Abstract
Previously available androgen receptor (AR) antagonists (bicalutamide, flutamide, and nilutamide) have limited activity against AR in prostate cancers that relapse after castration [castration resistant prostate cancer (CRPC)]. However, recent AR competitive antagonists such as MDV3100, generated through chemical modifications to the current AR ligands, appear to have increased activity in CRPC and have novel mechanisms of action. Using pharmacophore models and a refined homology model of the antagonist-liganded AR ligand binding domain, we carried out in silico screens of small molecule libraries and report here on the identification of a series of structurally distinct nonsteroidal small molecule competitive AR antagonists. Despite their unique chemical architectures, compounds representing each of six chemotypes functioned in vitro as pure AR antagonists. Moreover, similarly to MDV3100 and in contrast to previous AR antagonists, these compounds all prevented AR binding to chromatin, consistent with each of the six chemotypes stabilizing a similar AR antagonist conformation. Additional studies with the lead chemotype (chemotype A) showed enhanced AR protein degradation, which was dependent on helix 12 in the AR ligand binding domain. Significantly, chemotype A compounds functioned as AR antagonists in vivo in normal male mice and suppressed AR activity and tumor cell proliferation in human CRPC xenografts. These data indicate that certain ligand-induced structural alterations in the AR ligand binding domain may both impair AR chromatin binding and enhance AR degradation and support continued efforts to develop AR antagonists with unique mechanisms of action and efficacy in CRPC.
Most prostate cancer (PCa) patients respond initially to androgen deprivation therapy (surgical or medical castration) that suppresses androgen receptor (AR) activity, but they invariably relapse with tumors that express high levels of AR and AR-regulated genes despite castrate androgen levels in serum (1). Although a significant number of these castration-resistant prostate cancer (CRPC) patients respond to secondary therapies such as CYP17A1 inhibition that further suppress androgen synthesis (2), only a small proportion respond to currently available AR antagonists (flutamide, nilutamide, or bicalutamide) (Fig. 1A) (3). Some patients treated long term with these AR antagonists develop somatic mutations in the AR ligand binding domain (LBD) that markedly enhance the agonist activity of these drugs (4). However, wild-type AR (AR WT) is present in the majority of CRPC patients that relapse after androgen deprivation therapy, and the mechanistic basis for the limited effectiveness of AR antagonists in these patients remains to be firmly established (5). The diarylthiohydantoin AR antagonist MDV3100 was synthesized through chemical modifications to a potent nonsteroidal AR agonist (Fig. 1A), and appears substantially more active in CRPC than previous AR antagonists (6–8). In contrast to bicalutamide, which stimulates AR nuclear translocation and may acquire agonist activity in CRPC (9, 10), the MDV3100-liganded AR localizes primarily to the cytoplasm and does not have demonstrable agonist activity (6). These observations indicate that AR antagonists with novel mechanisms of action may provide significant therapeutic opportunities in CRPC.
Fig. 1.
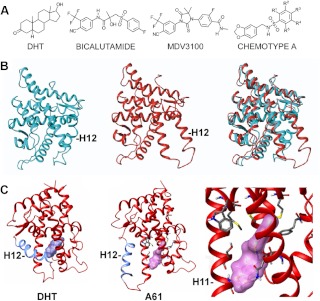
Structures of AR antagonists and homology model of AR in antagonist conformation. A, Structures of DHT, current AR antagonists, and the chemotype A chemical scaffold. In A61, R1 and R3 are Cl. In A89, R1 and R4 are Cl, and R3 is O-CH2-CH3. B, AR LBD in the agonist conformation and the refined homology model of AR LBD in an antagonist conformation, which features marked displacement of helix 12. These conformations superimpose to approximately 3.6 Å. C, Structure of DHT-liganded AR LBD and predicted structure of the chemotype A compound A61-liganded AR. Structures are rotated approximately 90° along the vertical axis compared with B. The right panel is a close-up of the A61-liganded AR LBD.
The AR contains an N-terminal transactivation domain (NTD), a central DNA binding domain (DBD), a C-terminal LBD that binds androgens [testosterone and dihydrotestosterone (DHT)], and a hinge region between the DBD and LBD that contributes to nuclear localization. Newly synthesized AR associates with a heat shock protein 90 chaperone complex that aids in folding the LBD into a conformation that can bind androgen, and in the absence of ligand, the AR undergoes proteasome mediated degradation. Androgen binding induces a shift in the positioning of helix 12 in the LBD and stabilizes AR in the agonist conformation that positions helix 12 adjacent to helices 3–5. This supports formation of an interface that initially binds a hydrophobic helix in the AR NTD (FQNLF) and subsequently binds to LxxLL motifs in coactivator proteins (11, 12). The agonist-liganded AR translocates to the nucleus, dimerizes, and binds to specific sequences [androgen responsive elements (ARE)] in AR target genes (13).
Crystallography studies have elucidated the structures of AR LBD bound to agonists and of mutant AR bound to antagonists in an agonist-like conformation, but structures of the AR LBD in an antagonist conformation have not been reported (14, 15). To facilitate the identification of compounds that may stabilize an antagonist conformation of the AR, we describe initially the use of homology modeling to generate a structure for AR in an antagonist conformation. We then describe the use of a computer-aided drug discovery and development platform, which leverages the combined power of molecular modeling with in silico screens of diverse drug-like small molecule libraries, for the de novo discovery of AR antagonists with novel mechanisms of action and activity in CRPC.
Materials and Methods
AR homology modeling
The refined homolog model of the AR LBD in an antagonized conformation was constructed using the comparative protein modeling suite ORCHESTRAR (SYBYL 8.0; Tripos International, St. Louis, MO). The crystal structure of agonist bound AR (PDB code 2AM9) as well as the crystal structure of progesterone receptor in complex with the corepressor nuclear receptor corepressor (NCoR) (PDB code 2OVM) was used as templates in generating and validating the model (16, 17). After searching the loops and fixing the side chains, the obtained structure was minimized with MMFF94 force field using the Biopolymer module. The AR model was submitted to PROCHECK and the quality of the protein structure evaluated using a Ramachandran plot (18). We determined that the refined homology model of the AR in the antagonized conformation had an RMSD of 3.627 Å when superimposed with the AR WT structure.
Ligand-based pharmacophore development
A training set of 23 active reference compounds (Supplemental Fig. 1) were subjected to pharmacophore alignment using the GALAHAD (19). Briefly, the molecules were aligned using the constellation of ligand pharmacophore descriptors, and single conformer pharmacophore models were generated using triplet tuplets. These triplet tuplet pharmacophores were generated using the point features including positive nitrogens, negative centers, hydrogen bond donors, acceptors atoms, and hydrophobic centers with the multiple edge lengths binned at 0.5 Å intervals. One cluster of our initial virtual repository of approximately 400,000 compounds was minimized using CONCORD (R. S. Pearlman, Concord, distributed by Tripos International) and subjected to the tuplet conformer generation (20). These three-dimensional pharmacophoric tuplet hypotheses were generated with the 100 random conformations of the active molecules, resulting in a hit list of 4130 compounds (the top 1% of the chemical repository).
Structure-based virtual screening
The hits from our pharmacophore screens were subjected to structure-based docking evaluations using our AR homology model and cross-docked into the variant AR crystal structure bound to bicalutamide using the Surflex 2.4 suite (21). Docking was carried out with the initial generation of protomol with threshold range of 0.50 Å. Furthermore, before docking, the ligands were energy minimized using the Tripos force field that uses Powell minimization and simplex optimization with a distance-dependent dielectric function and an energy gradient of 0.01 kcal/molÅ with a maximum of 1000 iterations. Our first-generation docking targeted the putative ligand-binding site within our homology model (Fig. 1B). The top compounds were further selected for use by in silico adsorption, distribution, metabolism, excretion, and toxicology (ADMET) evaluation (SARCHITECT 2.5; Strand Biosciences, Hebbal, Bangalore, India). The final compound ranking was based on our normalized total interaction energy from the docking as well as the ADMET ranking that used drug-like truPk and truTox properties (22). Supplemental Fig. 2, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org, gives an outline of the drug discovery schema and identification of the second-generation chemotype A compounds.
Reporter gene assays and chromatin immunoprecipitation (ChIP)
COS7 cells were transfected overnight using Lipofectamine (Life Technologies, Grand Island, NY) and then switched to medium with 10% steroid depleted fetal bovine serum [charcoal dextran stripped serum (CSS)] (Hyclone Laboratories, Logan, UT). Plasmids encoding steroid receptors and reporter genes have been described previously (9, 23, 24). The T887A, W741C, and ARΔH12 mutant constructs were generated using the QuikChange II site-directed mutagenesis system (Stratagene, La Jolla, CA) (24). Transfections were done in triplicate (mean and sem shown) and data are representative of at least three experiments. For anti-AR ChIP, VCaP cells in steroid depleted medium were pretreated with chemotype A compounds for 1 h and then stimulated with 1 nm DHT or vehicle for 2 h. After formalin fixation, chromatin extraction, and chromatin shearing, samples were immunoprecipitated with a polyclonal rabbit anti-AR antibody against the NTD or control IgG (Santa Cruz Biotechnology, Santa Cruz, CA). Bound DNA was determined by quantitative real-time PCR.
Immunoblotting and immunofluorescence
For drug treatments, cells were grown to 50–60% confluence in medium with 5% CSS for 2–3 d and then treated for 24 h or as indicated. Cells were separated into nuclear and cytoplasmic fractions (NE-PER nuclear extraction reagents; Pierce, Rockford, IL). Equal amounts of protein were immunoblotted using anti-prostate-specific antigen (PSA; 1:3000, polyclonal; BioDesign International, Saco, ME), anti-AR (1:2000, polyclonal; Upstate Biotechnology, Lake Placid, NY), anti-β-actin (1:5000, monoclonal; Abcam, Cambridge, MA), or anti-β-tubulin (1:2000; Upstate Biotechnology). For immunofluorescence, AR was detected indirectly using a fluorescent secondary antibody.
Xenografts
Tissue sections underwent antigen retrieval and were then blocked using 5% goat serum and avidin blocking solution (Vector Laboratories, Burlingame, CA). Anti-AR (Santa Cruz Biotechnology) or anti-Ki67 (Dako, Carpinteria, CA) was added overnight at 4 C, followed by biotinylated secondary antibody and streptavidin-horseradish peroxidase (Vector Laboratories). RNA was extracted from frozen sections containing nonnecrotic tumor. Quantitative real-time RT-PCR amplification was with Taqman one-step RT-PCR reagents and results were normalized to coamplified 18S RNA or glyceraldehyde-3-phosphate dehydrogenase.
Results
Homology modeling and in silico screening for AR antagonists
To permit the use of a structure-based virtual screen (SBVS), we first generated a refined homology model of the AR LBD in an antagonist conformation through integration of structure/function data derived from crystallographic studies of antagonist-bound glucocorticoid receptor (GR) and progesterone receptor (PR) (Fig. 1B) (17, 25, 26). This model, which features marked displacement of helix 12, was then used in SBVS to identify small molecules predicted to bind in the steroid binding pocket and stabilize this AR LBD conformation. These SBVS were used to refine small molecule hit lists initially generated using ligand-based pharmacophore models encapsulating functionally requisite steric and electronic features identified from a series of 23 known antagonists (Supplemental Fig. 1). These small molecules that then successfully docked into the AR LBD homology model were further filtered in silico for preferred drug-like properties using ADMET models. A diagram outlining these steps is shown in Supplemental Fig. 2.
Functional validation of in silico identified antagonists
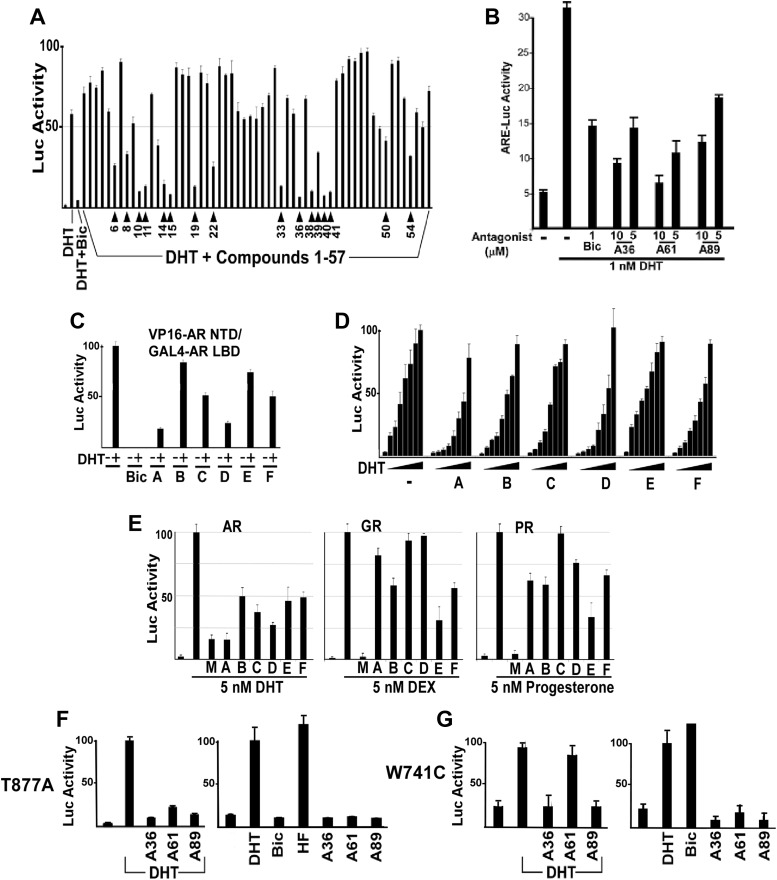
The 57 highest ranked nonsteroidal compounds were purchased, and 16 of these (at 50 μm) demonstrated greater than 50% inhibition of DHT-stimulated ARE-luciferase reporter gene activity, with no significant effect on an internal Renilla luciferase control (Fig. 2A). Several inhibited AR activity 80–90% (comparable with 10 μm bicalutamide), and none had demonstrable agonist activity in this reporter gene assay (Supplemental Fig. 3). The 16 compounds represented six chemical scaffolds that were distinct from those used by previously identified antagonists (chemotypes A-F), with 11 of the 16 belonging to the chemotype A series (Fig. 1A and Supplemental Fig. 4). This provided an initial structure activity relationship series for chemotype A that was used to further refine our ligand-based pharmacophores for subsequent ligand based virtual screens (LBVS). These screens identified 40 additional chemotype A compounds, which were also purchased and found to have a range of potency in reporter gene assay (data not shown). Two of the most potent chemotype A compounds from this second generation were A61 and A89 (R-groups corresponding to A61 and A89 are indicated in the Fig. 1A legend). In transiently transfected COS7 cells stimulated with 1 nm DHT, these compounds had an IC50 of approximately 5 μm vs. approximately 1 μm for bicalutamide (Fig. 2B). Figure 1C shows the structure of the DHT-liganded LBD vs. the predicted structure of the A61 liganded LBD.
Fig. 2.
In silico-identified compounds are competitive AR antagonists. A, Luciferase activity from COS7 cells transfected with AR and AR-responsive luciferase reporter plasmids followed by treatment for 24 h with DHT (10 nm) alone or in the presence of bicalutamide (Bic; 10 μm) or compounds 1–57 (50 μm). Numbered arrows indicate the 16 compounds selected for further study. B, IC50 for chemotype A antagonists and bicalutamide. Luciferase activity from COS7 cells transfected with AR and AR-responsive luciferase reporter plasmids followed by drug treatment with 1 nm DHT and the indicated compounds for 24 h. Approximate IC50 under these conditions for bicalutamide was 1 μm, and for chemotype A compounds was approximately 5 μm. C, AR N/C interaction stimulated by DHT (10 nm) alone or in the presence of Bic (10 μm) or chemotype A-F compounds (10 μm). D, AR response to increasing DHT concentrations (0–200 nm) in the presence of chemotype A-F compounds (10 μm). E, COS7 cells were transfected for 24 h with AR, GR, or PR expression vectors and ARE4-Luc reporter (recognized by AR, GR, and PR) and the control reporter and then stimulated for 24 h with 5 nm of DHT, dexamethasone (DEX), or progesterone, with/without 25 μm of the indicated antagonists (A36 for chemotype A). Firefly luciferase was corrected for internal control Renilla luciferase activity and normalized to no antagonist, which was set at 100. F, T877A mutant AR response to DHT (10 nm), Bic (10 μm), hydroxyflutamide (HF; 100 nm), and chemotype A compounds (10 μm). G, W741C mutant AR response to DHT (10 nm), Bic (10 μm), and chemotype A compounds (10 μm). Stimulation by bicalutamide was approximately 5-fold higher than by DHT.
To more directly confirm that the six chemotypes did not induce an agonist conformation, we assessed their effects on the interaction between the AR NTD and the C-terminal LBD (AR N/C interaction), which is mediated by the LxxLL-like motif in the AR NTD binding to the agonist-induced coactivator binding site in the LBD (11, 12). Cells were cotransfected with expression vectors encoding the VP16 transactivation domain fused to the AR NTD (VP16-AR NTD), the GAL4 DBD fused to the AR LBD (GAL4-AR LBD), and a GAL4 regulated luciferase reporter. As expected, the N/C interaction was stimulated by DHT but not by the antagonist bicalutamide (Fig. 2C). Significantly, none of the chemotype A-F compounds stimulated the N/C interaction, and they inhibited the DHT-stimulated AR N/C interaction to varying degrees consistent with their potency as AR antagonists.
To further assess whether the compounds were binding within the LBD steroid binding pocket, we determined whether inhibition was prevented by increasing DHT concentrations. Cells transfected with AR and luciferase reporter plasmids were treated with increasing concentrations of DHT (0–200 nm) in the presence of chemotype A-F compounds (10 μm). With the exception of chemotype E, each of the compounds at this concentration inhibited DHT stimulated AR activity (Fig. 2D). Moreover, this inhibition could be overcome by DHT, consistent with competitive binding to the AR ligand binding pocket.
We next conducted reporter gene assays with the GR and PR to see whether these compounds had cross-reactivity with these related steroid receptors. Chemotype C did not show cross-reactivity, whereas chemotype E inhibited both GR and PR to a similar or greater extent than AR (Fig. 2E). Chemotype A (compound A36) at this concentration (25 μm) inhibited PR and weakly inhibited GR but was substantially more active against AR. As expected, mifepristone strongly inhibited all three receptors. It should be noted that our screen did not attempt to remove the compounds that could react with other steroid receptors, so some degree of cross-reactivity is not surprising and is consistent with the binding to the steroid pocket. Both A61 and A89 are similar to A36 with respect to GR and PR cross-reactivity (data not shown).
Finally, we examined the effects of chemotype A antagonists on mutant AR identified in PCa patients. The T877A mutant AR is strongly activated by hydroxyflutamide, the active metabolite of the AR antagonist flutamide (14). The T877A mutation generates increased space in the ligand binding pocket and thus allows hydroxyflutamide to bind without causing the structural distortion that is the basis for its antagonist activity. Consistent with the molecular modeling indicating that chemotype A directly interferes with helix 12 movement into the agonist position (see Fig. 1C), chemotype A compounds (A36, A61, and A89) do not stimulate the T877A mutant AR and still function as antagonists of DHT-stimulated activity (Fig. 2F). We similarly found that the W741C mutant AR, which is strongly activated by bicalutamide due to loss of the tryptophan that is displaced by bicalutamide in the AR WT (15), was not activated by the chemotype A compounds and could still be inhibited by A36 and A89 (Fig. 2G). The W741C mutation impaired inhibition by A61, consistent with changes in the side groups affecting the affinity for the AR LBD.
Chemotype A-F compounds impair DNA binding and nuclear localization
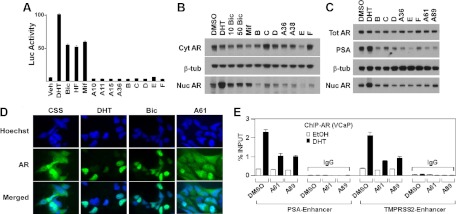
Although conventional AR antagonists including bicalutamide impair coactivator binding and enhance corepressor recruitment, they also stimulate AR nuclear localization and chromatin binding, which are mechanistic limitations that may result in partial agonist activity in CRPC (6, 9, 27). To address whether the chemotype A-F compounds support AR binding to DNA, we fused the VP16 transactivation domain to full-length AR (VP16-AR), giving it constitutive transcriptional activity. Consistent with our previous data (9), the VP16-AR yielded strong reporter activity indicative of DNA binding in the presence of DHT or antagonists (bicalutamide, hydroxyflutamide, and mifepristone) (Fig. 3A). In contrast, chemotype A-F compounds did not induce any reporter activity above background. We next fractionated an AR-negative PCa cell line (PC3) that was stably transfected with full-length AR WT (PC3-AR cells) to evaluate AR intracellular localization. As expected, nuclear AR levels in these cells cultured in steroid-depleted medium were greatly increased by DHT and modestly increased by bicalutamide and mifepristone (Fig. 3B). In contrast, treatment with the chemotype A-F antagonists did not increase nuclear AR and instead reduced nuclear AR.
Fig. 3.
Chemotype A-F compounds inhibit AR binding to DNA and AR nuclear localization. A, Luciferase activity from COS7 cells transfected with VP16-AR and AR-responsive luciferase reporter followed by treatment with DHT (10 nm), bicalutamide (Bic; 10 μm), hydroxyflutamide (HF; 100 nm), mifepristone (Mif; 100 nm), or chemotype A-F compounds (50 μm) for 24 h. B, AR protein in nuclear and cytoplasmic fractions of PC3-AR cells, cultured in steroid depleted medium, after treatment with DHT (10 nm), Bic (10 μm, 50 μm), Mif (100 nm), or chemotype A-F compounds (50 μm) for 6 h. C, PSA and total vs. nuclear AR protein levels in VCaP cells after treatment with DHT (10 nm) or chemotype A-F compounds (50 μm) for 6 h. D, Immunofluorescent labeling of AR in VCaP cells grown in steroid-depleted medium (medium with CSS) followed by treatment with DHT (10 nm), Bic (50 μm), or compound A61 (50 μm) for 6 h. E, VCaP cells cultured for 3 d in steroid depleted medium were treated with 25 μm A61, A89, or DMSO (vehicle) for 1 h. DHT or vehicle [ethanol (EtOH)] was then added for another 2 h, followed by anti-AR or control IgG ChIP and quantitative PCR for ARE in the PSA and TMPRSS2 enhancers. Results are expressed as percentage bound relative to total input DNA.
To further assess whether these compounds may have partial agonist activity, we examined their effect on AR activity and nuclear localization in the human VCaP PCa cell line, which expresses high levels of endogenous AR WT (28, 29). VCaP cells cultured in steroid hormone-depleted medium produce low levels of testosterone and DHT that partially activate AR to stimulate synthesis of PSA, but this PSA synthesis and the levels of nuclear AR are substantially increased by treatment with DHT (Fig. 3C). In contrast, the chemotype A-F compounds decreased nuclear AR and concomitantly reduced basal PSA expression. The effect of chemotype A on intracellular AR distribution in VCaP cells was further assessed by immunofluorescence. VCaP cells in steroid-depleted medium (CSS medium) exhibited both nuclear and cytoplasmic AR staining, and treatment with DHT or bicalutamide markedly increased AR nuclear localization (Fig. 3D). In contrast, chemotype A compounds (A61 and A89, not shown) did not enhance nuclear AR, with most cells showing no clear nuclear accumulation of AR (Fig. 3D).
Finally, we used ChIP to directly assess effects of chemotype A on chromatin binding by the endogenous AR WT in VCaP cells. VCaP cells cultured in steroid-depleted medium were treated with dimethylsulfoxide (DMSO; vehicle), A61, or A89 (25 μm), followed by treatment with DHT (1 nm) or vehicle for 2 h. We then immunoprecipitated with anti-AR or control IgG and used quantitative real-time PCR to measure AR associated with the ARE in the PSA and TMPRSS2 gene enhancers. Significantly, A61 and A89 did not stimulate AR chromatin binding and decreased DHT induced AR binding to both genes (Fig. 3E). These results further support the conclusion that the chemotype A-liganded AR does not bind to chromatin.
Chemotype A compounds enhance AR protein degradation
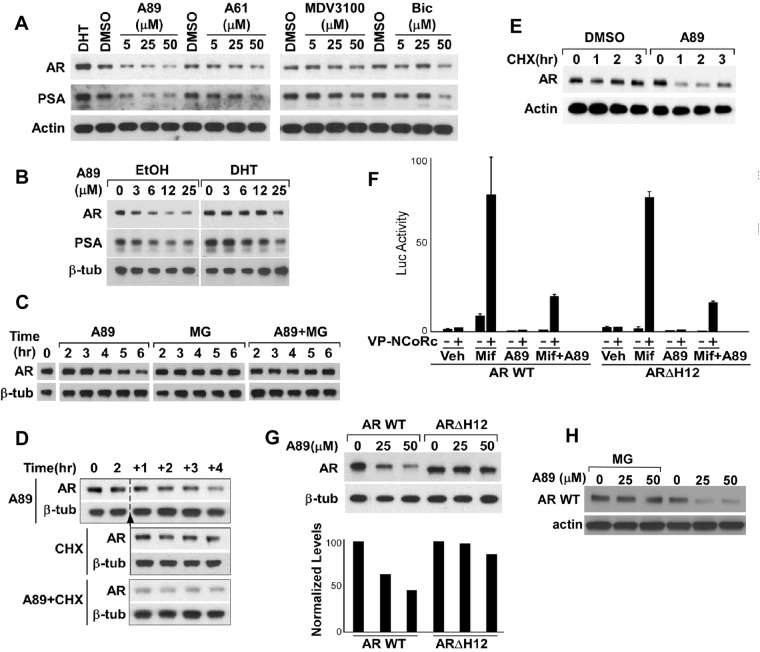
Similar to the results in VCaP cells, chemotype A compounds reduced nuclear AR in another CRPC cell line (C4-2 cells, derived from a castration resistant LNCaP xenograft) cultured in steroid-depleted medium (Supplemental Fig. 5) and also prevented DHT-stimulated nuclear localization (Supplemental Fig. 6). Significantly, although DHT binding increases the levels of AR protein by decreasing its rate of degradation, A61 and A89 prevented this DHT-stimulated increase in AR protein and caused a further reduction in basal cellular AR protein levels when added to the cells cultured in the steroid-depleted medium (Fig. 4A and Supplemental Figs. 5 and 6). This decrease in AR protein correlated with a decrease in PSA protein. MDV3100 and bicalutamide showed no clear effect on basal (in steroid depleted medium) PSA or the AR protein levels at concentrations (5 μm) that suppress DHT-stimulated AR activity, whereas modest decreases were observed at higher drug concentrations (25–50 μm) (Fig. 4A). The decline in AR protein in response to chemotype A appeared to be a direct consequence of AR binding because it was competitively blocked in the presence of DHT (Fig. 4B). This decrease in AR protein also was prevented by proteasome inhibitors (MG115 and MG132), implicating increased AR protein degradation as a further chemotype A mechanism of action (Fig. 4C).
Fig. 4.
Chemotype A compounds stimulate AR degradation. A, AR and PSA protein levels in C4-2 cells after treatment with DHT (10 nm), A61, A89, MDV3100, or bicalutamide (Bic) for 24 h. B, AR and PSA in C4-2 cells after treatment with A89 in the presence of DHT (10 nm) for 24 h. C, AR in C4-2 cells after treatment with A89 (50 μm), alone or in the presence of MG115 and MG132 proteasome inhibitors (MG; 10 μm each). D, AR in PC3-AR cells after treatment with CHX (100 nm) and A89 (50 μm). E, AR in VCaP cells after treatment with CHX (100 nm) and A89 (50 μm). F, Luciferase activity in COS7 cells transfected with either AR WT or ARΔΗ12, VP16-NCoRc, and an AR-responsive luciferase reporter followed by treatment with mifepristone (Mif; 100 nm) and A89 (50 μm), alone or in combination for 24 h. G, AR WT and ARΔΗ12 protein levels in transfected COS7 cells after treatment with A89 for 24 h. H, COS7 cells transfected with AR WT expression vector were treated overnight with A89, followed by the addition of MG115 and MG132 proteasome inhibitors (MG; 10 μm each) or vehicle for 2 h.
Because LNCaP and C4-2 cells express the T877A mutant AR, we next examined PC3-AR cells expressing AR WT and cultured in steroid-depleted medium. As observed in C4-2 cells, A89 caused a decrease in AR protein levels (Fig. 4D). Moreover, AR protein declined markedly in the A89-treated vs. control PC3-AR cells after 1 h treatment with cycloheximide (CHX) to block new protein synthesis (Fig. 4D), indicating that AR protein degradation was increased. Finally, using CHX to block new protein synthesis in VCaP cells cultured in steroid-depleted medium, we confirmed that A89 also increased the degradation of the endogenous AR WT (Fig. 4E). Together these data indicate that chemotype A binding to the AR LBD increases the basal (in steroid depleted medium) rate of AR degradation.
Chemotype A-induced AR degradation is helix 12 dependent
The estrogen receptor (ER)-α antagonist fulvestrant (Faslodex) appears to mediate ERα degradation by specifically repositioning helix 12. To assess the role of helix 12 in chemotype A-mediated AR degradation, we first determined whether helix 12 is required for A89 binding. Because this helix is required for AR agonist activity, we could not directly measure competitive antagonist activity on a truncated AR lacking helix 12. However, we showed previously that the AR antagonist mifepristone binds to the AR and recruits the corepressor protein NCoR in the absence of helix 12 (23, 24). Therefore, we used this mifepristone-stimulated NCoR recruitment as a readout to assay competitive A89 binding. COS7 cells were cotransfected with expression vectors for AR WT or an M886X variant truncated between helices 11 and 12 (AR ΔH12) along with vectors encoding the NCoR C-terminal AR binding region fused to the VP16 transactivation domain (VP16-NCoRc) and an AR-responsive luciferase reporter.
Consistent with our previous results, mifepristone strongly enhanced the interaction of VP16-NCoRc with both AR WT and AR ΔH12 (Fig. 4F). Significantly, A89 did not stimulate VP16-NCoRc recruitment, and it suppressed mifepristone-mediated VP16-NCoRc recruitment equivalently on both AR WT and AR ΔH12, indicating that A89 binding is not dependent on helix 12. We next transiently transfected COS7 cells with AR WT or AR ΔH12 plasmids and assayed for AR expression levels in response to A89. As expected, AR WT protein levels decreased in a dose-dependent manner after treatment with A89 (Fig. 4G) and could be restored by addition of proteasome inhibitors (Fig. 4H). In contrast, AR ΔΗ12 protein levels were not clearly decreased by A89 treatment, consistent with the A89-induced repositioning of helix 12 being a signal that targets AR for degradation.
Chemotype A compounds are efficacious in vivo in CRPC xenografts
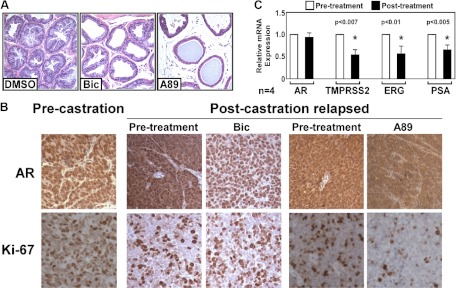
To evaluate the activity of the chemotype A compounds in vivo, we first treated male mice with compound A89 at 0.5–10 mg/d via ip injection for 7 d and found that the seminal vesicles underwent dose-dependent involution (a biomarker of AR antagonist activity) beginning at 2.5 mg/d (Supplemental Fig. 7). Compound A89 similarly caused atrophy of the androgen-sensitive prostate epithelium (Fig. 5A). We next evaluated A89 in VCaP xenografts that had relapsed after an initial response to castration. Consistent with our previous data (29), these relapsed VCaP xenografts expressed substantial nuclear and cytoplasmic AR and were refractory to bicalutamide, which enhanced nuclear AR expression (Fig. 5B). In contrast, A89 substantially decreased nuclear AR staining and decreased tumor cell proliferation as evidenced by Ki67 staining (Fig. 5B). Analysis of mRNA extracted from the biopsies of the relapsed VCaP xenografts before and after treatment with A89 further demonstrated a decrease in the expression of androgen-regulated genes including TMPRSS2, ERG (from the androgen regulated TMPRSS2-ERG fusion gene in VCaP), and PSA (Fig. 5C).
Fig. 5.
Chemotype A is efficacious in a CRPC xenograft model. A, Hematoxylin and eosin-stained sections of prostate ductal epithelium from mice treated with DMSO, bicalutamide (Bic), or A89 (10 mg/d). B, AR and Ki67 staining in pre- and postcastration relapsed VCaP xenograft tumors after treatment with A89 (5 mg/d) or bicalutamide (0.5 mg/d) for 7 d. C, mRNA levels of AR and AR-target genes in postcastration-relapsed VCaP xenograft tumors after treatment with A89 (5 mg/d) for 7 d.
Discussion
This study outlines our use of a broadly applicable computer-aided drug discovery and development platform to identify a series of AR antagonists that are both chemically and mechanistically unique. Significantly, despite the paucity of structural data for the AR LBD in an antagonist conformation, we were able to generate a refined homology model predictive of this antagonistic conformation. LBVS that used stringent ligand-dependent pharmacophore models developed around a training set of AR antagonists facilitated the rapid identification of a candidate hit list, which was then screened using the target-dependent SBVS and finally filtered using in silico ADMET models. Functional studies demonstrated that the 16 most active compounds representing chemotypes A-F were all AR competitive antagonists, although they had variable cross-reactivity with other steroid receptors because we did not select against this activity in the screening. Remarkably, the compounds were all devoid of detectable agonist activity. Consistent with this lack of agonist activity, chemotypes A-F all decreased nuclear AR levels and prevented chromatin binding as assessed using ChIP and using a constitutively active VP16-AR fusion protein that is robustly recruited by current AR antagonists. These data indicate that this virtual screening platform successfully identified small molecules with several distinct chemical architectures that all stabilize an antagonist conformation that is similar or identical to that specified by our homology model (Fig. 1B). Furthermore, although the structural features required for AR nuclear localization/chromatin binding have not been determined (and hence were not deliberately incorporated into the homology model), these data indicate that the LBD homology model reflects an AR conformation that is incompatible with these critical functions.
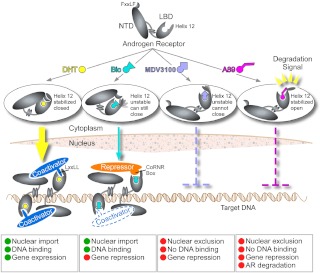
Studies aimed at defining the mechanism of action for these compounds further revealed that chemotype A compounds stabilize a conformation of the AR that is targeted for proteasome-mediated degradation (comparable studies of chemotypes B-F are currently in progress). Newly synthesized AR protein undergoes multiple rounds of heat shock protein 90-dependent refolding to maintain a conformation competent to bind androgen but in the absence of ligand the AR appears to undergo polyubiquitination and proteasome-mediated degradation. Structural features that target AR for protein degradation remain unclear, but our data suggest that the repositioning of helix 12 may expose a surface that is recognized by the degradation machinery, as appears to be the case for the fulvestrant-liganded ERα (30). Based on these findings, we propose that the functional consequences of antagonist binding may be dictated by the precise localization of helix 12 and that antagonist mediated stabilization of this helix in the degradation conformer may favor AR degradation (Fig. 6). However, further studies of additional AR antagonists, including others reported to stimulate AR nuclear export or degradation (31, 32), as well as further structural studies, clearly will be needed to determine the precise role of helix 12. In any case, these studies exemplify the utility of computational screening platforms and support the further development of AR antagonists that function through novel mechanisms of action.
Fig. 6.
Mechanisms of action for AR agonists and antagonists. Agonists stabilize a closed helix 12 position of the LBD that recruits the FxxLF motif of the NTD, resulting in AR N/C interaction, which facilitates AR nuclear import and DNA binding. Antagonists such as bicalutamide (Bic) do not stabilize helix 12 in a specific position and may allow a conformationally unstable helix 12 to transiently occupy the closed position and mediate AR nuclear import and DNA binding. However, this Bic-liganded AR preferentially recruits corepressors over coactivators. MDV3100 blocks helix 12 from closing, thereby preventing N/C interaction, nuclear import, and DNA binding. Chemotype A compounds stabilize helix 12 in a conformationally defined open position, preventing nuclear import and DNA binding. In addition, the positioning of helix 12 by chemotype A presents a degradation signal, resulting in increased AR degradation.
Supplementary Material
Acknowledgments
This work was supported by Grants R01 CA111803 and Prostate SPORE P50 CA090381 (to S.P.B.) from the National Institutes of Health, Grant PC060807 from the Department of Defense, and a Prostate Cancer Foundation Challenge Award. H.C.S. was supported by National Institutes of Health Grant T32 CA081156 and by Department of Defense Postdoctoral Training Award PC081332.
Disclosure Summary: The authors have nothing to disclose.
NURSA Molecule Pages†:
Ligands: Dihydrotestosterone | Bicalutamide.
Annotations provided by Nuclear Receptor Signaling Atlas (NURSA) Bioinformatics Resource. Molecule Pages can be accessed on the NURSA website at www.nursa.org.
- ADMET
- Adsorption, distribution, metabolism, excretion, and toxicology
- AR
- androgen receptor
- ARE
- androgen responsive element
- AR WT
- wild-type androgen receptor
- ChIP
- chromatin immunoprecipitation
- CHX
- cycloheximide
- CRPC
- castration-resistant prostate cancer
- CSS
- charcoal dextran stripped serum
- DBD
- DNA binding domain
- DHT
- dihydrotestosterone
- DMSO
- dimethylsulfoxide
- ER
- estrogen receptor
- GR
- glucocorticoid receptor
- ΔH12
- variant truncated between helices 11 and 12
- LBD
- ligand binding domain
- NCoR
- nuclear receptor corepressor
- NTD
- N-terminal transactivation domain
- PCa
- prostate cancer
- PR
- progesterone receptor
- PSA
- prostate-specific antigen
- SBVS
- structure-based virtual screen
- WT
- wild-type.
References
- 1. Attard G, Cooper CS, de Bono JS. 2009. Steroid hormone receptors in prostate cancer: a hard habit to break? Cancer Cell 16:458–462 [DOI] [PubMed] [Google Scholar]
- 2. Attard G, Reid AH, Olmos D, de Bono JS. 2009. Antitumor activity with CYP17 blockade indicates that castration-resistant prostate cancer frequently remains hormone driven. Cancer Res 69:4937–4940 [DOI] [PubMed] [Google Scholar]
- 3. Joyce R, Fenton MA, Rode P, Constantine M, Gaynes L, Kolvenbag G, DeWolf W, Balk S, Taplin ME, Bubley GJ. 1998. High dose bicalutamide for androgen independent prostate cancer: effect of prior hormonal therapy. J Urol 159:149–153 [DOI] [PubMed] [Google Scholar]
- 4. Taplin ME, Bubley GJ, Ko YJ, Small EJ, Upton M, Rajeshkumar B, Balk SP. 1999. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res 59:2511–2515 [PubMed] [Google Scholar]
- 5. Taplin ME, Rajeshkumar B, Halabi S, Werner CP, Woda BA, Picus J, Stadler W, Hayes DF, Kantoff PW, Vogelzang NJ, Small EJ. 2003. Androgen receptor mutations in androgen-independent prostate cancer: Cancer and Leukemia Group B Study 9663. J Clin Oncol 21:2673–2678 [DOI] [PubMed] [Google Scholar]
- 6. Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, Wasielewska T, Welsbie D, Chen CD, Higano CS, Beer TM, Hung DT, Scher HI, Jung ME, Sawyers CL. 2009. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324:787–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jung ME, Ouk S, Yoo D, Sawyers CL, Chen C, Tran C, Wongvipat J. 2010. Structure-activity relationship for thiohydantoin androgen receptor antagonists for castration-resistant prostate cancer (CRPC). J Med Chem 53:2779–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, Rathkopf D, Shelkey J, Yu EY, Alumkal J, Hung D, Hirmand M, Seely L, Morris MJ, Danila DC, Humm J, Larson S, Fleisher M, Sawyers CL. 2010. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet 375:1437–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Masiello D, Cheng S, Bubley GJ, Lu ML, Balk SP. 2002. Bicalutamide functions as an androgen receptor antagonist by assembly of a transcriptionally inactive receptor. J Biol Chem 277:26321–26326 [DOI] [PubMed] [Google Scholar]
- 10. Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. 2004. Molecular determinants of resistance to antiandrogen therapy. Nat Med 10:33–39 [DOI] [PubMed] [Google Scholar]
- 11. He B, Gampe RT, Jr, Kole AJ, Hnat AT, Stanley TB, An G, Stewart EL, Kalman RI, Minges JT, Wilson EM. 2004. Structural basis for androgen receptor interdomain and coactivator interactions suggests a transition in nuclear receptor activation function dominance. Mol Cell 16:425–438 [DOI] [PubMed] [Google Scholar]
- 12. Hur E, Pfaff SJ, Payne ES, Grøn H, Buehrer BM, Fletterick RJ. 2004. Recognition and accommodation at the androgen receptor coactivator binding interface. PLoS Biol 2:E274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lamont KR, Tindall DJ. 2010. Androgen regulation of gene expression. Adv Cancer Res 107:137–162 [DOI] [PubMed] [Google Scholar]
- 14. Sack JS, Kish KF, Wang C, Attar RM, Kiefer SE, An Y, Wu GY, Scheffler JE, Salvati ME, Krystek SR, Jr, Weinmann R, Einspahr HM. 2001. Crystallographic structures of the ligand-binding domains of the androgen receptor and its T877A mutant complexed with the natural agonist dihydrotestosterone. Proc Natl Acad Sci USA 98:4904–4909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bohl CE, Gao W, Miller DD, Bell CE, Dalton JT. 2005. Structural basis for antagonism and resistance of bicalutamide in prostate cancer. Proc Natl Acad Sci USA 102:6201–6206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nicholson RI, Gee JM, Bryant S, Francis AB, McClelland RA, Knowlden J, Wakeling AE, Osborne CK. 1996. Pure antiestrogens. The most important advance in the endocrine therapy of breast cancer since 1896. Ann NY Acad Sci 784:325–335 [DOI] [PubMed] [Google Scholar]
- 17. Madauss KP, Grygielko ET, Deng SJ, Sulpizio AC, Stanley TB, Wu C, Short SA, Thompson SK, Stewart EL, Laping NJ, Williams SP, Bray JD. 2007. A structural and in vitro characterization of asoprisnil: a selective progesterone receptor modulator. Mol Endocrinol 21:1066–1081 [DOI] [PubMed] [Google Scholar]
- 18. Kahraman A, Morris RJ, Laskowski RA, Thornton JM. 2007. Shape variation in protein binding pockets and their ligands. J Mol Biol 368:283–301 [DOI] [PubMed] [Google Scholar]
- 19. Richmond NJ, Willett P, Clark RD. 2004. Alignment of three-dimensional molecules using an image recognition algorithm. J Mol Graph Model 23:199–209 [DOI] [PubMed] [Google Scholar]
- 20. Abrahamian E, Fox PC, Naerum L, Christensen IT, Thøgersen H, Clark RD. 2003. Efficient generation, storage, and manipulation of fully flexible pharmacophore multiplets and their use in 3-D similarity searching. J Chem Inf Comput Sci 43:458–468 [DOI] [PubMed] [Google Scholar]
- 21. Jain AN. 2003. Surflex: fully automatic flexible molecular docking using a molecular similarity-based search engine. J Med Chem 46:499–511 [DOI] [PubMed] [Google Scholar]
- 22. Subramanian K. 2005. truPK—human pharmacokinetic models for quantitative ADME prediction. Expert Opin Drug Metab Toxicol 1:555–564 [DOI] [PubMed] [Google Scholar]
- 23. Hodgson MC, Astapova I, Cheng S, Lee LJ, Verhoeven MC, Choi E, Balk SP, Hollenberg AN. 2005. The androgen receptor recruits nuclear receptor CoRepressor (N-CoR) in the presence of mifepristone via its N and C termini revealing a novel molecular mechanism for androgen receptor antagonists. J Biol Chem 280:6511–6519 [DOI] [PubMed] [Google Scholar]
- 24. Hodgson MC, Shen HC, Hollenberg AN, Balk SP. 2008. Structural basis for nuclear receptor corepressor recruitment by antagonist-liganded androgen receptor. Mol Cancer Ther 7:3187–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kauppi B, Jakob C, Färnegardh M, Yang J, Ahola H, Alarcon M, Calles K, Engström O, Harlan J, Muchmore S, Ramqvist AK, Thorell S, Ohman L, Greer J, Gustafsson JA, Carlstedt-Duke J, Carlquist M. 2003. The three-dimensional structures of antagonistic and agonistic forms of the glucocorticoid receptor ligand-binding domain: RU-486 induces a transconformation that leads to active antagonism. J Biol Chem 278:22748–22754 [DOI] [PubMed] [Google Scholar]
- 26. Frego L, Davidson W. 2006. Conformational changes of the glucocorticoid receptor ligand binding domain induced by ligand and cofactor binding, and the location of cofactor binding sites determined by hydrogen/deuterium exchange mass spectrometry. Protein Sci 15:722–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shang Y, Myers M, Brown M. 2002. Formation of the androgen receptor transcription complex. Mol Cell 9:601–610 [DOI] [PubMed] [Google Scholar]
- 28. Loberg RD, St John LN, Day LL, Neeley CK, Pienta KJ. 2006. Development of the VCaP androgen-independent model of prostate cancer. Urol Oncol 24:161–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cai C, Wang H, Xu Y, Chen S, Balk SP. 2009. Reactivation of androgen receptor-regulated TMPRSS2:ERG gene expression in castration-resistant prostate cancer. Cancer Res 69:6027–6032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Osborne CK, Wakeling A, Nicholson RI. 2004. Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action. Br J Cancer 90(Suppl 1):S2–S6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bruno RD, Vasaitis TS, Gediya LK, Purushottamachar P, Godbole AM, Ates-Alagoz Z, Brodie AM, Njar VC. 2011. Synthesis and biological evaluations of putative metabolically stable analogs of VN/124–1 (TOK-001): head to head anti-tumor efficacy evaluation of VN/124–1 (TOK-001) and abiraterone in LAPC-4 human prostate cancer xenograft model. Steroids 76:1268–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Narayanan R, Yepuru M, Szafran AT, Szwarc M, Bohl CE, Young NL, Miller DD, Mancini MA, Dalton JT. 2010. Discovery and mechanistic characterization of a novel selective nuclear androgen receptor exporter for the treatment of prostate cancer. Cancer Res 70:842–851 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.