Abstract
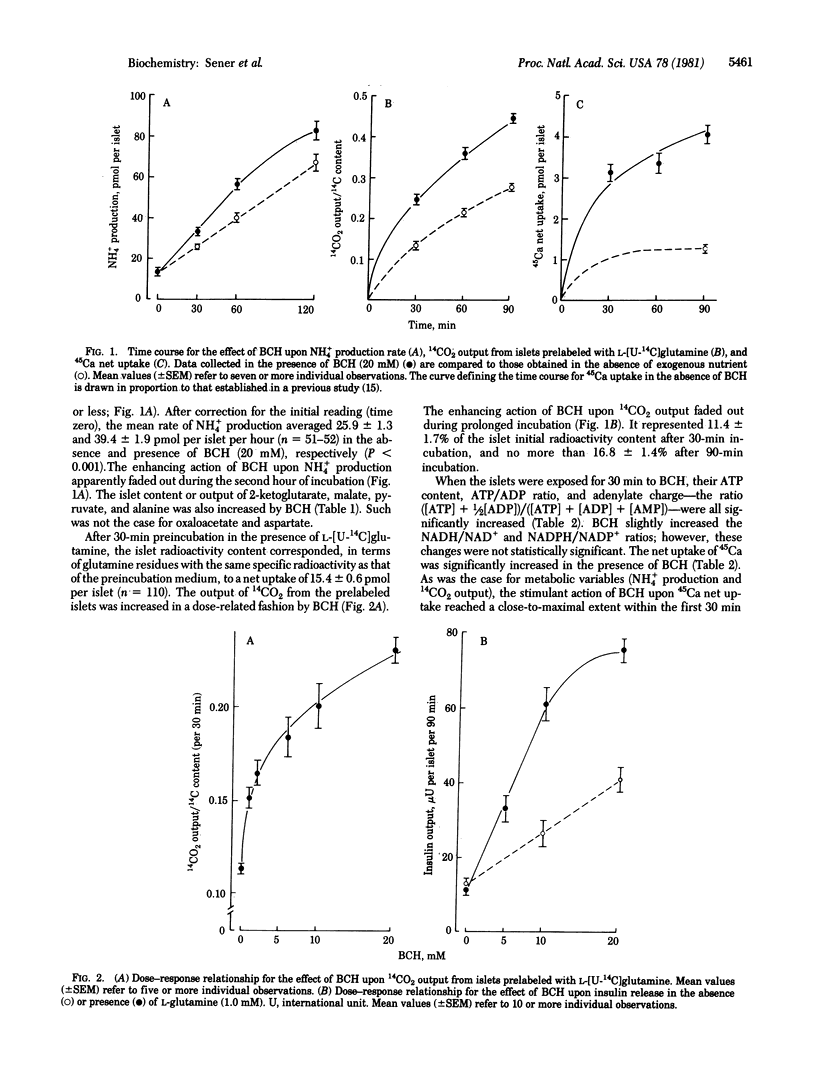
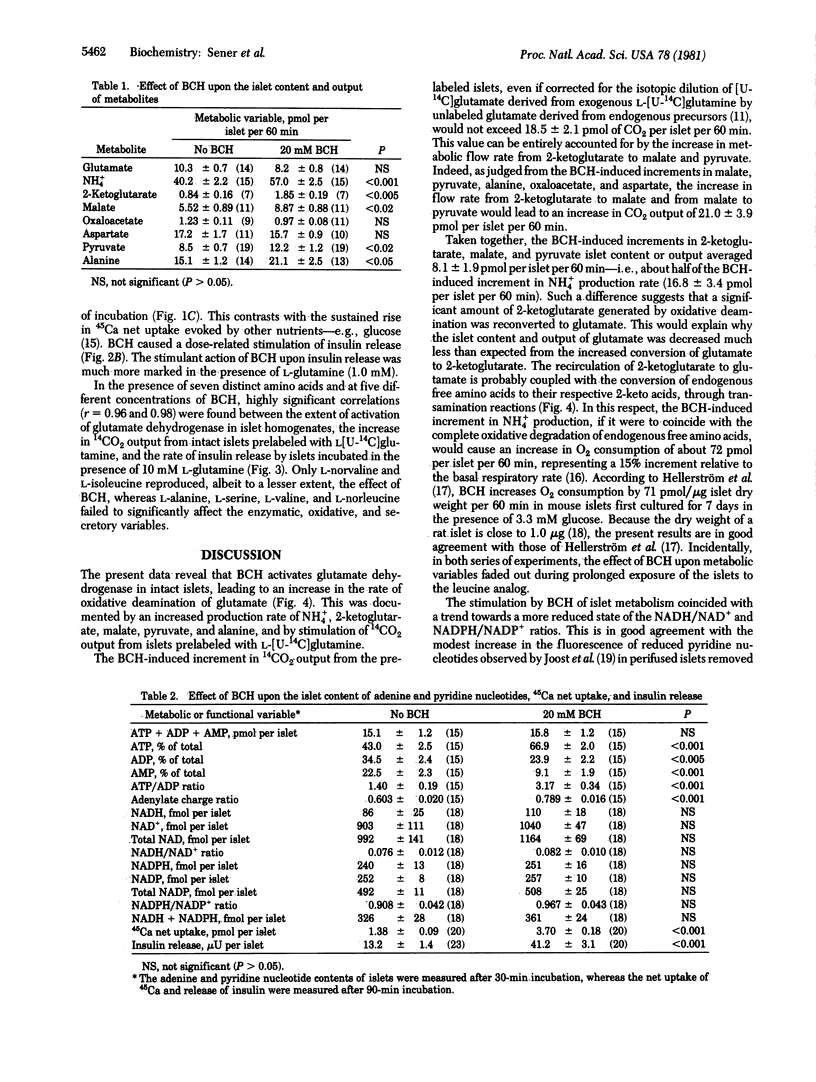
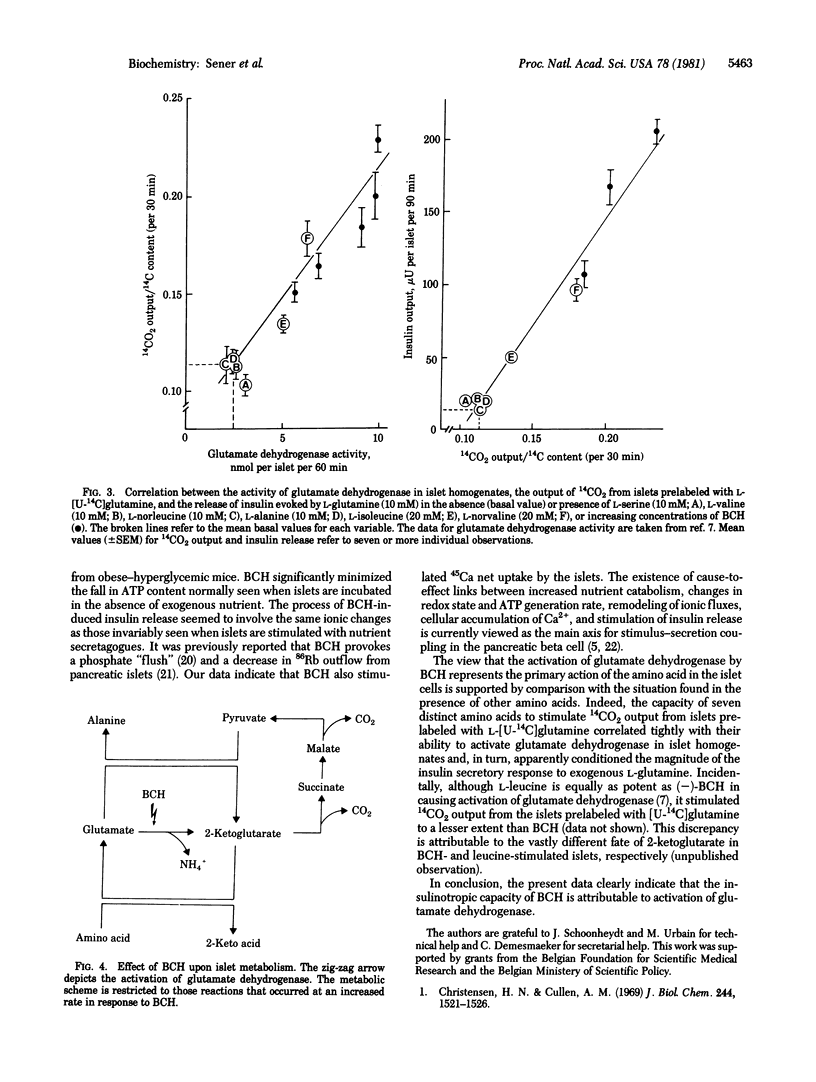
The leucine analog beta-2-aminobicyclo[2.2.1]heptane-2-carboxylic acid (BCH) activates glutamate dehydrogenase [L-glutamate:NAD+ oxidoreductase (deaminating), EC 1.4.1.2] in pancreatic islet homogenates. In intact islets, BCH increased the islet content or output of NH4+, 2-ketoglutarate, malate, pyruvate, and alanine. BCH caused a dose-related increase in 14CO2 output from islets prelabeled with L-[U-14C]glutamine. BCH increased the islet content of ATP and stimulated both 45Ca net uptake and insulin release. The capacity of seven distinct amino acids to activate glutamate dehydrogenase tightly correlated with their ability to augment 14CO2 output from islets prelabeled with [U-14C]-glutamine and to stimulate insulin release in the presence of L-glutamine. The activation of glutamate dehydrogenase by BCH may thus account for the insulin-releasing capacity of the leucine analog.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J. Glucoreceptor mechanisms and the control of insulin release and biosynthesis. Diabetologia. 1980 Jan;18(1):5–15. doi: 10.1007/BF01228295. [DOI] [PubMed] [Google Scholar]
- Christensen H. N., Cullen A. M. Behavior in the rat of a transport-specific, bicyclic amino acid. Hypoglycemic action. J Biol Chem. 1969 Mar 25;244(6):1521–1526. [PubMed] [Google Scholar]
- Christensen H. N., Hellman B., Lernmark A., Sehlin J., Tager H. S., Täljedal I. B. In vitro stimulation of insulin release by non-metabolizable, transport-specific amino acids. Biochim Biophys Acta. 1971 Aug 13;241(2):341–348. doi: 10.1016/0005-2736(71)90034-4. [DOI] [PubMed] [Google Scholar]
- Fajans S. S., Quibrera R., Pek S., Floyd J. C., Jr, Christensen H. N., Conn J. W. Stimulation of insulin release in the dog by a nonmetabollizable amino acid. Comparison with leucine and arginine. J Clin Endocrinol Metab. 1971 Jul;33(1):35–41. doi: 10.1210/jcem-33-1-35. [DOI] [PubMed] [Google Scholar]
- Freinkel N., Younsi C. E., Dawson R. M. Insulin release and phosphate ion efflux from rat pancreatic islets induced by L-leucine and its nonmetabolizable analogue, 2-aminobicyclo[2.2.1]heptane-2-carboxylic acid. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3403–3407. doi: 10.1073/pnas.73.10.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerström C., Andersson A., Welsh M. Respiration of the pancreatic B-cell: effects of glucose and 2-aminonorbornane-2-carboxylic acid. Horm Metab Res Suppl. 1980;Suppl 10:37–43. [PubMed] [Google Scholar]
- Hutton J. C., Malaisse W. J. Dynamics of O2 consumption in rat pancreatic islets. Diabetologia. 1980 May;18(5):395–405. doi: 10.1007/BF00276821. [DOI] [PubMed] [Google Scholar]
- Joost H. G., Panten U., Ishida H., Poser W., Hasselblatt A. Effects of bicyclic leucine analogues on insulin release from the perfused rat pancreas and fluorescence of reduced pyridine nucleotides from perifused pancreatic islets. Life Sci. 1975 Jan 15;16(2):247–254. doi: 10.1016/0024-3205(75)90022-3. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Malaisse-Lagae F., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. 3. Uptake of 45 calcium by isolated islets of Langerhans. Endocrinology. 1971 Jan;88(1):72–80. doi: 10.1210/endo-88-1-72. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Brisson G., Malaisse-Lagae F. The stimulus-secretion coupling of glucose-induced insulin release. I. Interaction of epinephrine and alkaline earth cations. J Lab Clin Med. 1970 Dec;76(6):895–902. [PubMed] [Google Scholar]
- Malaisse W. J., Hutton J. C., Kawazu S., Sener A. The stimulus-secretion coupling of glucose-induced insulin release. Metabolic effects of menadione in isolated islets. Eur J Biochem. 1978 Jun 1;87(1):121–130. doi: 10.1111/j.1432-1033.1978.tb12357.x. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Hutton J. C., Sener A., Levy J., Herchuelz A., Devis G., Somers G. Calcium antagonists and islet function: VII. Effect of calcium deprivation. J Membr Biol. 1978 Jan 18;38(3):193–208. doi: 10.1007/BF01871922. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Sener A., Carpinelli A. R., Anjaneyulu K., Lebrun P., Herchuelz A., Christophe J. The stimulus-secretion coupling of glucose-induced insulin release. XLVI. Physiological role of L-glutamine as a fuel for pancreatic islets. Mol Cell Endocrinol. 1980 Nov;20(2):171–189. doi: 10.1016/0303-7207(80)90080-5. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Sener A., Herchuelz A., Hutton J. C. Insulin release: the fuel hypothesis. Metabolism. 1979 Apr;28(4):373–386. doi: 10.1016/0026-0495(79)90111-2. [DOI] [PubMed] [Google Scholar]
- Sener A., Kawazu S., Hutton J. C., Boschero A. C., Devis G., Somers G., Herchuelz A., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. Effect of exogenous pyruvate on islet function. Biochem J. 1978 Oct 15;176(1):217–232. doi: 10.1042/bj1760217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sener A., Malaisse W. J. L-leucine and a nonmetabolized analogue activate pancreatic islet glutamate dehydrogenase. Nature. 1980 Nov 13;288(5787):187–189. doi: 10.1038/288187a0. [DOI] [PubMed] [Google Scholar]
- Sener A., Malaisse W. J. The metabolism of glucose in pancreatic islets. Diabete Metab. 1978 Jun;4(2):127–133. [PubMed] [Google Scholar]


