Nicolaou P, Bantounas I, Saito M, Iseki S, Uney JB, Phylactou LA. 2008. Twist induces reversal of myotube formation. Differentiation 76:182–192 [DOI] [PubMed] [Google Scholar]
- 50. Niebyl JR, Blake DA, White RD, Kumor KM, Dubin NH, Robinson JC, Egner PG. 1980. The inhibition of premature labor with indomethacin. Am J Obstet Gynecol 136:1014–1019 [DOI] [PubMed] [Google Scholar]
- 51. Pauley KM, Chan EK. 2008. MicroRNAs and their emerging roles in immunology. Ann NY Acad Sci 1143:226–239 [DOI] [PubMed] [Google Scholar]
- 52. van Rooij E, Liu N, Olson EN. 2008. MicroRNAs flex their muscles. Trends Genet 24:159–166 [DOI] [PubMed] [Google Scholar]
- 53. Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. 2003. Cytokines, prostaglandins and parturition—a review. Placenta 24(Suppl A):S33–S46 [DOI] [PubMed] [Google Scholar]
- 54. Olson DM, Zaragoza DB, Shallow MC, Cook JL, Mitchell BF, Grigsby P, Hirst J. 2003. Myometrial activation and preterm labour: evidence supporting a role for the prostaglandin F receptor—a review. Placenta 24(Suppl A):S47–S54 [DOI] [PubMed] [Google Scholar]
- 55. Havelock JC, Keller P, Muleba N, Mayhew BA, Casey BM, Rainey WE, Word RA. 2005. Human myometrial gene expression before and during parturition. Biol Reprod 72:707–719 [DOI] [PubMed] [Google Scholar]
- 56. Spoelstra NS, Manning NG, Higashi Y, Darling D, Singh M, Shroyer KR, Broaddus RR, Horwitz KB, Richer JK. 2006. The transcription factor ZEB1 is aberrantly expressed in aggressive uterine cancers. Cancer Res 66:3893–3902 [DOI] [PubMed] [Google Scholar]
- 57. Meis PJ. 2005. 17 Hydroxyprogesterone for the prevention of preterm delivery. Obstet Gynecol 105:1128–1135 [DOI] [PubMed] [Google Scholar]
- 58. Bracken CP, Gregory PA, Kolesnikoff N, Bert AG, Wang J, Shannon MF, Goodall GJ. 2008. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res 68:7846–7854 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.