Abstract
Disturbed sleep-wake cycle and circadian rhythmicity are associated with cancer, but the underlying mechanisms are unknown. Employing a tissue-isolated human breast xenograft tumor nude rat model, we observed that glycogen synthase kinase 3β (GSK3β), an enzyme critical in metabolism and cell proliferation/survival, exhibits a circadian rhythm of phosphorylation in human breast tumors. Exposure to light-at-night suppresses the nocturnal pineal melatonin synthesis, disrupting the circadian rhythm of GSK3β phosphorylation. Melatonin activates GSK3β by inhibiting the serine-threonine kinase Akt phosphorylation, inducing β-catenin degradation and inhibiting epithelial-to-mesenchymal transition, a fundamental process underlying cancer metastasis. Thus, chronic circadian disruption by light-at-night via occupational exposure or age-related sleep disturbances may contribute to cancer incidence and the metastatic spread of breast cancer by inhibiting GSK3β activity and driving epithelial-to-mesenchymal transition in breast cancer patients.
Epithelial-to-mesenchymal transition (EMT) is a developmental process in which epithelial cells acquire a mesenchymal phenotype and become migratory (1). EMT-like events can promote cancer progression, enhancing invasion and metastasis (2, 3). Cellular changes that characterize EMT include loss of cell-cell adhesions, reorganization of cytoskeleton, acquisition of a spindle-like morphology, and increased cell motility and invasiveness. Reduced expression of E-cadherin, a structural component of adherent junctions that are critical for epithelial cell polarity and adhesion, is considered a hallmark of EMT, and many EMT-inducing factors trigger epithelial cell reorganization by impairing the expression or function of E-cadherin (2, 4).
One molecule implicated in E-cadherin repression is β-catenin, a transcription factor associated with the Wingless (Wnt) signaling pathway. The Wnt/β-catenin pathway is tightly linked to EMT, with β-catenin being an essential component of the adherent junctions bound to the cytoplasmic domain of E-cadherin (5). On the other hand, when dissociated from the adherent junctions, β-catenin can also translocate to the nucleus and complex with the transcription factor T-cell factor/lymphocyte enhancer factor (TCF/LEF) to induce the transcription of Wnt target genes, including the zinc finger protein Snail, a transcriptional repressor of E-cadherin, and vimentin, an intermediate filament protein and marker of mesenchymal cells (5–7). Cytoplasmic β-catenin is constantly degraded by a destruction complex consisting of axin, adenomatous polyposis coli, glycogen synthase kinase 3β (GSK3β), and casein kinase 1 (CK1) (5). Activated GSK3β phosphorylates β-catenin triggering its ubiquitin/proteosome-mediated degradation. Inhibition of GSK3β by either serine-threonine kinase Akt or Wnt signaling stabilizes β-catenin, promoting its nuclear translocation and dimerization with TCF/LEF to induce the transcription of Wnt target genes (5). Interestingly, Snail, the E-cadherin repressor, can be regulated by GSK3β-mediated ubiquitination similar to β-catenin (8). These findings put GSK3β as a focal point in the EMT signaling pathway due to its ability to regulate both Snail and β-catenin.
Numerous endocrine factors have been shown to influence the development and growth of breast cancer, including steroid hormones, growth factors (9, 10), and melatonin (MLT) (11), an indoleamine hormone synthesized and secreted by the pineal gland in the dark phase of the light-dark cycle (12). Pineal production of MLT is suppressed by light, thus the blood levels of MLT are low during the day and high during the night (12). The nocturnal surge of MLT acts as a biological timing signal driven by a central pacemaker residing in the suprachiasmatic nuclei (SCN) (13, 14) and is acutely suppressed by exposure to light during the night (12, 13). At physiological concentrations, MLT appears to be involved in the regulation of a wide range of biological and pathological processes (12, 14). It has been well established that MLT exerts an antiproliferative effect in human breast cancer cells in vitro (15) and inhibits the growth of mammary tumors in animal models (11, 16). The antiproliferative effect of MLT appears to be mediated primarily through the MLT receptor 1A (MT1), a G protein-coupled receptor (17). Recently, we (18), and others (19), have demonstrated that MLT also has antiinvasive properties in human breast cancer cells. The antiinvasive action of MLT is mediated, partially, through inhibition of p38 MAPK and its downstream regulation of matrix metalloproteinase (MMP)2 and MMP9 (18). In addition, MLT's effect on breast cancer cell invasion/metastasis appears to involve its regulation of E-cadherin and β-integrin (19).
To determine whether MLT regulates EMT to affect breast cancer cell invasion, we examined its actions on GSK3β and EMT. Using a tissue-isolated human breast xenograft tumor nude rat model (20), we demonstrated that MLT activates GSK3β by inhibiting Akt phosphorylation and activity, blocking Akt-mediated phosphorylation and inactivation of GSK3β. Up-regulation of GSK3β signaling by MLT leads to reduced levels of active β-catenin, and repression of β-catenin-mediated transactivation, which subsequently restores E-cadherin expression and represses vimentin expression in human breast cancer cells with an EMT phenotype. Collectively, these data suggest that MLT plays an important role in the regulation of EMT in breast cancer. Moreover, using the same tissue-isolated human breast xenograft tumor nude rat model, we demonstrated that the inactive form (phosphorylated at Ser9) of GSK3β exhibits a circadian rhythm in human breast tumors. Exposure to light-at-night, which suppresses the nocturnal MLT synthesis, disrupts the circadian rhythm of GSK3β phosphorylation. These findings have provided a potential mechanism linking circadian disruption with cancer.
Materials and Methods
Chemicals and reagents
All chemicals and tissue culture reagents were purchased from Sigma-Aldrich (St. Louis, MO). Cell culture medium, DMEM, RPMI 1640, and fetal bovine serum (FBS) were purchased from GIBCO (now part of Invitrogen Corp., Carlsbad, CA). The FuGENE HD transfection reagent was purchased from Roche (Indianapolis, IN). Human recombinant IGF-I and Wnt3a were purchased from PeproTech, Inc. (Rocky Hill, NJ). Human recombinant TGF-β was purchased from R&D Systems (Minneapolis, MN).
Cell lines and cell culture
Three human breast cancer cell lines were used in these studies. The parental MCF-7 human breast cancer cell line was obtained from the laboratory of the late William L. McGurie (San Antonio, TX). The MCF-7/steroid receptor-negative (SR−) human breast cancer cell line was established from SR− tumors that evolved over several passages from a subset of MCF-7 xenografts that had become estrogen unresponsive (20). MCF-7/constitutively active Src (caSrc) cell line (stably transfected with caSrc kinase) was kindly provided by Bin Shan (Tulane University). All cell lines, except MCF-7/caSrc cells, were cultured in RPMI 1640 medium supplemented with 10% FBS (GIBCO), 50 mm minimum essential medium nonessential amino acids, 1 mm sodium pyruvate, 2 mm glutamine, 10 mm basal medium eagle, 100 mg/ml streptomycin, and 100 U/ml penicillin. The MCF-7/caSrc cells were cultured in DMEM with the same supplements as described above. These cell lines were routinely maintained at 37 C in a humidified atmosphere of 5% CO2 and 95% air.
Protein extraction and Western blot analysis
Cells were harvested and then lysed in a protein extraction buffer containing Tris [50 mm (pH 7.4)], EDTA (20 mm), Nonidet P-40 (0.5%), NaCl (150 mm), phenylmethylsulfonyl fluoride (0.3 mm), NaF (1 mm), Na3VO4 (1 mm), dithiothreitol (1 mm), aprotinin (1 μg/ml), leupeptin (1 μg/ml), and pepstatin (1 μg/ml). The cell lysates were centrifuged for 10 min at 10,000 × g at 4 C. Protein concentrations of the supernatants were determined using a protein assay kit (Bio-Rad Laboratories, Inc. Hercules, CA). Total protein (80 μg per sample) was electrophoretically separated on a 10% SDS-PAGE and electroblotted onto a Hybond membrane. After incubation with 5% nonfat milk in Tris-buffered saline containing 0.1% Tween 20, the immunoblots were probed with antibodies to E-cadherin, phospho-Akt (Ser473), phospho-Akt (Thr308), phospho-GSK3β (Ser9), phospho-Erk1/2 (Thr202/Tyr204), phospho-p38 (Thr180/Tyr182), Snail, β-catenin (Cell Signaling Technology, Inc., Danvers, MA), phospho-GSK3β (Tyr216), active β-catenin (Millipore Corp., Billerica, MA), or vimentin (Becton Dickinson Biosciences, Franklin Lakes, NJ). The same blots were stripped and reprobed with antibodies to Akt, GSK3β, Erk1/2, p38 (Cell Signaling Technology, Inc.), tubulin, or glyceraldehyde-3-phosphate dehydrogenase (Millipore Corp.), respectively.
Tumor lysate extraction
Frozen tumors were pulverized and manually homogenized in 50 mm HEPES (pH 7.5), 150 mm NaCl, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate, 0.1% sodium deoxycholate, 1 mm Na3VO4, 100 nm okadaic acid, and 1× protease inhibitor cocktail Set I (Calbiochem/EMD Biosciences, Billerica, MA). Tumor lysates were centrifuged at 15,300 × g for 20 min. Supernatants were aliquoted and stored at −80 C.
Immunofluorescence staining
MCF-7 cells were seeded in four-well chamber slides at a density of 4 × 104 cells/well and transiently transfected with the pcDNA3.1-MT1 MLT receptor DNA construct. Sixteen hours after transfection, cells were subjected to serum starvation for 24 h. Cells were then pretreated with MLT (10−8 m) for 30 min before incubation with MLT and TGF-β (10 ng/ml) for an additional 48 h. After incubation cells were fixed with 4% paraformaldehyde in PBS for 10 min at room temperature, permeabilized with 0.3% Triton X-100 in PBS/5% normal goat serum for 1 h, and incubated overnight with anti-E-cadherin antibody (Cell Signaling Technology, Inc.). The secondary antibody used was Alexa Fluor 488 goat antirabbit secondary antibody (Molecular Probes, Grand Island, NY).
Fluorescence images were acquired using a Nikon Eclipse 80i microscope and a SensiCam QE camera (Cooke Corp., Romulus, MI) and IPLab version 3.65a software (Scanalytics, Rockville, MD). Exposure conditions were optimized for the brightest field of the specified conditions and held constant for subsequent exposures.
Transient transfection
MCF-7 cells were seeded in six-well plates at a density of 3 × 106 cells per well in RPMI 1640 supplemented with 10% FBS. After 24 h of serum starvation, cells were then transfected with 0.7 μg of super-pTOPFLASH (8 × TCF/LEF binding sites) or pFOPFLASH (8 × mutant TCF/LEF binding sites) luciferase reporter construct (provided by Gill Morris at Tulane University; originally from Randall Moon at University of Washington, Seattle, WA) and 1.0 μg of pcDNA3-β-catenin, pcDNA3.1-MT1, or empty vector, using the FuGENE HD transfection reagent according to the manufacture's instructions.
Luciferase reporter assays
TOP/FOPFLASH luciferase activities present in cellular lysates were assayed using the Luciferase Assay System (Promega, Madison, WI) according to the manufacture's instructions and were normalized with β-galactosidase activities.
Wound healing assays
The migratory property of MCF-7/SR− cells was examined using the CytoSelect Wound Healing Assay kit (Cell Biolabs, San Diego, CA) according to the manufacture's instructions. Briefly, cell suspension was added to the well with a plastic insert in place. The insert was removed from the well after a monolayer of cells formed, creating a wound gap of 0.9 mm. Cells were then treated with MLT (10−8 m) or vehicle and incubated for another 48 h.
Transwell cell migration assay
MCF-7/SR− cells in log phase of growth were serum-starved for 24 h before seeding, detached by brief trypsinization, and resuspended in medium containing the appropriate treatment. Cells (4 × 105 cells/ml in 0.5 ml of medium supplemented with 0.5% BSA) were added in suspension to the upper chamber of the inserts (Becton Dickinson Biosciences), and medium (0.75 ml, supplemented with 10% FBS as chemoattractant) containing the same treatment was added to the bottom well. After incubation for 24 h, the nonmigrating cells were removed from the upper surface of the membrane, and cells migrated to the under surface of the membrane were stained with a Diff-Quick staining kit (Dade Behring, now part of Siemens Healthcare Diagnostics, Deerfield, IL) and counted microscopically at ×100 magnification. Five fields per membrane were randomly selected and counted in each group.
Collection of MLT-supplemented human donor whole blood and tissue-isolated tumor perfusions in situ
Three healthy premenopausal female volunteers (mean age, 22.6 ± 0.6 yr) were recruited at Tulane University, regardless of race and ethnic background. All subjects were on regular sleep patterns and were free of any medication (MLT supplements, birth control pills, and/or β-blockers). All subjects signed approved institutional review board consent forms. Antecubital venous blood was drawn from each female subject during the daytime both before and after an oral MLT supplement of 300 μg, which results in pharmacologic blood levels 1 h after administration. MCF-7/SR− xenografts were implanted in nude rats in a tissue-isolated manner as previously described (20). Sets (three tumors per perfusion) of tissue-isolated MCF-7/SR− human breast cancer xenografts were perfused in situ for 1 h as previously described (20) with whole blood collected from donor human subjects either before or after MLT supplement.
Circadian rhythm of GSK3β phosphorylation and Snail expression and disruption by light-at-night
Weanling female nude rats (Hsd:RH-Foxn1rnu) between 35 and 50 g were housed in light exposure chambers on a 12-h light, 12-h dark (12L:12D) cycle [lights on 0600–1800 h, i.e. Zeitgeber time (ZT)0–ZT12] as previously described (21). Animals were allowed to acclimate for 2 wk and then separated into two groups of 36 animals each. Group A (control) remained on a 12L:12D (total darkness) lighting regimen. Group B (light-at-night) were subjected to 12L:12L with a light intensity of 0.2 lux or 0.08 μW/cm2 present at night. Two weeks later, MCF-7/SR− human breast cancer xenografts were implanted into these animals in a tissue-isolated manner, as described previously (20). When tumors reached sufficient size for measurement, six tumors were collected at 4-h interval time points over a 24-h period (ZT2, ZT6, ZT10, ZT14, ZT18, and ZT22). Tumor lysates were assayed for phospho-GSK3β and Snail by Western blot analysis.
Nocturnal light exposure studies in human subjects and effects on Akt and GSK3β phosphorylation
At Thomas Jefferson University, healthy male volunteers (n = 3; mean age, 26.3 ± 4.0 yr) were recruited, regardless of race and ethnic background. All subjects were on regular sleep patterns and were free of any medication (MLT supplements and/or β-blockers). All subjects signed approved institutional review board consent forms. Antecubital venous blood sample was drawn from each subject during the daytime (1000–1400 h), nighttime (0200 h) after 2 h of complete darkness, and nighttime (0330 h) after 90 min of ocular, bright, white light exposure at 580 μW/cm2 (i.e. 2800 lux) at eye level reflected from the wall. Sets (two tumors per perfusion) of tissue-isolated PC3 human prostate cancer xenografts were perfused in situ for 1 h as previously described (20) with these donor blood samples.
Assay of blood hormone levels in rats and human subjects
Rat serum MLT levels were measured via RIA as previously described (20).
Statistical analysis
Data are represented as the mean ± sem. The statistical significance at 95% confidence level was determined by one-way ANOVA followed by a Student-Newman-Keuls post hoc test analysis using Statview software (SAS Institute, Inc., Cary, NC).
Results
MLT activates GSK3β by blocking Akt-repression of GSK3β activity
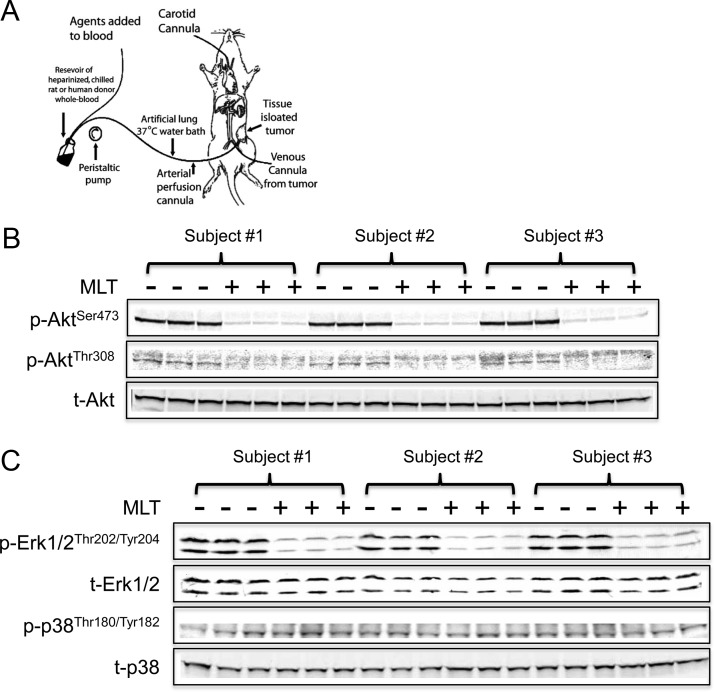
To determine whether MLT regulates EMT to affect breast cancer cell invasion, we examined its actions on GSK3β. The kinase activity of GSK3β requires phosphorylation at Tyr216 in the activation loop (22). Akt inhibits GSK3β activity by phosphorylation of GSK3β at Ser9 (23). Dauchy et al. (24) and others (25) reported that MLT can repress Akt activity in a variety of human malignancies. We employed an in vivo model to examine MLT's effect on Akt (26), in which tissue-isolated MCF-7/SR− human breast tumor xenografts were perfused in situ for 1 h with daytime (low MLT) human donor blood collected from female human subjects either before or after receiving an oral MLT supplement (Fig. 1A). Figure 1B shows that Akt phosphorylation at Ser473 was almost completely abolished in tumors perfused with the postsupplement MLT-rich human donor blood as compared with those perfused with the corresponding MLT-deficient presupplement daytime human blood. The Thr308-phosphorylation signal of Akt was very low but was also decreased in tumors perfused with the postsupplement blood (Fig. 1B). We also examined the effect of MLT on other signaling pathways, including Erk1/2 and p38 MAPK pathways. As shown in Fig. 1C, phosphorylation of Erk1/2, like that of Akt, was dramatically suppressed in tumors perfused with the postsupplement MLT-rich human blood as compared with the presupplement group. However, MLT supplementation did not have any significant effect on the phosphorylation of p38 MAPK, which has been previously shown to be down-regulated by MLT in human breast cancer cells (Fig. 1C) (18).
Fig. 1.
MLT inhibits Akt phosphorylation in human breast cancer cells. A, Schematic representation of tissue-isolated tumor perfusion model. MLT-rich postsupplement human female daytime blood suppressed Akt phosphorylation (B) and Erk1/2 phosphorylation (C) in tissue-isolated MCF-7/SR− human breast cancer xenografts. Daytime venous blood was drawn from three females both before (−) and after (+) an oral MLT supplementation of 300 μg, which results in pharmacologic blood levels 1 h after administration. Separate sets of three tissue-isolated MCF-7/SR− xenografts were perfused with pre- or postsupplement blood for 60 min. Western blot analyses examined the phosphorylation of Akt (B) and Erk1/2 and p38 (C). p-, Phospho; t-, total-.
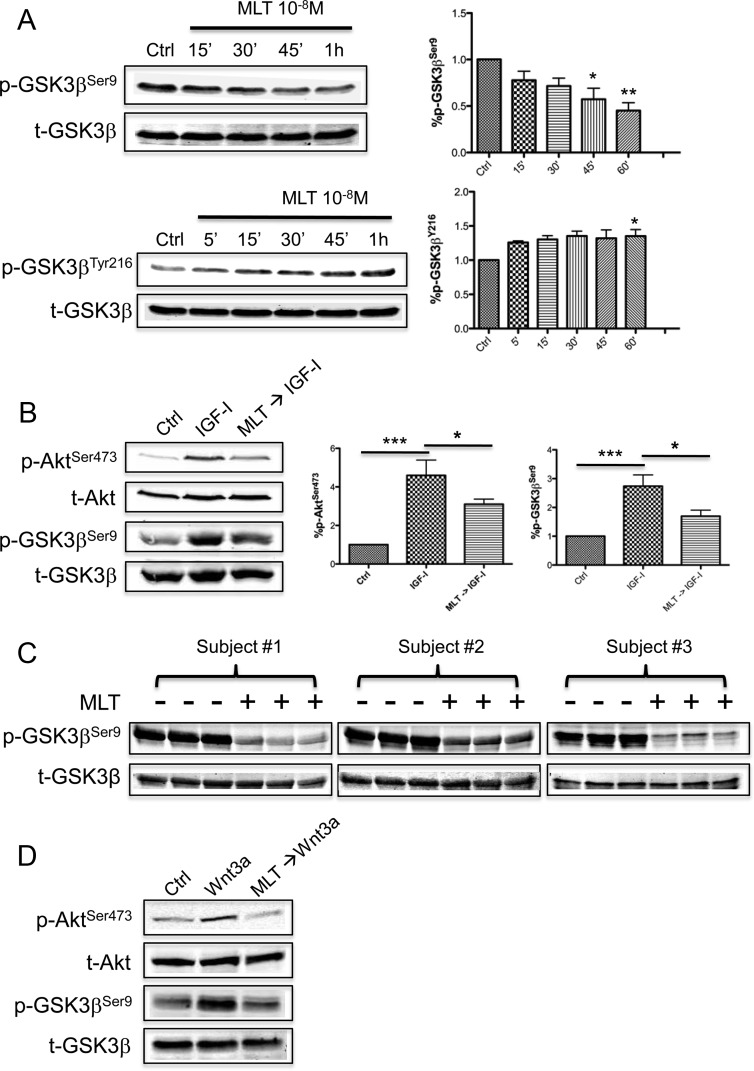
Because MLT inhibits Akt phosphorylation, follow-up studies examined whether MLT could block Akt's repressive phosphorylation of GSK3β. As shown in Fig. 2A, phosphorylation of GSK3β at Ser9 (inactivating phosphorylation site) was significantly repressed after MLT administration in parental MCF-7 (SR+) cells, whereas the Tyr216 phosphorylation (activating phosphorylation site) was enhanced. Figure 2B shows that IGF-I stimulated Akt phosphorylation in MCF-7 cells, inducing a concurrent increase in GSK3β phosphorylation (Ser9), and that a 30-min pretreatment with MLT blocked IGF-I-mediated Akt phosphorylation of GSK3β. Employing our in vivo tissue-isolated MCF-7/SR− human breast tumor xenograft model system, an even more pronounced suppression of GSK3β phosphorylation at Ser9 was observed in tumors perfused with the postsupplement MLT-rich human donor blood compared with tumors perfused with the MLT-deficient presupplement blood from the same female subject (Fig. 2C).
Fig. 2.
MLT activates GSK3β in human breast cancer cells. A, Regulation of GSK3β phosphorylation by MLT in MCF-7 human breast cancer cells. Cells were treated with MLT for the times indicated and Western blot analyses used to examine GSK3β phosphorylation at Ser9 and Tyr216. The band intensity of phospho-GSK3β was quantified and normalized to that of total-GSK3β. The data are expressed as percent phospho-GSK3β (ratio of phospho-GSK3β to total-GSK3β). n = 3; *, P < 0.05; **, P < 0.001 (ANOVA with Student-Newman-Keuls post hoc test). Data are mean ± sem. B, MLT (30-min pretreatment 10−8 m) blocked IGF-I (50 ng/ml)-induced GSK3β phosphorylation in MCF-7 cells. The band intensity of phospho-Akt and phospho-GSK3β was quantified and normalized to that of total-Akt and total-GSK3β, respectively. The data are expressed as percent phospho-Akt and phospho-GSK3β. n = 3; *, P < 0.05; ***, P < 0.0001 (ANOVA with Student-Newman-Keuls post hoc test). Data are mean ± sem. C, MLT-rich postsupplement human blood suppressed GSK3β phosphorylation at Ser9 in tissue-isolated MCF-7/SR− human breast cancer xenografts. D, MLT (30-min pretreatment 10−8 m) blocked the inhibitory effect of Wnt3a (200 ng/ml) signaling on GSK3β in MCF-7 cells. Ctrl, 0.00001% ethanol; p-, phospho-; t-, total-.
GSK3β activity has been reported to be repressed by the Wnt pathway allowing accumulation of cytoplasmic/nuclear β-catenin (5, 27). In MCF-7 cells, administration of recombinant human Wnt3a stimulated Akt phosphorylation at Ser473 and induced GSK3β phosphorylation at Ser9 (Fig. 2D). Pretreatment of MCF-7 cells with MLT abolished the actions of Wnt3a (Fig. 2D) on GSK3β phosphorylation, demonstrating that MLT can block both Akt- and Wnt-induced repression of GSK3β activity.
MLT activation of GSK3β leads to repression of β-catenin
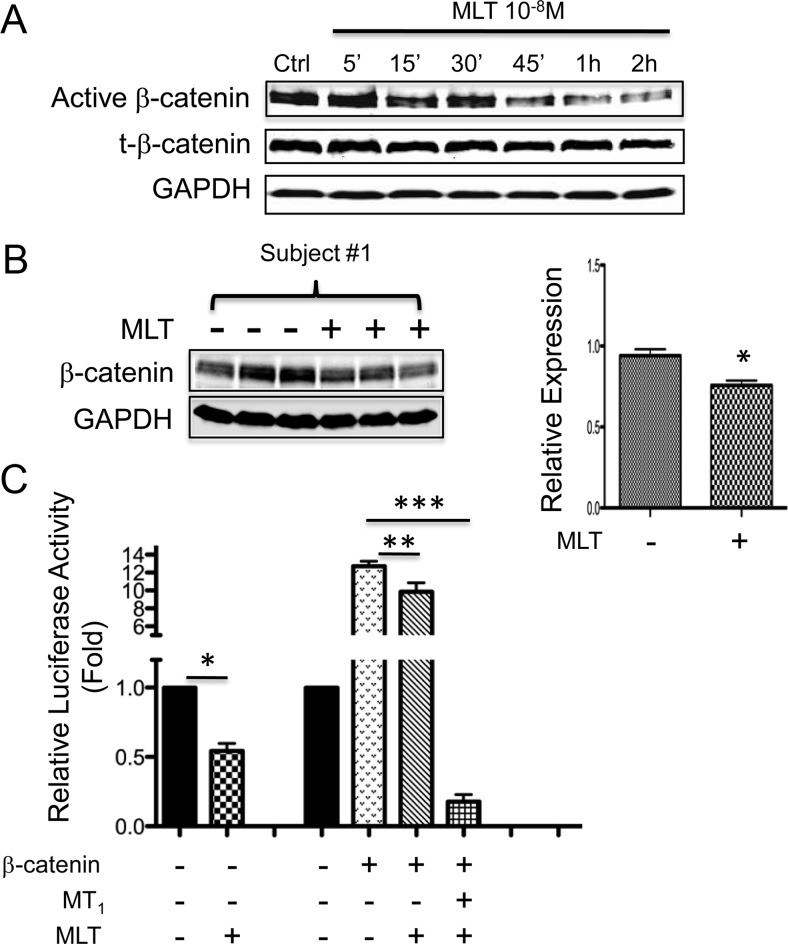
To address whether MLT activation of GSK3β further leads to down-regulation of β-catenin, we examined the effect of MLT on β-catenin expression and transcriptional activity. Figure 3A shows that MLT administration induced a rapid reduction in the levels of both active (nonphosphorylated) and total β-catenin in MCF-7 cells. Decreased levels of total β-catenin were also observed in vivo in tissue-isolated MCF-7/SR− tumor xenografts perfused with the postsupplement MLT-rich human donor blood, as compared with the corresponding presupplement group (Fig. 3B). Next, we examined the effect of MLT on the TCF/LEF-driven transcription using a TOPFLASH luciferase reporter assay. MLT administration significantly repressed basal transcriptional activity as well as the β-catenin-induced TOPFLASH activity in MCF-7 cells (Fig. 3C). The effect of MLT on TCF/LEF-driven transcription was substantially enhanced by overexpression of the MT1 receptor. As shown in Fig. 3C, β-catenin-induced TOPFLASH activity was almost completely abolished in cells transfected with the MT1 receptor and treated with MLT (Fig. 3C).
Fig. 3.
Activation of GSK3β by MLT represses β-catenin expression and activity. A, MLT repressed the expression of β-catenin. MCF-7 cells were treated with MLT for the times indicated and active (nonphosphorylated) and total (t-) β-catenin were measured by Western blot analysis. B, Western blot analysis shows MLT-rich postsupplement human blood decreased β-catenin protein levels in tissue-isolated MCF-7/SR− human breast cancer xenografts. Right panel, The band intensity of β-catenin was quantified and normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). n = 3; *, P = 0.0209 vs. control (two-tailed student's t test). Data shown are representative of the three sets of perfusions. C, MLT repressed β-catenin/TCF/LEF-driven transcription in luciferase reporter assay. MCF-7 cells were transiently transfected with a super-pTOPFLASH (8× TCF/LEF binding sites) or pFOPFLASH (8× mutant TCF/LEF binding sites) luciferase reporter construct, empty vector, or expression plasmids for β-catenin and MT1, and treated with MLT (10−8 m) or vehicle (0.00001% ethanol) for 48 h. Data were calculated as the ratio of TOP/FOP activity and expressed as fold of control. n = 3; *, P = 0.0182 vs. control (two-tailed student's t test); **, P < 0.01; ***, P < 0.0001 (ANOVA with Student-Newman-Keuls post hoc test). Data are mean ± sem. Ctrl, 0.00001% ethanol.
MLT blocks EMT in human breast cancer cells
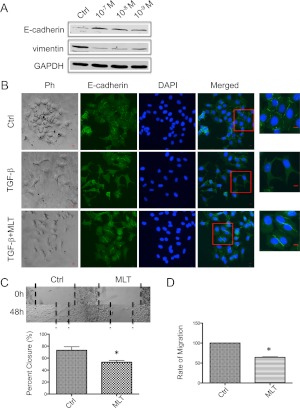
To determine whether MLT regulates EMT phenotypes, we first examined its effect on the expression of commonly used epithelial marker E-cadherin and mesenchymal marker vimentin. The effect of MLT administration on E-cadherin and vimentin expression was examined in MCF-7/caSrc cells that were stably transfected with the caSrc kinase and exhibit low E-cadherin and elevated vimentin expression (28). Protein expression levels of E-cadherin were significantly increased, and vimentin expression was almost completely abolished (Fig. 4A) after MLT administration (10−9 to 10−7 m) in MCF-7/caSrc cells.
Fig. 4.
MLT regulates EMT in breast cancer cells. A, MLT regulated protein expression of E-cadherin and vimentin in human breast cancer cells. MCF-7/caSrc cells were treated with MLT as denoted or vehicle (Ctrl, 0.00001% ethanol.) for 5 d. Western blot analyses examined E-cadherin and vimentin expression. B, Phase-contrast microscopy (Ph) and immunofluorescence staining of E-cadherin (green) in MCF-7 cells. Serum-starved MCF-7 cells were treated with TGF-β (10 ng/ml) for 48 h to induce EMT, with or without the presence of MLT (10−8 m). Cells are counterstained with DAPI to visualize nuclei (blue). Magnified images of boxed regions are shown on the right. Scale bar, 10 μm. Higher-magnification phase-contrast images are shown in Supplemental Fig. 1. Ctrl, 0.00001% ethanol. C, MLT suppressed MCF-7/SR− cell migration in wound healing assays. Wounds were generated as described in Materials and Methods. Cells were treated with MLT (10−8 m) or vehicle (Ctrl, 0.00001% ethanol.) for 48 h. Data are presented as % closure. Percent closure (%) = migrated cell surface area/total surface area × 100. *, P = 0.0407 vs. diluent-treated cells by two-tailed student's t test (n = 3). D, MLT inhibited the migration of MCF-7/SR− cells treated with MLT (10−8 m) or vehicle (Ctrl, 0.00001% ethanol.) for 24 h in transwell assays. Data are presented as % of control (100%). *, P = 0.00167 vs. diluent-treated cells by two-tailed student's t test (n = 3). Data are mean ± sem. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase; DAPI, 4′,6-diamidino-2-phenylindole.
The effect of MLT on cell morphology and subcellular localization of E-cadherin was also examined by immunofluorescence staining. TGF-β was used to induce EMT in MCF-7 cells. Incubation of MCF-7 cells with TGF-β (10 ng/ml) for 48 h induced a fibroblast-like morphology with loss of cellular contact and a reduction/loss of epithelial staining of E-cadherin. In contrast, cells treated with TGF-β in presence of MLT (10−8 m) exhibited higher levels of epithelial staining of E-cadherin with less-fibroblastic morphology, as compared with cells treated with TGF-β alone (Fig. 4B and Supplemental Methods and Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). The ability of MLT to regulate EMT phenotype was further confirmed by wound healing assay and transwell migration assay. Administration of MLT significantly reduced the migration rate of MCF-7/SR− cells (Fig. 4, C and D).
GSK3β phosphorylation at Ser9 is circadian-regulated and disrupted by light-at-night
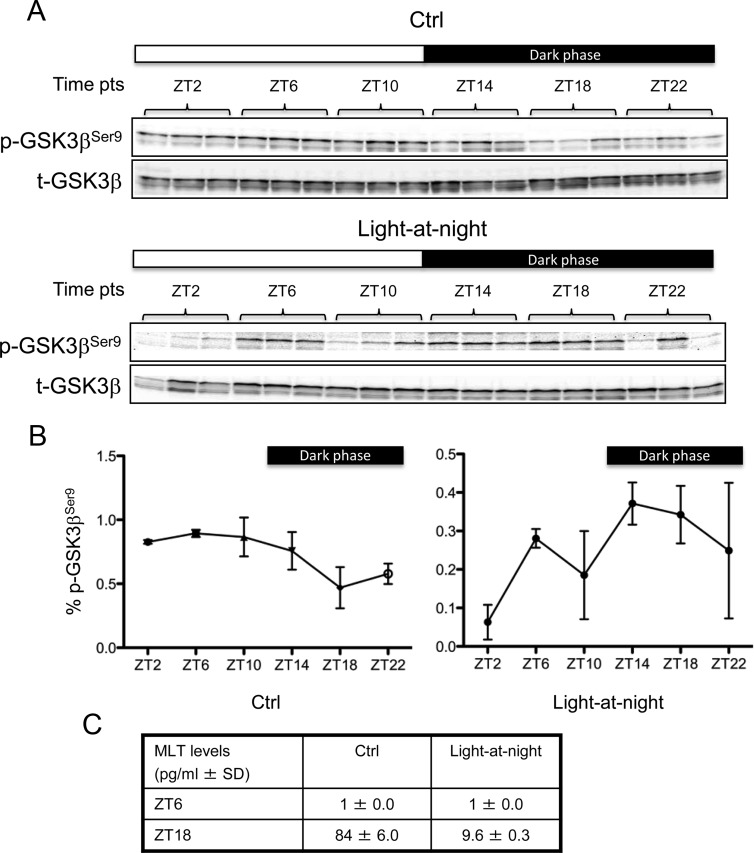
Approximately 15–20% of all mammalian genes are under circadian control, including a number of cancer associated genes, and the expression of a number of these genes is modified by light-at-night (29). Given that GSK3β phosphorylation is regulated by MLT, an output of the SCN (central pace maker), we further investigated whether there are circadian-dependent changes in the expression/phosphorylation of GSK3β. Breast cancer cells grown in culture lack central circadian regulation; thus, we employed our tissue-isolated tumor xenograft model system to examine whether GSK3β expression/phosphorylation is circadian-regulated and whether this circadian regulation can be disrupted by the host's exposure to light-at-night. Tissue-isolated MCF-7/SR− tumor xenografts in nude rats housed under a control 12L:12D cycle or exposed to light-at-night (12L:12L, with a light intensity of 0.20 lux present at night) were sampled at 4-h intervals over a 24-h period starting at ZT2 (i.e. 2 h after lights on). In control tumors, GSK3β phosphorylation at Ser9 exhibited a circadian rhythm with peak levels at midlight phase (ZT6) when MLT levels are low (1 ± 0.0 pg/ml) falling gradually to its nadir around middark phase (ZT18) when MLT levels are highest (84 ± 6.0 pg/ml) (Fig. 5, A–C). In contrast, circadian disruption by exposure to light-at-night almost completely abolished the nocturnal MLT surge (9.6 ± 0.3 pg/ml at ZT18) (Fig. 5C) and dysregulated the circadian rhythm of GSK3β phosphorylation (Fig. 5, A and B), resulting in dark phase levels of Ser9-phosphorylated GSK3β equivalent to the daytime high levels.
Fig. 5.
Circadian rhythm of GSK3β phosphorylation in human breast cancer and disruption by light-at-night. A, Tissue-isolated MCF-7/SR− tumors were implanted in nude rats exposed to either control 12L:12D cycle (Ctrl) (upper panels) or light-at-night (12L:12L with a light intensity of 0.20 lux present at night, lower panels). Tumors were harvested at 4-h intervals over a 24-h period starting at ZT2. Levels of phospho-GSK3β (Ser9) and total-GSK3β were determined by Western blot analysis. The experiment was done in triplicate. B, The band intensity of phospho-GSK3β (Ser9) was quantified and normalized to that of total-GSK3β. The data are expressed as percent phospho-GSK3β (ratio of phospho-GSK3β to total-GSK3β). Each data point represents the average of three tumors. Error bars represent sd. C, Serum levels of MLT in the rat blood at ZT6 and ZT18 were measured by RIA assays. p-, Phospho-; t-, total-.
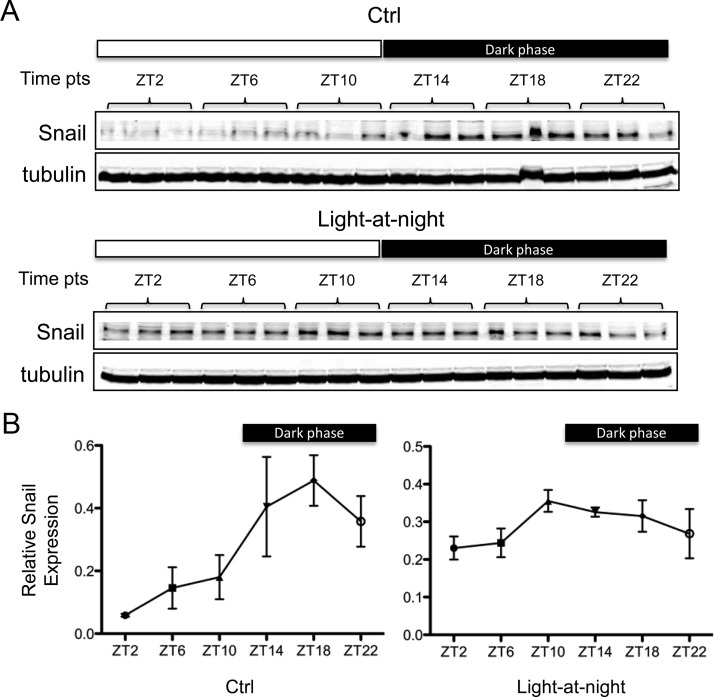
We also examined the expression of the β-catenin-driven, EMT-associated gene Snail as a readout of circadian regulation of GSK3β in these tissue-isolated tumor xenografts. As shown in Fig. 6, expression of Snail protein also exhibited a very pronounced circadian rhythm with peak levels detected during the dark phase and lower levels during the daytime. Furthermore, Snail expression was constitutively elevated in response to the host's exposure to light-at-night.
Fig. 6.
Circadian rhythmic expression of Snail protein in human breast cancer. A, Expression levels of Snail protein in tumors described in Fig. 5 were determined by Western blot analysis. The experiment was done in triplicate. B, The band intensity of Snail was quantified and normalized to that of tubulin. Each data point represents the average of three tumors. Error bars represent sd. Ctrl, 12L:12D; Light-at-night, 12L:12L with a light intensity of 0.20 lux present at night.
Exposure to light-at-night in human male subjects affects Akt and GSK3β phosphorylation in human prostate tumors
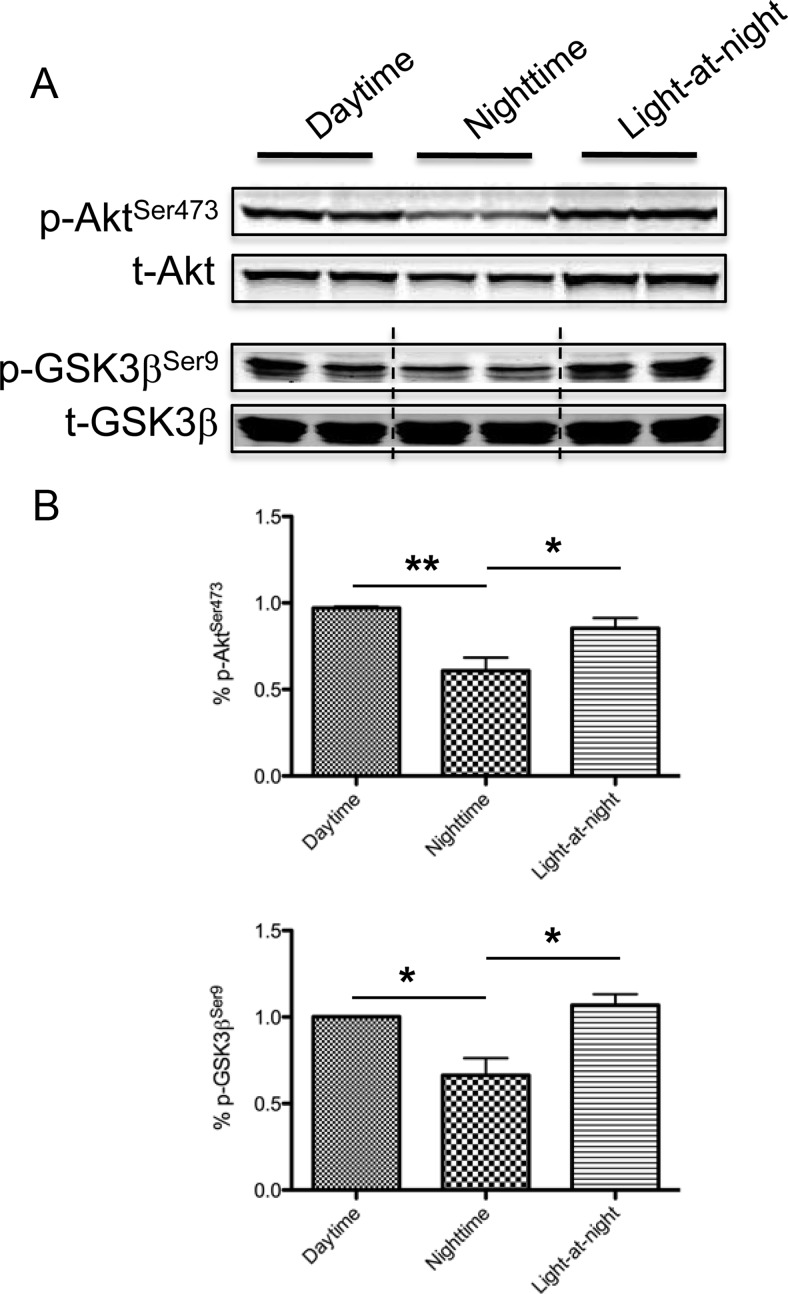
To address whether light-at-night-induced disruption of MLT's circadian rhythm is of biological consequence, we collected human donor blood from male human subjects during the daytime, when MLT levels are low, the nighttime, when MLT levels are high, and the nighttime after 90-min ocular exposure to bright, white light (light-at-night). Tissue-isolated PC3 human prostate cancer xenografts were perfused with blood drawn under these different conditions (daytime, nighttime, and light-at-night), and tumor lysates were assayed for phosphorylation of Akt and GSK3β. As shown in Fig. 7, tumors perfused with blood drawn during the nighttime exhibited significantly reduced levels of phospho-Akt and GSK3β at its Ser9 inhibitory site as compared with those perfused with daytime blood. The inhibitory effect of the nighttime blood on Akt and GSK3β phosphorylation was reversed in tumors perfused with nighttime blood drawn from male subjects exposed to light-at-night.
Fig. 7.
Exposure to light-at-night in human male subjects induces disregulation of Akt and GSK3β phosphorylation in human prostate cancer. A, Separate sets of two tissue-isolated PC3 human prostate cancer xenografts were perfused with human venous blood drawn from male human subjects during the daytime, nighttime, and nighttime after 90 min of ocular, bright, white light exposure at 580 μW/cm2 (i.e. 2800 lux) (light-at-night). Western blot analyses examined the phosphorylation of Akt and GSK3β. B, The band intensity of phospho-Akt and phospho-GSK3β was quantified and normalized to that of total-Akt and total-GSK3β, respectively. The data are expressed as percent phospho-Akt and GSK3β. Each data point represents the average of two tumors. n = 3; *, P < 0.05; **, P < 0.01 (ANOVA with Student-Newman-Keuls post hoc test). Error bars represent sem. p-, Phospho-; t-, total-.
Discussion
GSK3β is a ubiquitously expressed protein kinase that acts as a key regulator in the signaling networks that govern EMT (5). Unlike most other protein kinases, GSK3β is constitutively active, and its activity is inhibited upon cellular responses to stimuli, such as growth factors (5). The best-characterized regulators of GSK3β are Akt and the Wnt signaling pathway. Akt inhibits GSK3β activity by phosphorylating Ser9 of GSK3β (23). Using a tissue-isolated tumor perfusion model, we have demonstrated that MLT inhibits Akt activity in human breast cancer cells, as judged from its phosphorylation at Ser473 and Thr308. Both Ser473-phosphorylation and Thr308-phosphorylation of Akt were significantly suppressed in MCF-7/SR− xenografts perfused with blood from women receiving MLT supplementation (Fig. 1), resulting in blockade of GSK3β phosphorylation at Ser9 (Fig. 2C). These in vivo results were further confirmed by in vitro studies using parental SR+ MCF-7 cells, in which MLT induces a gradual decrease in the Ser9-phosphorylation of GSK3β (Fig. 2A). In addition, we observed a modest increase in GSK3β phosphorylation at Tyr216 (the activating phosphorylation) in response to MLT administration in MCF-7 cells (Fig. 2A). Collectively, these results demonstrate that MLT suppresses Akt phosphorylation/activity and up-regulates GSK3β activity and signaling by inhibiting its Ser9-phosphorylation while stimulating the Tyr216 phosphorylation.
To test whether MLT regulates other signaling pathways, we also examined the effect of MLT on the phosphorylation of Erk1/2 and p38 MAPK using the tissue-isolated tumor perfusion model. Levels of phospho-Erk1/2 were significantly suppressed by the postsupplement MLT-rich human blood (Fig. 1C). In contrast, p38 phosphorylation was not affected by MLT supplementation, indicating that in vivo MLT specifically regulates phosphorylation of Akt, GSK3β and Erk1/2 in the tissue-isolated MCF-7/SR− xenografts in nude rats. Previously, we have reported that MLT suppresses p38 phosphorylation in human breast cancer cells in vitro (18). The discrepancy between the current study on p38 and our previous report may be caused in part by cell line variance (MCF-7/SR− vs. MCF-7/6 cells) and that MLT's action on p38 is cell-context dependent. Furthermore, tumor cells in a circadian-regulated environment (in a circadian complete host) are differentially modulated by MLT compared with noncircadian-regulated cells in long-term culture (30).
In line with the in vitro and in vivo results on MLT regulation of Akt and GSK3β, MLT administration was able to block IGF-I-induced Akt phosphorylation in MCF-7 cells and prevent the concurrent stimulation of GSK3β phosphorylation at Ser9 (Fig. 2B). Thus, MLT inhibits Akt phosphorylation and attenuates Akt's repressive action on GSK3β, allowing GSK3β activation. Interestingly, the Wnt signaling pathway has been shown to cross talk with the Akt pathway to regulate GSK3β (27, 31). In our study, we observed an induction of Akt phosphorylation and concomitant increase in Ser9-phosphorylation of GSK3β in MCF-7 cells upon Wnt3a stimulation, which is indicative of a cross talk between the Wnt and Akt signaling pathways (Fig. 2D). The effects of Wnt3a on Akt and GSK3β were blocked by MLT administration (Fig. 2D), demonstrating that MLT can block both Akt- and Wnt-induced phosphorylation/repression of GSK3β activity (Fig. 8). Further studies are needed to determine whether MLT can modulate the canonical Wnt signaling involving activation of the Frizzled receptors to control GSK3β localization and thus accessibility to its substrates (5).
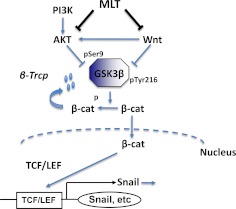
Fig. 8.
Schematic pathway of MLT's action on GSK3β and EMT. PI3K, Phosphatidylinositol 3 kinase; β-cat, β-catenin; p, Phosphate group.
Recently, a new mechanism of GSK3β regulation was proposed. The p38 MAPK was shown to inactivate GSK3β by inducing an inhibitory phosphorylation at Ser389 (32). We ruled out the possibility of implication of p38 in MLT regulation of GSK3β, at least in MCF-7/SR− cells, because MLT had no effect on p38 phosphorylation as examined in the circadian-complete tissue-isolated MCF-7/SR− xenograft nude rat perfusion model (Fig. 1C). However, p38 may be involved in MLT's regulation of GSK3β in other cell lines.
We further demonstrated that MLT activation of GSK3β leads to reduced intracellular levels of β-catenin, as well as repression of the β-catenin/TCF/LEF-mediated transcription (Fig. 3). MLT was able to repress not only the basal TOPFLASH activity but also the TOPFLASH activity induced by β-catenin overexpression. The greatest effect of MLT on inhibiting TOPFLASH transcription was observed with the elevated expression of the MT1 MLT receptor (Fig. 3C), suggesting that MT1 receptor is important in driving this process. The MT1 is a G protein-coupled receptor that has been shown to couple to various G proteins (Gαi2, Gαi3, Gαq, and Gα11) (33). Further studies are needed to determine the specific Gα protein(s) to which MT1 couples to regulate β-catenin. The involvement of MT2 receptor was not examined, because we have previously shown that MT2 is not expressed in MCF-7 breast cancer cell line (34).
The ability of MLT to regulate Akt, GSK3β, and β-catenin led to our hypothesis that MLT plays an important role in the regulation of EMT. Thus, we further examined whether MLT alters EMT phenotypes. Key phenotypic changes in cells undergoing EMT include decreased expression of E-cadherin and increased expression of vimentin (2, 4). Because parental MCF-7 cells express very low levels of vimentin protein, we used a MCF-7/caSrc clone (27) that exhibits EMT-like features, including low levels of E-cadherin and elevated levels of vimentin. MLT administration restored the expression of E-cadherin and suppressed the expression of vimentin in these cells (Fig. 4A), which is indicative of an inhibitory effect on EMT. The effect of MLT on E-cadherin expression is mediated, at least partially, at the transcriptional level, because MLT increased the mRNA levels of E-cadherin as measured by real-time RT-PCR analyses (Supplemental Methods and Supplemental Fig. 2). Thus, MLT, via blockade of Akt-mediated repression of GSK3β and decreased β-catenin levels/activity, regulates the expression of the EMT markers E-cadherin and vimentin. These results are consistent with the previous report by Cos et al. (19), who showed that MCF-7 cells incubated with MLT (10−9 m) exhibited elevated levels of E-cadherin as determined by immunohistochemistry.
The ability of MLT administration to alter subcellular localization of E-cadherin (Fig. 4B) and significantly reduce the migration rate of MCF-7/SR− cells (Fig. 4, C and D), the definitive phenotypic change associated with EMT, confirms that MLT, via the regulation of GSK3β, represses EMT and inhibits the migratory behavior of breast cancer cells. It is important to note that although the maximal effects of MLT on the protein expression of EMT markers (E-cadherin and vimentin) were observed after 5 d of MLT administration (Fig. 4A), we started to see changes in the mRNA levels of E-cadherin as early as 3 h (data not shown). The phenotypic changes (E-cadherin localization and cell migration) were observed within 48 h of treatment (Fig. 4, B–D). Previous studies have demonstrated that MLT acts through multiple mechanisms to inhibit breast cancer cell invasion/metastasis, which include reinforcement of cell adhesion via increasing the expression of E-cadherin and β-integrin (19), and suppression of the degradation of extracellular matrix via inhibiting p38 MAPK and its downstream targets MMP2 and MMP9 (18). The regulation of GSK3β and EMT represents an additional mechanism underlying the antiinvasive/metastatic action of MLT.
MLT is a well-established output of the SCN, the central pace maker, whose production by the pineal gland is under circadian control (12–14); thus, it follows that phosphorylation/activity of Akt and GSK3β, being regulated by MLT, should exhibit circadian-dependent daily variations. Indeed, our collaborators have observed a circadian control of the phosphorylated Akt (Ser473) in the tissue-isolated MCF-7/SR− xenografts, which peaked at midlight phase (ZT6), and were low during the dark phase (21). Here, using the same MCF-7/SR− xenografts, we demonstrated that the phosphorylation of GSK3β (Ser9) also displays a circadian rhythm, with a peak at midlight phase (ZT6) and a nadir at middark phase (ZT18) (Fig. 5, A and B). The peak levels of GSK3β phosphorylation (Ser9) were detected at the same time as Akt phosphorylation (21). It is important to note that based on the circadian rhythm of its phosphorylation at the Ser9 inhibitory site, GSK3β activity is suppressed during the light phase (ZT2–10) and up-regulated during the dark phase (ZT14–ZT22) in presence of high circulating MLT levels (Fig. 5C). In contrast, circadian disruption by exposure of the host animal to light-at-night almost completely abolished the nocturnal MLT surge (9.6 ± 0.3 vs. 84 ± 6.0 pg/ml in 12L:12D at ZT18) (Fig. 5C) and dysregulated the circadian rhythm of GSK3β phosphorylation (Fig. 5, A and B), resulting in dark phase levels of Ser9- phosphorylated GSK3β elevated to the daytime high levels. The ability of blood from women receiving MLT supplementation to suppress the daytime levels of Akt phosphorylation (Fig. 1B) clearly demonstrates its role in regulating GSK3β phosphorylation and activity. Because dim light-at-night appears to specifically disrupt MLT rhythm (20), these data suggest that the circadian regulation of GSK3β phosphorylation is mediated through MLT.
Next, we examined whether the circadian rhythmic activity of GSK3β induced circadian regulation in the levels of its target gene Snail. Snail is a direct substrate of the kinase activity of GSK3β (8). Meanwhile, it is also an indirect target of GSK3β via the β-catenin-mediated transcriptional regulation (6). The protein levels of Snail exhibit a very pronounced circadian rhythm in MCF-7/SR− xenografts (Fig. 6). Interestingly, the peak levels of Snail protein were detected at the middark phase (ZT18) when GSK3β is most active, which then began to fall at the late-dark phase (ZT22) and reached its nadir at the early-light phase (ZT2). The 8-h lag period observed between the start of the up-shift of GSK3β activity (ZT14) and the start of down-shift of Snail levels (ZT22), as well as that observed between the peak of GSK3β activity (ZT18) and the nadir of Snail levels (ZT2), may reflect a delayed response due to the GSK3β/β-catenin-mediated transcriptional/translational processing. This may seem paradoxical as the Snail protein has a short half-life controlled by GSK3β-directed ubiquitination/degradation (8). A potential explanation for this paradox is that GSK3β is a unique kinase whose phosphorylation of substrates requires a priming phosphorylation by another kinase (5). In the case of Snail, it has been reported that a “priming” phosphorylation by CK1 is essential for the subsequent GSK3β phosphorylation (35). Thus, the rhythm of Snail protein expression driven by the posttranslational control mechanism alone may potentially be determined by three factors: rhythm of GSK3β activity, rhythm of CK1 activity, and the availability/proportion of the primed pool of Snail protein at a given time. Given the complex nature of the control mechanism underlying Snail expression, it is plausible that the pattern of Snail's circadian rhythm may not necessarily follow that of GSK3β. Further support of a circadian regulation of Snail is seen as exposure of the host animal to light-at-night almost completely abolishes the circadian rhythm of Snail protein expression (Fig. 6).
Breast cancer incidence is five times higher in industrialized nations characterized by excessive usage of artificial light during the night vs. underdeveloped countries (36), and it has been hypothesized that exposure to light-at-night via its suppression of nocturnal MLT production (13) may represent a unique risk factor for breast cancer in industrialized countries (37). Recently, night shift work, and ostensibly exposure to light-at-night, has been designated by the World Health Organization as a probable (group 2A) carcinogen (38). A major unanswered question is how circadian disruption influences cell proliferation in tumors, as well as other aspects of tumor progression, including invasion/metastasis. We have previously reported that exposure to light-at-night induces suppression of nocturnal MLT, stimulating breast tumor fatty acid uptake and metabolism and activating the insulin-like growth factor-I receptor/phosphoinositide-dependent protein kinase 1 signaling pathway, promoting breast tumor growth in vivo (20, 21). In the present studies, we further demonstrated that exposure of human subjects to light-at-night disrupts the normal regulation of the Akt/GSK3β axis by MLT in human PC3 prostate xenograft tumors (Fig. 7). Altogether, these results indicated that light-at-night-induced disruption of the MLT circadian rhythm has significant and far-reaching biological consequences. In these studies, we also present our data from PC3 human prostate cancer xenografts, because we have observed similar inhibitory effects of MLT on the phosphorylation of Akt and GSK3β and other kinases in prostate cancer cells as well as in breast cancer cells (data not shown). Our data in PC3 human prostate cancer xenografts supports our hypothesis that light-at-night-induced disruption of MLT circadian rhythm has a major impact on the normal regulation of Akt and GSK3β by MLT and that MLT regulation of Akt and GSK3β occurs in multiple cancers, ruling out the possibility of MLT's actions being cell line or tumor type specific. Moreover, the data generated in the PC3 xenograft model demonstrate that MLT's regulation of Akt and GSK3β may impact the invasive capacity of a number of malignancies, including breast and prostate cancer.
In summary, our findings demonstrate the existence of a circadian rhythm in GSK3β phosphorylation/activity in human breast cancer orchestrated by the central circadian pacemaker via its output, MLT. It has been previously reported that the GSK3β activity displays a 24-h cycle in the SCN and liver (39). Disruption of the GSK3β circadian rhythm has the potential to cause major perturbations in the regulation of breast cancer EMT and metastasis via deregulation of β-catenin target genes, such as Snail and the Snail-related zinc-finger transcription factor, Slug. Given that many GSK3β substrates, including c-Myc, Cyclin D1, β-catenin, and the recently identified coactivator steroid receptor coactivator-3 (5, 40–42), function as protooncogenes, it becomes clearer that exposure to light-at-night, via its suppression of MLT and disruption of GSK3β's rhythm of phosphorylation/activity, contributes to the increased breast cancer risk observed in rotating night shift workers (43). Furthermore, given that MLT's actions on GSK3β and E-cadherin mRNA expression happen within hours, the repression of nighttime MLT by light-at-night, and the subsequent up-regulation of Akt activity and down-regulation of GSK3β activity, may potentially drive EMT of breast cancer cells and increase the metastatic spread of breast cancer among high-risk individuals, including breast cancer patients occupationally exposed to light-at-night and elderly breast cancer patients who display sleep disruption that involves light-at-night-induced MLT suppression.
The primary goal of this study is to understand the molecular basis for the association between circadian disruption and breast cancer. We believe that these data support the association between light-at-night-induced circadian disruption and the increased breast cancer risk: light-at-night disrupts the circadian rhythm of GSK3β via perturbation of the nocturnal surge of MLT, the biological timing signal. Our findings also link the circadian clock, which imposes a temporal regulation of many physiological functions, to the GSK3β pathway, which is a key gatekeeper of EMT.
Supplementary Material
Acknowledgments
We thank Dr. Joan Brugge for generously providing the ca-Src construct and Dr. Gilbert F. Morris and Dr. Randall Moon for kindly providing the TOPFLASH and FOPFLASH constructs as gifts.
This work was supported by the National Institutes of Health Grant R01 CA-054152–14 and an Army Department of Defense grant (to S.M.H.), an American Association for Laboratory Animal Science GLAS grant (R.T.D. and D.E.B.), and the Institute for Integrative Health (Baltimore, MD) (G.C.B.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- caSrc
- Constitutively active Src
- CK1
- casein kinase 1
- EMT
- epithelial-to-mesenchymal transition
- FBS
- fetal bovine serum
- GSK3β
- glycogen synthase kinase 3β
- 12L:12D
- 12 h light, 12 h dark
- MLT
- melatonin
- MMP
- matrix metalloproteinase
- MT1
- melatonin receptor 1A
- SCN
- suprachiasmatic nucleus
- SR−
- steroid receptor-negative
- TCF/LEF
- T-cell factor/lymphocyte enhancer factor
- Wnt
- Wingless
- ZT
- Zeitgeber time.
References
- 1. Hay ED. 2005. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn 233:706–720 [DOI] [PubMed] [Google Scholar]
- 2. Thiery JP. 2002. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2:442–454 [DOI] [PubMed] [Google Scholar]
- 3. Willipinski-Stapelfeldt B, Riethdorf S, Assmann V, Woelfle U, Rau T, Sauter G, Heukeshoven J, Pantel K. 2005. Changes in cytoskeletal protein composition indicative of an epithelial-mesenchymal transition in human micrometastatic and primary breast carcinoma cells. Clin Cancer Res 11:8006–8014 [DOI] [PubMed] [Google Scholar]
- 4. Tomaskovic-Crook E, Thompson EW, Thiery JP. 2009. Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res 11:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Es JH, Barker N, Clevers H. 2003. You Wnt some, you lose some: oncogenes in the Wnt signaling pathway. Curr Opin Genet Dev 13:28–33 [DOI] [PubMed] [Google Scholar]
- 6. ten Berge D, Koole W, Fuerer C, Fish M, Eroglu E, Nusse R. 2008. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell 3:508–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gilles C, Polette M, Mestdagt M, Nawrocki-Raby B, Ruggeri P, Birembaut P, Foidart JM. 2003. Transactivation of vimentin by β-catenin in human breast cancer cells. Cancer Res 63:2658–2664 [PubMed] [Google Scholar]
- 8. Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. 2004. Dual regulation of Snail by GSK-3β-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol 6:931–940 [DOI] [PubMed] [Google Scholar]
- 9. Dickson RB, Johnson MD, el-Ashry D, Shi YE, Bano M, Zugmaier G, Ziff B, Lippman ME, Chrysogelos S. 1993. Breast cancer: influence of endocrine hormones, growth factors and genetic alterations. Adv Exp Med Biol 330:119–141 [DOI] [PubMed] [Google Scholar]
- 10. Osborne CK. 1998. Steroid hormone receptors in breast cancer management. Breast Cancer Res Treat 51:227–238 [DOI] [PubMed] [Google Scholar]
- 11. Hill SM, Frasch T, Xiang S, Yuan L, Duplessis T, Mao L. 2009. Molecular mechanisms of melatonin anticancer effects. Integr Cancer Ther 8:337–346 [DOI] [PubMed] [Google Scholar]
- 12. Reiter RJ, Tan DX, Fuentes-Broto L. 2010. Melatonin: a multitasking molecule. Prog Brain Res 181:127–151 [DOI] [PubMed] [Google Scholar]
- 13. Brainard GC, Rollag MD, Hanifin JP. 1997. Photic regulation of melatonin in humans: ocular and neural signal transduction. J Biol Rhythms 12:537–546 [DOI] [PubMed] [Google Scholar]
- 14. Reiter RJ. 1991. Melatonin: the chemical expression of darkness. Mol Cell Endocrinol 79:153–158 [DOI] [PubMed] [Google Scholar]
- 15. Hill SM, Blask DE. 1988. Effects of the pineal hormone melatonin on the proliferation and morphological characteristics of human breast cancer cells (MCF-7) in culture. Cancer Res 48:6121–6126 [PubMed] [Google Scholar]
- 16. Teplitzky SR, Kiefer TL, Cheng Q, Dwivedi PD, Moroz K, Myers L, Anderson MB, Collins A, Dai J, Yuan L, Spriggs LL, Blask DE, Hill SM. 2001. Chemoprevention of NMU-induced rat mammary carcinoma with the combination of melatonin and 9-cis-retinoic acid. Cancer Lett 168:155–163 [DOI] [PubMed] [Google Scholar]
- 17. Yuan L, Collins AR, Dai J, Dubocovich ML, Hill SM. 2002. MT1 melatonin receptor overexpression enhances the growth suppressive effect of melatonin in human breast cancer cells. Mol Cell Endocrinol 192:147–156 [DOI] [PubMed] [Google Scholar]
- 18. Mao L, Yuan L, Slakey LM, Jones FE, Burow ME, Hill SM. 2010. Inhibition of breast cancer cell invasion by melatonin is mediated through regulation of the p38 mitogen-activated protein kinase signaling pathway. Breast Cancer Res 12:R107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cos S, Fernández R, Güézmes A, Sánchez-Barceló EJ. 1998. Influence of melatonin on invasive and metastatic properties of MCF-7 human breast cancer cells. Cancer Res 58:4383–4390 [PubMed] [Google Scholar]
- 20. Blask DE, Brainard GC, Dauchy RT, Hanifin JP, Davidson LK, Krause JA, Sauer LA, Rivera-Bermudez MA, Dubocovich ML, Jasser SA, Lynch DT, Rollag MD, Zalatan F. 2005. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res 65:11174–11184 [DOI] [PubMed] [Google Scholar]
- 21. Wu J, Dauchy RT, Tirrell PC, Wu SS, Lynch DT, Jitawatanarat P, Burrington CM, Dauchy EM, Blask DE, Greene MW. 2011. Light at night activates IGF-1R/PDK1 signaling and accelerates tumor growth in human breast cancer xenografts. Cancer Res 71:2622–2631 [DOI] [PubMed] [Google Scholar]
- 22. Hughes K, Nikolakaki E, Plyte SE, Totty NF, Woodgett JR. 1993. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J 12:803–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785–789 [DOI] [PubMed] [Google Scholar]
- 24. Dauchy RT, Blask DE, Dauchy EM, Davidson LK, Tirrell PC, Greene MW, Tirrell RP, Hill CR, Sauer LA. 2009. Antineoplastic effects of melatonin on a rare malignancy of mesenchymal origin: melatonin receptor-mediated inhibition of signal transduction, linoleic acid metabolism and growth in tissue-isolated human leiomyosarcoma xenografts. J Pineal Res 47:32–42 [DOI] [PubMed] [Google Scholar]
- 25. Martín V, Herrera F, Carrera-Gonzalez P, García-Santos G, Antolín I, Rodriguez-Blanco J, Rodriguez C. 2006. Intracellular signaling pathways involved in the cell growth inhibition of glioma cells by melatonin. Cancer Res 66:1081–1088 [DOI] [PubMed] [Google Scholar]
- 26. Dauchy RT, Sauer LA. 1986. Preparation of “tissue-isolated” rat tumors for perfusion: a new surgical technique that preserves continuous blood flow. Lab Anim Sci 36:678–681 [PubMed] [Google Scholar]
- 27. Sonderegger S, Haslinger P, Sabri A, Leisser C, Otten JV, Fiala C, Knöfler M. 2010. Wingless (Wnt)-3A induces trophoblast migration and matrix metalloproteinase-2 secretion through canonical Wnt signaling and protein kinase B/AKT activation. Endocrinology 151:211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anbalagan M, Carrier L, Glodowski S, Hangauer D, Shan B, Rowan BG. 2012. KX-01, a novel Src kinase inhibitor directed toward the peptide substrate site, synergizes with tamoxifen in estrogen receptor α positive breast cancer. Breast Cancer Res Treat 132:391–409 [DOI] [PubMed] [Google Scholar]
- 29. Cailotto C, Lei J, van der Vliet J, van Heijningen C, van Eden CG, Kalsbeek A, Pévet P, Buijs RM. 2009. Effects of nocturnal light on (clock) gene expression in peripheral organs: a role for the autonomic innervation of the liver. PLoS One 4:e5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xiang S, Mao L, Duplessis T, Yuan L, Dauchy R, Dauchy E, Blask DE, Frasch T, Hill SM. 2012. Oscillation of clock and clock controlled genes induced by serum shock and regulated by melatonin in human breast epithelial and breast cancer cells. Breast Cancer (Auckl) 6:137–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fukumoto S, Hsieh CM, Maemura K, Layne MD, Yet SF, Lee KH, Matsui T, Rosenzweig A, Taylor WG, Rubin JS, Perrella MA, Lee ME. 2001. Akt participation in the Wnt signaling pathway through Dishevelled. J Biol Chem 276:17479–17483 [DOI] [PubMed] [Google Scholar]
- 32. Thornton TM, Pedraza-Alva G, Deng B, Wood CD, Aronshtam A, Clements JL, Sabio G, Davis RJ, Matthews DE, Doble B, Rincon M. 2008. Phosphorylation by p38 MAPK as an alternative pathway for GSK3β inactivation. Science 320:667–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lai L, Yuan L, Chen Q, Dong C, Mao L, Rowan B, Frasch T, Hill SM. 2008. The Gαi and Gαq proteins mediate the effects of melatonin on steroid/thyroid hormone receptor transcriptional activity and breast cancer cell proliferation. J Pineal Res 45:476–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ram PT, Dai J, Yuan L, Dong C, Kiefer TL, Lai L, Hill SM. 2002. Involvement of the mt1 melatonin receptor in human breast cancer. Cancer Lett 179:141–150 [DOI] [PubMed] [Google Scholar]
- 35. Xu Y, Lee SH, Kim HS, Kim NH, Piao S, Park SH, Jung YS, Yook JI, Park BJ, Ha NC. 2010. Role of CK1 in GSK3β-mediated phosphorylation and degradation of snail. Oncogene 29:3124–3133 [DOI] [PubMed] [Google Scholar]
- 36. Parkin DM, Läärä E, Muir CS. 1988. Estimates of worldwide frequency of sixteen major cancers in 1980. Int J Cancer 41:184–197 [DOI] [PubMed] [Google Scholar]
- 37. Stevens RG. 1987. Electric power use and breast cancer: a hypothesis. Am J Epidemiol 125:556–561 [DOI] [PubMed] [Google Scholar]
- 38. Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Altieri A, Benbrahim-Tallaa L, Cogliano V. 2007. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol 8:1065–1066 [DOI] [PubMed] [Google Scholar]
- 39. Iitaka C, Miyazaki K, Akaike T, Ishida N. 2005. A role for glycogen synthase kinase-3β in the mammalian circadian clock. J Biol Chem 280:29397–29402 [DOI] [PubMed] [Google Scholar]
- 40. Henriksson M, Bakardjiev A, Klein G, Lüscher B. 1993. Phosphorylation sites mapping in the N-terminal domain of c-myc modulate its transforming potential. Oncogene 8:3199–3209 [PubMed] [Google Scholar]
- 41. Diehl JA, Cheng M, Roussel MF, Sherr CJ. 1998. Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev 12:3499–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu RC, Feng Q, Lonard DM, O'Malley BW. 2007. SRC-3 coactivator functional lifetime is regulated by a phospho-dependent ubiquitin time clock. Cell 129:1125–1140 [DOI] [PubMed] [Google Scholar]
- 43. Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Colditz GA. 2001. Rotating night shifts and risk of breast cancer in women participating in the nurses' health study. J Natl Cancer Inst 93:1563–1568 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.