Abstract
Most diseases, injuries, and other health conditions experienced by working people are multifactorial, especially as the workforce ages. Evidence supporting the role of work and personal risk factors in the health of working people is frequently underused in developing interventions. Achieving a longer, healthy working life requires a comprehensive preventive approach. To help develop such an approach, we evaluated the influence of both occupational and personal risk factors on workforce health. We present 32 examples illustrating 4 combinatorial models of occupational hazards and personal risk factors (genetics, age, gender, chronic disease, obesity, smoking, alcohol use, prescription drug use). Models that address occupational and personal risk factors and their interactions can improve our understanding of health hazards and guide research and interventions.
Work and workplace hazards are known to compromise the health of workers and represent a significant national financial, social, medical, and emotional burden, but health is also affected by an array of individual risk factors such as genetics, age, gender, obesity, smoking, alcohol use, and the use of prescription drugs.1,2 Despite their awareness of these hazards, decision-makers and stakeholders do not strongly emphasize taking a holistic view of the health of working people.
Historically, work has been compartmentalized from other human activities. This separation is in part because of legislative limitations with respect to worker safety and health and the practice of limiting liability and determining the cause of injury or illness among workers.3 Although some work-related conditions are de facto triggers for compensation in various jurisdictions and the historical practice has been to take workers “as is” (with existing disabilities and propensities for injury), some compensation and tort systems apportion the cause of an injury or illness among various work-related and non-work-related causes and compensate only work-related causes.4,5 However, determining the extent to which workers' illnesses or disabilities are influenced by work and nonwork factors is not a precise science.
THE PROBLEM
Most of the diseases, injuries, and other health conditions experienced by working people are multifactorial. The underlying evidence for the role of various risk factors in the overall health of working people is frequently underused in developing interventions, and most research focuses on a single risk factor through the lens of a single discipline or topic. For example, an investigator interested in smoking may treat all other factors as confounders or effect modifiers when assessing smoking–disease relationships. Thus, smoking is the primary focus, and the overall impact of all risk factors is not directly considered or studied.
Similarly, in assessing workplace risk factors, personal risk factors (PRFs) are treated as confounders or sources of bias, and the complete range of workplace risk factors and PRFs that affect the health of working people are rarely comprehensively studied. This is partly because society tends to appropriate resources to address certain specific problems such as smoking, drinking, and occupational disease. Rarely do societal programs focus on research and interventions addressing the composite effect of those risk factors.
Understanding the interactions between risk factors may help to target and determine the effectiveness of health protection and health promotion interventions. Specific problem-driven research focuses on a marginal effect that is averaged over the other risk factors in a given context. Such problem-driven research, although beneficial in understanding a specific risk factor, has led to a lack of comprehensive research on the combined role of PRFs and occupational risk factors (ORFs) in work-related illness and injury. ORFs and PRFs are not only potential confounders or effect modifiers of associations of each risk factor with disease, but they may also be on a causal pathway to each other. For example, shift work may be associated with higher rates of obesity or smoking, or the use of prescription drugs may interact with workplace chemical exposures in affecting various organ systems.
To isolate the effects of risk factors, epidemiologists usually study them in isolation while assuming that other factors are constant or ensuring that they are part of a uniformly distributed background (and hence they are disregarded in terms of interfering with the assessment of this single factor). One challenge in epidemiological research is to identify major modifying factors when they are not uniformly distributed.6 Determination of effect modification requires analyses that include interaction terms in statistical models or stratification based on candidate variables. Identifying effect modification is important because failure to do so can lead to misinterpretation of exposure–disease relationships and to inefficiencies, including incorrect targeting, in developing interventions.7,8
The overarching rationale for considering the interaction of ORFs and PRFs is that the health of the contemporary workforce is critical to the well-being of the nation and its international competitiveness.9–11 The growing burden of illness and injury and the subsequent increased use of health care services are driving up health care costs.12 Ultimately, the impact of shortages in skilled labor and rising health care costs on productivity and profitability can affect business and national economic health.2 Many developed nations with an aging population face the challenge of increasing workforce participation, especially among older workers.13 As a means of meeting this challenge, governmental policies are being implemented to increase the age of full retirement to balance the ratio of dependent to employed individuals (the dependency ratio).14
We address various ways in which ORFs and PRFs can combine or interact and develop a conceptual approach to describing the interaction of these 2 types of risk factors among workers. The goal is to begin to develop a theoretical framework for considering the health of working people in a comprehensive manner.
THE INITIAL FRAMEWORK
We used 4 basic conceptual models to evaluate the relationships among ORFs, PRFs, and disease outcomes (including both illness and injury) in working populations. The roles of PRFs and ORFs in causing illness and injury can be very complex. We focus on an initial framework in which to consider issues associated with these 2 risk factor categories.
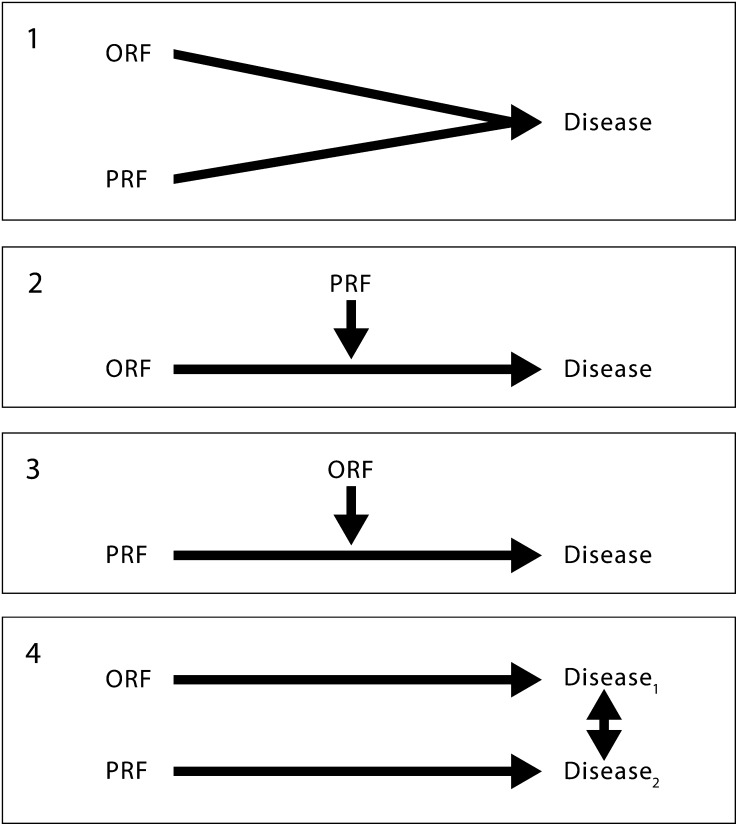
The models presented here are not meant to delineate specific molecular-, cellular-, or organ-level etiological steps; epidemiological mechanisms; or statistical relationships with respect to the diseases discussed. Rather, we developed these models to describe theoretical frameworks through which PRFs and ORFs affect health outcomes (Figure 1). They were adapted from previous work,15,16 specifically the work of Ottman17 on gene–environment interactions. Conceptual models have been used in epidemiology to represent causal relations among factors, to identify potential confounders or sources of bias, and to categorize effect modifiers.18–22
FIGURE 1—
Conceptual models delineating PRF and ORF effects.
Note. ORF = occupational risk factor; PRF = personal risk factor. Model 1 depicts a model in which the PRF and ORF are independent of each other with respect to their impact on disease. Models 2, 3, and 4 present a framework in which PRFs and ORFs can have interaction effects on disease. In models 2 and 3, PRFs and ORFs affect the same disease or disease stage, whereas in model 4 risk factors can affect different diseases or disease stages that can affect each other or disease stages that can exacerbate or compound the disease. In some cases, placement of examples in one model versus another can change on the basis of scientific information or interpretation of that information. References to disease may also include injury.
The 8 PRFs assessed were genetics, age, gender, chronic disease, obesity, smoking, alcohol use, and prescription drug use. We selected these PRFs because they represent common risk factors for various diseases. They are not meant to be exhaustive but to illustrate how most PRFs can be assessed with respect to their interaction with ORFs.
LITERATURE SEARCH STRATEGY
We employed a 2-stage search strategy for identifying examples of PRFs and ORFs. Initially, we searched combinations of general ORFs, PRFs, and work-related terminology in all fields using the PubMed database; the search strategy terminology is listed in Table 1.
TABLE 1—
Initial Search Strategy for Evaluating the Literature on Occupational and Personal Risk Factors for Occupational Illness and Injury
| PRF Category | Previous 2 Years | Previous 5 Years | All Yearsa |
| Genetics | Genetics and work | ||
| Age | Age and work | ||
| Gender | Gender and work | ||
| Chronic disease | Stress and work: terms in title only (preceding 3 y); work-related diseases, injuries (preceding y only) | Chronic diseases/conditions and work; stress, acute and work; stress, chronic and work | Preexisting conditions, occupational exposure, occupational diseases and work; preexisting conditions (terms in title/abstract) and workplace terms in title field only |
| Obesity | Exercise, occupational exposure, occupational diseases and work; physical fitness, occupational exposure, occupational diseases and work | ||
| Smoking | Smoking, occupational exposure, occupational diseases and work | Smoking and work: terms in title field only | |
| Alcohol | Alcohol and work (title field only) | ||
| Prescription drug use | Prescription drugs, occupational exposure, occupational diseases and work; prescription drugs and work: terms in title field only |
Note. PRF = personal risk factor. Literature searches for each personal risk factor combined with various occupational risk factors were conducted. Search strategies differed in number of years back in the literature the search was conducted, and this was dictated by individual characteristics of the PRF or occupational risk factor being searched. General searches refer to search terms that were not specific to any single PRF.
General searches for all years included the following terms: work, work-related, workplace, worksite; workforce, workers; occupation, occupations, occupational; employment, employee; job; health behaviors and work (title field only); lifestyle and work (title field only); multifactorial etiologies, occupational exposures, occupational diseases and work.
We then identified articles addressing specific diseases and disease processes through further investigation of primary sources in relevant journal articles and review articles, again using all fields in the PubMed database. This next stage of the literature search focused on specific hazards and health effects terms derived from articles identified in the first stage of the search.
We based the examples used to illustrate each type of model for each PRF examined on studies that were peer-reviewed, original research articles; meta-analyses; or systematic reviews of studies that tested hypotheses and noted statistically significant effect sizes based on relative risks or odds ratios.
THE MODELS
According to model 1 (Figure 1), a PRF and an ORF can both cause the same disease with possibly independent effects. Here we define an independent effect to mean that a given level of effect is seen if there is no relationship other than an additive one between the 2 sets of factors that cause a particular outcome.23 Examples for model 1 may be transitory because further research might suggest that other models are more suitable.
Models 2 and 3 conceptualize ORFs and PRFs, alternately, as effect-modifying variables that affect a disease association. Thus, in an ORF–disease association a PRF would be an effect modifier. Conversely, a PRF–disease association could be modified by an ORF. Model 4 illustrates the situation in which ORFs and PRFs affect different diseases or disease stages with subsequent interactions between multiple diseases or disease stages.
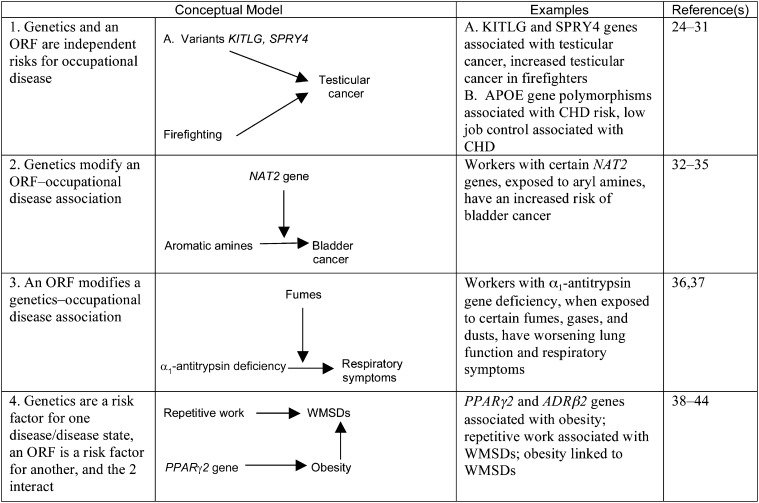
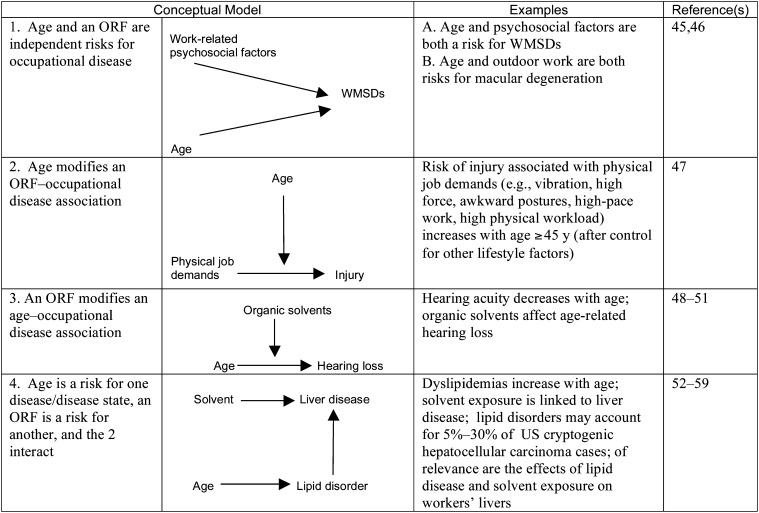
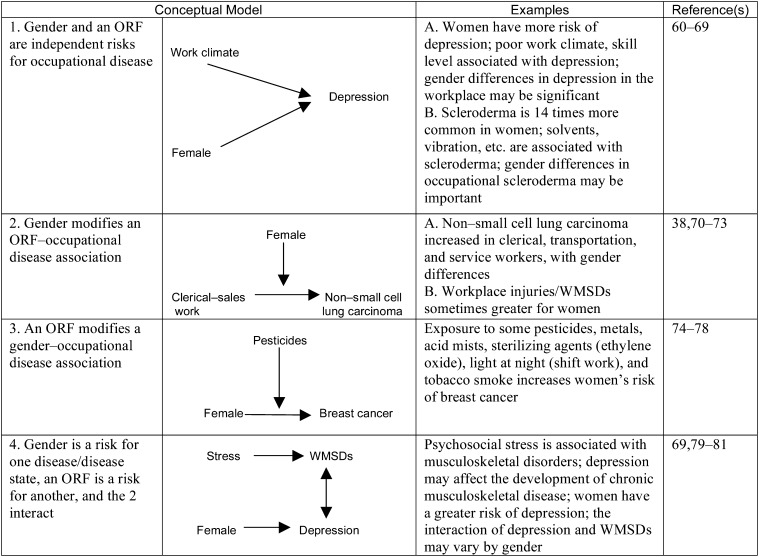
Models 2, 3, and 4 can all contain interaction effects of risk factors on outcomes. We define an interaction effect to mean that a given magnitude of effect would be observed if there is a relationship different from an additive one between the 2 sets of factors. Although many interaction effects may be important in a given model, not all interaction effects have meaningful consequences. This concept is important in situations in which ORFs and PRFs interact with each other but such interactions have only minimal effects on disease outcomes. Inclusion of such interactions may allow more accurate characterization and description of disease mechanisms. For each of the 8 PRFs, 4 models of interaction with ORFs are identified in Figures 2 through 9.15,24–164 In the case of each PRF considered, these figures present examples with descriptions and references for each type of model.
FIGURE 2—
Examples of 4 Conceptual Models of the Relationships Between Genetics and Occupational Risk Factors
Note. CHD = coronary heart disease; ORF = occupational risk factor; WMSD = work-related musculoskeletal disease. Bidirectional and unidirectional arrows indicate flow of effect in the models exemplified.
FIGURE 3—
Examples of 4 Conceptual Models of the Relationships Between Age and Occupational Risk Factors
Note. ORF = occupational risk factor; WMSD = work-related musculoskeletal disease. Bidirectional and unidirectional arrows indicate flow of effect in the models exemplified.
FIGURE 4—
Examples of 4 Conceptual Models of the Relationships Between Gender and Occupational Risk Factors
Note. ORF = occupational risk factor; WMSD = work-related musculoskeletal disease. Bidirectional and unidirectional arrows indicate flow of effect in the models exemplified.
FIGURE 5—
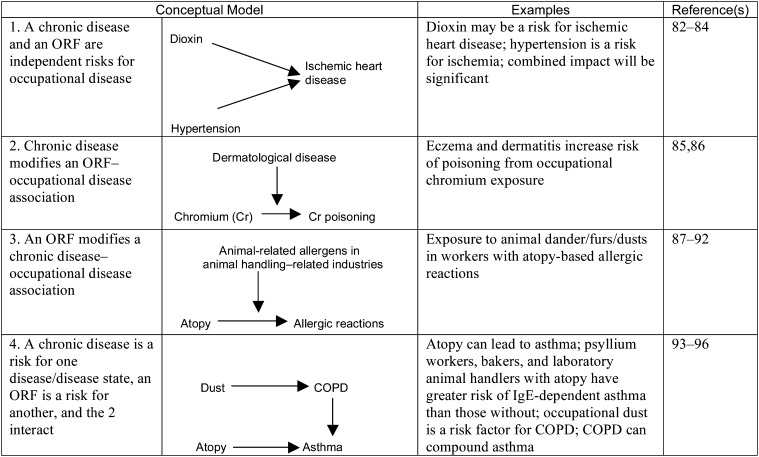
Examples of 4 Conceptual Models of the Relationships Between Chronic Disease and Occupational Risk Factors
Note. COPD = chronic obstructive pulmonary disease; ORF = occupational risk factor. Bidirectional and unidirectional arrows indicate flow of effect in the models exemplified.
FIGURE 6—
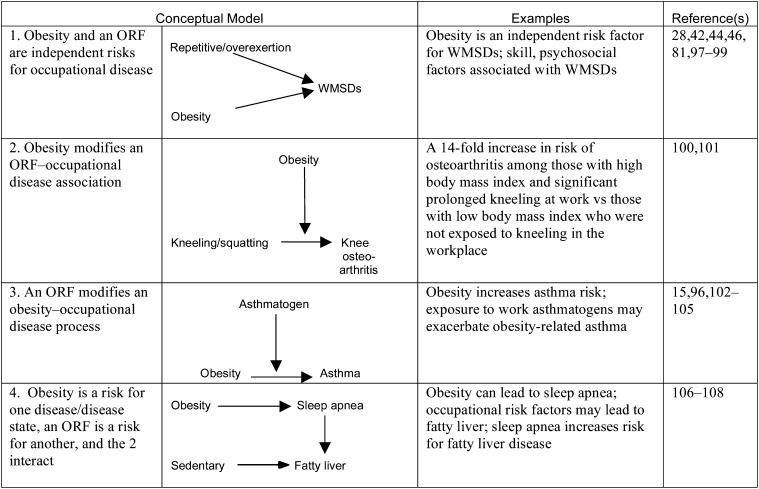
Examples of 4 Conceptual Models of the Relationships Between Obesity and Occupational Risk Factors
Note. ORF = occupational risk factor; WMSD = work-related musculoskeletal disease. Bidirectional and unidirectional arrows indicate flow of effect in the models exemplified.
FIGURE 7—
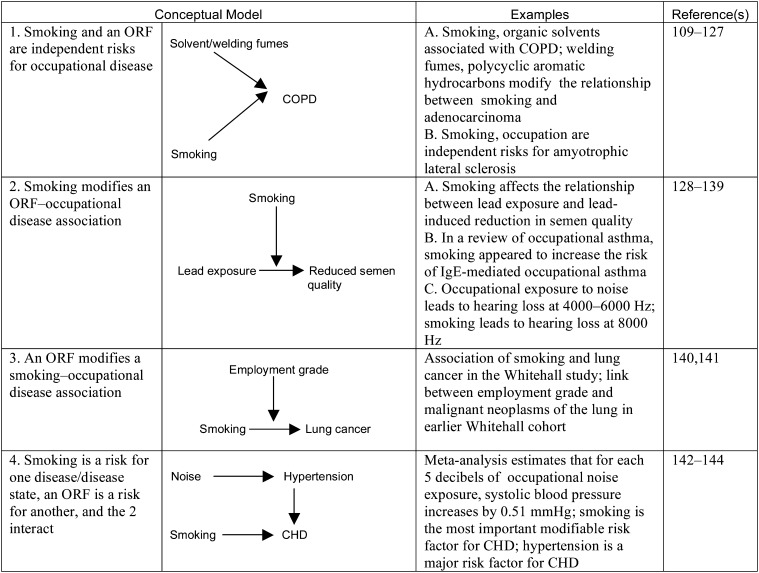
Examples of 4 Conceptual Models of the Relationships Between Smoking and Occupational Risk Factors
Note. CHD = coronary heart disease; COPD = chronic obstructive pulmonary disease; ORF = occupational risk factor. Bidirectional and unidirectional arrows indicate flow of effect in the models exemplified.
FIGURE 8—
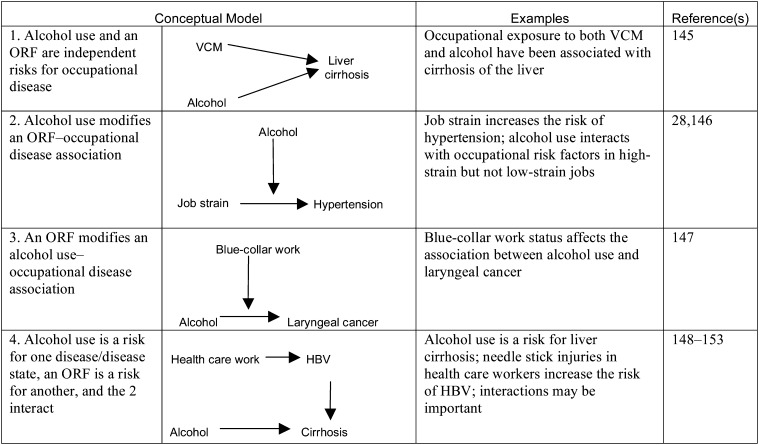
Examples of 4 Conceptual Models of the Relationships Between Alcohol Use and Occupational Risk Factors
Note. HBV = hepatitis B virus; ORF = occupational risk factor; VCM = vinyl chloride monomer. Bidirectional and unidirectional arrows indicate flow of effect in the models exemplified.
FIGURE 9—
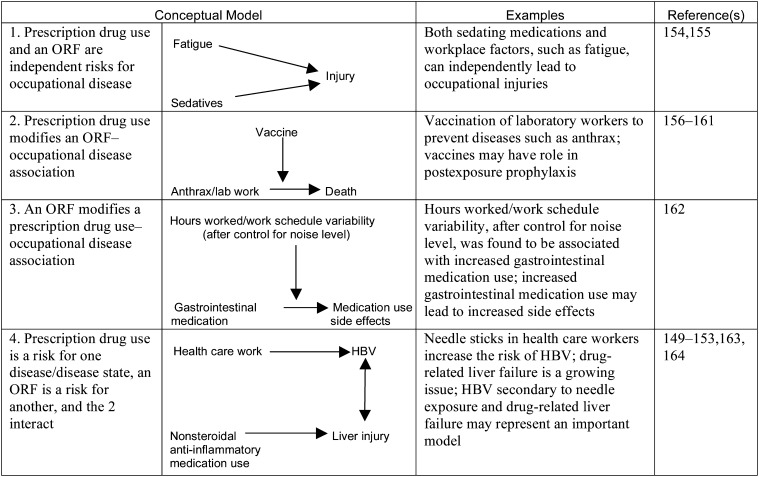
Examples of 4 Conceptual Models of the Relationships Between Prescription Drug Use and Occupational Risk Factors
Note. HBV = hepatitis B virus; ORF = occupational risk factor; PRF = personal risk factor. Bidirectional and unidirectional arrows indicate flow of effect in the models exemplified.
Genetics
Inherited genetic factors can contribute to variable responses of workers to occupational hazards.165–171 In most cases, inherited genetic factors alone may not lead to adverse outcomes; however, such factors combined with an occupational risk factor can alter risk.
For example, among chemical industry workers, polymorphism in the NAT2 gene itself does not cause bladder cancer, but in workers with a particular NAT2 genotype, exposure to aromatic amines increases the risk of bladder cancer.33,34,36 Furthermore, other NAT2 polymorphisms may be protective for bladder cancer in workers exposed to benzidine in the absence of other aryl amine exposures (such as 2-naphthylamine or 4-aminobiphenyl), indicating that genetic variations in the same gene can have different effects on a disease outcome according to exposure and mechanism of action.172 Increasingly, patterns of genes within genomes may be associated with various PRF and ORF combinations.173,174
Age
Age is a widely studied effect modifier with a complex biology.175 Age influences people's susceptibility to disease or dysfunction. Generally, the incidence of disease increases with age, but aging and disease are not synonymous.176 Aging can influence workers' susceptibility or resistance to various hazards. Becker et al.45 presented data supporting age, among other factors, as an independent risk factor for work-related musculoskeletal diseases. Factors such as high perceived job stress and non-work-related stress may be strongly associated with these diseases as well.47 In the complex disease process involved in work-related musculoskeletal diseases, modeling the effects of age and psychosocial work factors on disease outcomes (Figure 3) illustrates a way to refine targets for intervention and prevention.
The variable development of occupational illness and injury according to age will have implications for disease prevention in an aging workforce. In particular, given the societal and economic pressures of maintaining working populations with larger and larger numbers of older workers, understanding the role of age in the development of disease and the subsequent impact on occupational illness and injury will be crucial in early disease intervention, health promotion, and workplace interventions for aging workers.
Gender
Similar to age, gender has often been used to stratify the workforce into subpopulations with different ORF–disease risk profiles. Despite its importance, epidemiological studies often ignore the impact of gender, although within occupations the magnitude of an ORF can vary by gender.74,177 Classic occupational epidemiology has paid less attention to women's health issues. Recent studies have begun including gender interactions, but more effort is needed in this regard.178 Rarely have studies taken into account the potential interactions between gender, social class, employment status, and family roles.179 In general, methods have not been systematized or used to quantify gender differences in clinical research.180
Chronic Disease
All individuals enter the workplace with a set of characteristics that may affect their vulnerability to occupational risk factors. These characteristics may include a broad range of chronic diseases, many of which vary according to the age of the employee (Table 2).181a Some chronic diseases, such as hypertension, also can be risk factors for other diseases such as ischemic heart disease.
TABLE 2—
Common Chronic Diseases or Conditions Present in the Workforce, by Age
| Age < 45 Years | Age 45–54 Years | Age 55–64 Years | Age Not Specified |
| Asthma | Stress | Coronary heart disease | Allergies |
| Depression | Hypertension | Respiratory infections | |
| Anxiety | Arthritis | Migraines/other headaches | |
| Musculoskeletal pain | Bipolar disorder (with depression) | ||
| Diabetes | Hypercholesterolemia (with coronary heart disease) | ||
| Cancer |
It is likely that some chronic conditions, such as skin diseases (e.g., eczema, psoriasis) and autoimmune disorders (e.g., inflammatory bowel disease), are undercounted yet constitute a significant disease burden in the workforce. Coexisting conditions may interact with occupational risk factors.182 Workers are often healthier than the rest of the population, in part because continued employment requires good health or the development of disease causes workers to leave employment.183–185 This “healthy worker effect” may influence the study of risk factor interactions, with workers having lower rates of chronic disease than the population at large has. One US study suggested that, after adjustment for this effect, the exposure–outcome association (in this case, the association between arsenic and ischemic heart disease) became stronger with a statistically significant increasing trend.186
Obesity
Obesity is rapidly increasing in most developed countries. It is a contributing factor in cardiovascular disease, diabetes, asthma, some cancers, and many other diseases and is associated with workplace absenteeism and reduced productivity.187–189 Obesity appears to have genetic and environmental determinants.190 Lack of physical activity and high consumption of energy-dense foods are the primary causes of obesity.
Occupational hazards and obesity are part of a complex matrix of risk factors that are a function of technological development as well as social, economic, and demographic factors. Numerous studies have reported increases in body weight among shift workers.15,191 In addition, a relatively large number of studies have demonstrated the association of job stress with body mass index.15,192,193 Long work hours have also been associated with higher body mass indexes.191,193 Obesity has been related to decreased participation in the workforce and other life activities.194
Smoking
Smoking is an extremely significant determinant of adverse health outcomes. It has been shown to be an independent variable, a confounder, and an effect modifier in occupational epidemiological relationships.195–201 Smoking as an exposure is a risk factor for many diseases, including heart disease and cancer.
In the workplace, shift work has been shown to affect workers' rates of smoking.196 Other occupational risk factors demonstrated to affect smoking rates include work at sea,197 construction and cleaning work,198 and work-related stress.199 Quantification of the role of smoking in occupational health has been difficult but is becoming increasingly exact.200,201 Smoking may also exert a healthy worker effect, with workers who have a stronger smoking history and possibly shorter work lives affecting the interpretation of studies of interactions with ORFs.
Alcohol Use
Alcohol consumption is highly prevalent in many countries and is associated with extensive morbidity and mortality.202,203 It has been estimated that alcohol misuse contributes extensively to lost workdays and lost productivity.204 Workplace harassment has been reported to lead to alcohol misuse,205 suggesting that occupational hazards can lead to increased alcohol consumption. In addition to alcohol consumption as a general risk factor, an estimated 8.9 million workers in the United States consume alcohol during the workday and 2.3 million do so before beginning the workday.206 Because of the significant health effects of alcohol consumption, loss of workforce members as a result of alcohol use may be another healthy worker effect that influences the understanding of interactions among personal and occupational hazards.
Prescription Drug Use
Prescription drug use has the potential to interact with ORFs, but detailed knowledge of its occupational safety and health impact remains limited. This interaction also may be related to the widespread presence of prescription drug use in developed and developing societies.207 An aging workforce can be expected to have an increased need for acute and chronic pharmacological regimens.207 Prescription drug use (the exposure, or PRF) can thus possibly lead to a range of occupational outcomes.
The PRFs associated with prescription drug use may reflect adverse side effects from single medications, interactions between drugs, or polypharmacy. For example, workplace musculoskeletal trauma can lead to greater consumption of prescription nonsteroidal anti-inflammatory agents and narcotics, increasing the risk of adverse side effects from these drugs.208
Exposures during the manufacturing and processing of drugs can lead to adverse outcomes such as allergy and urticaria.209 An additional factor for pharmaceutical industry workers is the impact of concomitant exposures to chemicals that can interact with prescribed drugs. Rates of prescription drug use vary by occupation, with high-stress occupations associated with increased use of psychotropic prescription drugs.210 Use of prescription medicine can also ameliorate the effects of occupational risk factors.211 A high-stress job can exacerbate hypertension, but workers' use of antihypertensive medications can result in work performance improvements and reductions in absenteeism.212
A precondition may lead to both the use of a prescription drug and an adverse outcome, the so-called effect modification by proxy.21 The drug is not the cause of the adverse outcome but is a modifier, by proxy, of the effect of interest.213 Differentiation of true effect modifiers from effect modifiers by proxy will be a critical issue in evaluating prescription drugs as PRFs, and future research should take this into consideration.
OVERVIEW
We have described and illustrated 4 conceptual models for the evaluation of the role of ORFs and PRFs in the development of disease. At an initial stage, a model theorizing an isolated ORF–disease relationship is potentially useful in developing interventions in the workplace. The seemingly reasonable nature of such a model, however, is perhaps a result mainly of the state of knowledge at a given point in time. In the case of many diseases, such a rudimentary model is inappropriate because the relationship between risk factors and disease is shown by epidemiological and other data to be complex.
Model 1 represents independent effects of ORFs and PRFs on outcomes. Some examples of such effects, such as the roles of genetic variants in testicular cancer and the link between firefighting and testicular neoplasms, highlight the potential independent action of PRFs and ORFs on certain diseases. However, other examples used to illustrate model 1 (Figures 2–9), such as the effects of fatigue and sedatives on workplace injuries, could easily require more complex modeling frameworks as research provides new insights. This potentiality underscores the fluidity required in such modeling efforts.
Models 2 and 3 represent interaction effects (effect modification) of ORFs and PRFs in the etiology of occupational disease. Both of these models illustrate effect modification. Assessing effect modification may be useful in at least 3 ways.7,8,21,22 First, understanding effect modification may define subgroups most in need of intervention. Second, effect modification may help elucidate how the joint biological effects of 2 exposures inhibit or enhance each other. Third, effect modification may reveal different mechanisms. For example, as discussed in the section on genetics, recent investigations have suggested that certain aromatic amines, such as benzidine, use genetic pathways for the development of cancer that are different from those of other compounds such as 2-naphthylamine and 4-aminobiphenyl.
Model 4 represents more complicated effects of ORFs and PRFs on occupational illness and injury. Although our examples focus on model 4 in its basic form, this model can also be described in an expanded format, which enables consideration of more complex relationships between ORFs and PRFs. For example, in some contexts, obesity can be considered a PRF that is a result of an ORF, such as shift work, that in turn can subsequently interact with that same ORF to affect another adverse outcome, such as cardiovascular endpoints. However, presenting examples of the expanded version of this model and discussing in detail various other statistical analysis issues were beyond the scope of the present work.
Future work should explore statistical and epidemiological considerations of basic and complex modeling approaches and issues associated with interactive effects. Other areas of investigation should include the role of particular statistical approaches in analyzing models with PRFs and ORFs, including, but not limited to, the utility of various regressions and other techniques. Assessment of the etiological fraction or relative strength of PRFs and ORFs was also beyond the scope of this study.
A Comprehensive Approach
From the vantage point of public health in the workplace, different exposures to workplace hazards leading to multiple adverse outcomes compound the medical burden on individual workers as well as the burden on the workforce as a whole.1 The modeling of independent versus interactive effects of ORFs and PRFs on occupational illness and injury is the initial step in the process of defining the causes of illness and injury. In examining mechanisms, risk factors, or outcomes, modeling of this nature represents a theoretical framework for a comprehensive approach to the overall health of working people.
The bulk of our investigation involved assessing the scientific literature to obtain examples of the relationship of 8 PRFs (genetics, age, gender, chronic disease, obesity, smoking, alcohol use, and prescription drug use) to disease, individually and in relation to work, with a general focus on epidemiological studies. It should be noted that, in several cases, applying selection criteria to articles that might conventionally be considered disparate but were found to be relevant to a particular disease process illustrated the potential to link different domains of information to model ORFs, PRFs, and occupational disease and develop new hypotheses for subsequent analyses.
Although the examples we identified were not necessarily the only possible interactions with a particular PRF, or even the most important from a clinical or public health perspective, they do illustrate a range of important health conditions, many with significant societal burdens. Furthermore, these examples provide a roadmap for melding scientific and clinical knowledge that may have been divided by disciplinary boundaries so that we can develop broader models of occupational illness and injury.
The placement of an ORF, PRF, and disease process grouping in a particular model is also subject to the current level of scientific knowledge. This issue is reflected in various models for several of the PRFs presented here, including gender and prescription drugs. More research evaluating the impact of PRFs on the relationship of ORFs to occupational disease is needed. For example, given the extensive acute and chronic use of pharmacological agents in modern society, there is a need for studying the impact of this PRF and its role as an independent or modifying variable, which has significant implications for modern occupational health.
In the example of the role of smoking and noise in coronary heart disease shown in Figure 7 (model 4), differences in disease mechanism may exist, with interventions and health promotions varyingly affected. However, more complex models incorporating multiple PRFs and ORFs may be more informative in identifying high-risk combination scenarios.
There is value in considering both ORFs and PRFs in epidemiological studies. At the design stage of such investigations, methodological issues regarding direct effects, confounding, effect modification, exposure misclassification, and conceptualization of the term “interaction,” from a statistical as well as a biological perspective, should be explored, with rationales and models supported empirically or theoretically. For example, studies of gene–environment interactions, such as recent explorations representing environmental exposures with the concept of the “exposome” (a measure of all exposures of an individual in a lifetime and how those exposures relate to disease) and the interaction of such representations with genetic factors evaluated in genomic-wide association studies, may suggest future contexts in which to further evaluate the nature of interactions between ORFs and PRFs.169,214
These methodological considerations are germane not only to occupational epidemiological and other biological study designs but also to risk evaluation and assessment, interventional paradigms, and health promotion in the workplace. Such design, analysis, intervention, and promotion development requires careful consideration of the occurrence, direction, and magnitude of effects to optimally judge study designs or data relevant to risk or outcome analyses.215 In addition, collection of PRF data will likely increase the cost of research on risk factor interactions. However, a more thorough appraisal of the health of the workforce may lead to more effective interventions.
Other Logistical Considerations
Evaluations of ORFs and PRFs in occupational safety and health research should be reinforced by other logistical considerations. Occupational factors need to be regularly included in medical records, particularly as new electronic record formats are being developed.216 Clinicians may be able to provide a more thorough appraisal of a patient's condition with an occupational history.217 Knowledge of the interaction of risk factors may foster enhanced management of occupational illness and injury. Occupational medicine clinicians may use information about risk factor interactions to better address workplace safety and health problems, particularly with respect to understanding and addressing health issues arising from exposures in the workplace versus those arising from PRFs. From the perspective of general medical practitioners, broader information about PRF and ORF interactions may assist in addressing general health issues in populations in which health prevention and promotion are a major focus.217
Workers' compensation efforts may necessitate a new category of cause, effect, and risk determination with implications for the use of worker's compensation and health care resources. Ethical, legal, and social issues relevant to long-held beliefs and approaches regarding disease causation, compensation, blame, and liability will also need to be considered. In addition, the recent passage of the Patient Protection and Affordable Care Act, which allows employers to offer a health plan premium differential based on employees meeting standards such as not smoking, reaching recommended weight levels, and having normal blood pressure, underscores a variety of ethical, legal, and social issues even as it promotes the broader use of comprehensive health promotion programs in workplaces.218 Nonetheless, with an aging workforce and potential workforce shortages, there is a need to consider a comprehensive approach to the health of the workforce and to invest in studying its ramifications.
Globally, explaining the distribution of health and disease exclusively in terms of risk factors in individuals only partly addresses the health of the workforce. There is a need for contextual or multilevel analyses that address group- or macro-level variables given that various economic, social, cultural, and environmental group-level characteristics have been shown to be strongly related to the health of the workforce.219–223
Modeling that considers both PRFs and ORFs would provide a foundation for an integrated worklife approach that combines protection from workplace hazards and health promotion.181b,224–228 This approach could include, for example, the development of wellness programs at worksites or funded by employers. These types of programs have been demonstrated to result in a positive return on investment for both workers and employers.2,12,229,230 Nonetheless, attention to PRFs and wellness should not be a reason for employers to fail to provide a safe and healthy workplace or to blame workers for occupational health and safety problems.11,15
Conclusions
We have presented 32 examples of disease processes for 8 PRFs and 4 models, demonstrating an extensive catalog of combinations and interactions of ORFs and PRFs among workers. These examples clearly demonstrate the utility of new representations of PRF–ORF combinations and their impact on our understanding of disease with respect to hypothesis generation, study design, risk evaluation and assessment, workplace intervention, clinical evaluation, and health promotion in working populations. The models and examples offered here highlight the value of conceptual representations of relationships between ORFs, PRFs, and disease to drive more fully developed approaches to control occupational illness and injury and develop a comprehensive view of workforce health.
Models that combine ORFs and PRFs contain an inherent flexibility to model greater disease complexity; can guide various stages of epidemiological investigation, data analysis, and intervention development; and possess the capacity to incorporate intricate variables and analyses. Employing models and approaches that maximize consideration of factors impinging on the health of the workforce will allow researchers and practitioners to move beyond the historically fractionated approach to occupational illness and injury.
Thus, a comprehensive approach to the health of working people can form the basis for research and investigation into occupational illness and injury, address issues important for maintaining a healthy workforce despite pressures from factors such as aging and unsustainable dependency ratios, and contribute to the fostering of an integrated work life to better protect worker safety and health and fortify national and societal well-being.
Acknowledgments
We thank the following individuals for comments on earlier versions of this article: Benjamin C. Amick III, A. John Bailer, L. Casey Chosewood, William E. Halperin, Mary K. Schubauer-Berigan, Christine W. Sofge, Paolo Vineis, Elizabeth Ward, and Naomi Swanson. We also acknowledge the efforts of Devin Baker, John Lechliter, Sherry Fendinger, Brenda Proffitt, and Vanessa B. Williams regarding logistical issues in preparation of the article.
Note. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Human Participant Protection
Because no human participants were involved in this research, no protocol approval was needed.
References
- 1.Schulte PA. Characterizing the burden of occupational injury and disease. J Occup Environ Med. 2005;47(6):607–622 [DOI] [PubMed] [Google Scholar]
- 2.Loeppke R. The value of health and the power of prevention. Int J Workplace Health Manag. 2008;1(2):95–108 [Google Scholar]
- 3.Ringen K, Smith WJ. Occupational diseases and equity issues. Va J Nat Resources L. 1983;2:213–231 [Google Scholar]
- 4.Richter J. Taking the worker as you find him. Md J Contemp Leg Issues. 1997;8:189–236 [Google Scholar]
- 5.Poulter SR. Genetic testing in toxic injury litigation: the path to scientific certainty or blind alleys. Jurimetrics. 2001;41:211–239 [Google Scholar]
- 6.Schoenbach VJ, Rosamund WD. Understanding the Fundamentals of Epidemiology: An Evolving Text. Chapel Hill, NC: University of North Carolina; 2000 [Google Scholar]
- 7.Kleinbaum DG, Kupper LL, Morgenstern H. Epidemiologic Research: Principles and Quantitative Methods. New York, NY: John Wiley & Sons Inc; 1982 [Google Scholar]
- 8.Vineis P, Kreibel D. Causal models in epidemiology: past inheritance and genetic future. Environ Health. 2006;5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tompa E. The impact of health on productivity: macro and microeconomic evidence and policy implications. : Sharpe A, Banting K, The Review of Economic Performance and Social Progress 2002: Towards a Social Understanding of Productivity. Vol. 2 Ottawa, Ontario, Canada: Centre for the Study of Living Standards; 2002:181–202 [Google Scholar]
- 10.Davis K, Collins SR, Doty MMet al. Health and productivity among U.S. workers. Issue Brief (Commonw Fund). 2005;856:1–10 [PubMed] [Google Scholar]
- 11.Schulte P, Vainio P. Well-being at work—overview and perspective. Scand J Work Environ Health. 2010;36(5):422–429 [DOI] [PubMed] [Google Scholar]
- 12.American College of Occupational and Environmental Medicine Healthy workforce/healthy economy: the role of health, productivity, and disability management in addressing the nation's health care crisis. J Occup Environ Med. 2009;51(1):114–119 [DOI] [PubMed] [Google Scholar]
- 13.van den Berg TIJ, Elders LAM, de Zwart BCHet al. The effects of work-related and individual factors on the Work Ability Index: a systematic review. Occup Environ Med. 2009;66(4):211–220 [DOI] [PubMed] [Google Scholar]
- 14.Holzmann R. Toward a Reformed and Coordinated Pension System in Europe: Rationale and Potential Structure. Washington, DC: World Bank; 2004 [Google Scholar]
- 15.Schulte PA, Wagner GR, Ostry Aet al. Work, obesity, and occupational safety and health. Am J Public Health. 2007;97(3):428–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulte PA, Wagner GR, Downes A, Miller DB. A framework for the concurrent consideration of occupational hazards and obesity. Ann Occup Hyg. 2008;52(7):555–566 [DOI] [PubMed] [Google Scholar]
- 17.Ottman R. An epidemiologic approach to gene-environment interaction. Genet Epidemiol. 1990;7(3):177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearl J. Causal diagrams for empirical research. Biometrika. 1995;82(4):669–688 [Google Scholar]
- 19.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48 [PubMed] [Google Scholar]
- 20.Hernan MA, Hernandez-Diaz S, Werler MMet al. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;155(2):176–184 [DOI] [PubMed] [Google Scholar]
- 21.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed Philadelphia, PA: Lippincott Williams & Wilkins; 2008 [Google Scholar]
- 22.VanderWeele TJ, Robbins JM. Directed acyclic graphs, sufficient causes, and the properties of conditioning on a common effect. Am J Epidemiol. 2007;166(9):1096–1104 [DOI] [PubMed] [Google Scholar]
- 23.Weinberg CR. Less is more, except when less is less: studying joint effects. Genomics. 2009;93(1):10–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanetsky PA, Mitra N, Vardhanabhuti Set al. Common variation in KITLG and at 5q31.3 predisposes to testicular germ cell cancer. Nat Genet. 2009;41(7):811–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bates MN. Registry-based case-control study of cancer in California firefighters. Am J Ind Med. 2007;50(5):339–344 [DOI] [PubMed] [Google Scholar]
- 26.Bennet AM, Di Angelantonio E, Ye Zet al. Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA. 2007;298(11):1300–1311 [DOI] [PubMed] [Google Scholar]
- 27.Ma F, Fleming LE, Lee DJ, Trapido E, Gerace TA. Cancer incidence in Florida professional firefighters, 1981 to 1999. J Occup Environ Med. 2006;48(9):883–888 [DOI] [PubMed] [Google Scholar]
- 28.Belkic KL, Landsbergis PA, Schnall PL, Baker D. Is job strain a major source of cardiovascular disease risk? Scand J Work Environ Health. 2004;30(2):85–128 [DOI] [PubMed] [Google Scholar]
- 29.Kuper H, Marmot M. Job strain, job demands, decision latitude, and risk of coronary heart disease within the Whitehall II study. J Epidemiol Community Health. 2003;57(2):147–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bosma H, Marmot MG, Hemingway H, Nicholson AC, Brunner E, Stansfeld SA. Low job control and risk of coronary heart disease in Whitehall II (prospective cohort) study. BMJ. 1997;314(7080):558–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosma H, Peter R, Siegrist J, Marmot M. Two alternative job stress models and the risk of coronary heart disease. Am J Public Health. 1998;88(1):68–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kellen E, Zeegers M, Paulussen Aet al. Does occupational exposure to PAHs, diesel and aromatic amines interact with smoking and metabolic genetic polymorphisms to increase the risk on bladder cancer? The Belgian Case Control Study on Bladder Cancer Risk. Cancer Lett. 2007;245(1–2):51–60 [DOI] [PubMed] [Google Scholar]
- 33.Hung RJ, Boffetta P, Brennan Pet al. GST, NAT, SULT1A1, CYP1B1 genetic polymorphisms, interactions with environmental exposures and bladder cancer risk in a high-risk population. Int J Cancer. 2004;110(4):598–604 [DOI] [PubMed] [Google Scholar]
- 34.Golka K, Prior V, Blaszkewicz M, Bolt HM. The enhanced bladder cancer susceptibility of NAT2 slow acetylators towards aromatic amines: a review considering ethnic differences. Toxicol Lett. 2002;128(1–3):229–241 [DOI] [PubMed] [Google Scholar]
- 35.Vineis P, Marinelli D, Autrup Het al. Current smoking, occupation, N-acetyltransferase-2 and bladder cancer: a pooled analysis of genotype-based studies. Cancer Epidemiol Biomarkers Prev. 2001;10(12):1249–1252 [PubMed] [Google Scholar]
- 36.Ambroise D, Wild P, Moulin JJ. Update of a meta-analysis on lung cancer and welding. Scand J Work Environ Health. 2006;32(1):22–31 [DOI] [PubMed] [Google Scholar]
- 37.Senn O, Russi EW, Imboden M, Probst-Hensch NM. Alpha(1)-antitrypsin deficiency and lung disease: risk modification by occupational and environmental inhalants. Eur Respir J. 2005;26(5):909–917 [DOI] [PubMed] [Google Scholar]
- 38.Hooftman WE, van der Beek AJ, Bongers PM, van Mechelen W. Is there a gender difference in the effect of work-related physical and psychosocial risk factors on musculoskeletal symptoms and related sickness absence? Scand J Work Environ Health. 2009;35(2):85–95 [DOI] [PubMed] [Google Scholar]
- 39.Nordander C, Ohlsson K, Akesson Iet al. Risk of musculoskeletal disorders among females and males in repetitive/constrained work. Ergonomics. 2009;52(10):1226–1239 [DOI] [PubMed] [Google Scholar]
- 40.Roquelaure Y, Ha C, Rouillon Cet al. Risk factors for upper-extremity musculoskeletal disorders in the working population. Arthritis Rheum. 2009;61(10):1425–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ochoa MC, Marti A, Azcona Cet al. Gene-gene interaction between PPAR gamma 2 and ADR beta 3 increases obesity risk in children and adolescents. Int J Obes Relat Metab Disord. 2004;28(suppl 3):S37–S41 [DOI] [PubMed] [Google Scholar]
- 42.Koleva M, Kostova V. Occupational and personal risk factors for musculo-skeletal disorders in fertilizer plant workers. Cent Eur J Public Health. 2003;11(1):9–13 [PubMed] [Google Scholar]
- 43.Miranda H, Viikari-Juntura E, Martikainen R, Takala EP, Riihimaki H. A prospective study of work related factors and physical exercise as predictors of shoulder pain. Occup Environ Med. 2001;58(8):528–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roquelaure Y, Mariel J, Dano C, Fanello S, Penneau-Fontbonne D. Prevalence, incidence and risk factors of carpal tunnel syndrome in a large footwear factory. Int J Occup Med Environ Health. 2001;14(4):357–367 [PubMed] [Google Scholar]
- 45.Becker J, Nora DB, Gomes Iet al. An evaluation of gender, obesity, age and diabetes mellitus as risk factors for carpal tunnel syndrome. Clin Neurophysiol. 2002;113(9):1429–1434 [DOI] [PubMed] [Google Scholar]
- 46.Bongers PM, Kremer AM, Laak J. Are psychosocial factors, risk factors for symptoms and signs of the shoulder, elbow, or hand/wrist? A review of the epidemiological literature. Am J Ind Med. 2002;41(5):315–342 [DOI] [PubMed] [Google Scholar]
- 47.Chau N, Bhattacherjee A, Kunar BM, Lorhandicap G. Relationship between job, lifestyle, age and occupational injuries. Occup Med (Lond). 2009;59(2):114–119 [DOI] [PubMed] [Google Scholar]
- 48.Fuente A, Slade MD, Taylor Tet al. Peripheral and central auditory dysfunction induced by occupational exposure to organic solvents. J Occup Environ Med. 2009;51(10):1202–1211 [DOI] [PubMed] [Google Scholar]
- 49.Vyskocil A, Leroux T, Truchon Get al. Occupational ototoxicity of n-hexane. Hum Exp Toxicol. 2008;27(6):471–476 [DOI] [PubMed] [Google Scholar]
- 50.Vyskocil A, Leroux T, Truchon Get al. Ototoxicity of trichloroethylene in concentrations relevant for the working environment. Hum Exp Toxicol. 2008;27(3):195–200 [DOI] [PubMed] [Google Scholar]
- 51.Gates GA, Mills JH. Presbycusis. Lancet. 2005;366(9491):1111–1120 [DOI] [PubMed] [Google Scholar]
- 52.Lindbohm ML, Sallmen M, Kyyronen P, Kauppinen T, Pukkala E. Risk of liver cancer and exposure to organic solvents and gasoline vapors among Finnish workers. Int J Cancer. 2009;124(12):2954–2959 [DOI] [PubMed] [Google Scholar]
- 53.Page JM, Harrison SA. NASH and HCC. Clin Liver Dis. 2009;13(4):631–647 [DOI] [PubMed] [Google Scholar]
- 54.Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer. 2009;115(24):5651–5661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caldwell S, Park SH. The epidemiology of hepatocellular cancer: from the perspectives of public health problem to tumor biology. J Gastroenterol. 2009;44:96–101 [DOI] [PubMed] [Google Scholar]
- 56.Brunzell J, Failor RA. Diagnosis and treatment of dyslipidemia. Available at: http://www.acpmedicine.com/acp/chapters/ch0906.htm. Accessed August 20, 2011
- 57.Nunes de Paiva MJ, Pereira Bastos de Siqueira ME. Increased serum bile acids as a possible biomarker of hepatotoxicity solvents in car repainting shops. Biomarkers. 2005;10(6):456–463 [DOI] [PubMed] [Google Scholar]
- 58.Caldwell SH, Crespo DM, Kang HS, Al-Osaimi AMS. Obesity and hepatocellular carcinoma. Gastroenterology. 2004;127(suppl 1):S97–S103 [DOI] [PubMed] [Google Scholar]
- 59.Brautbar N, Williams J. Industrial solvents and liver toxicity: risk assessment, risk factors and mechanisms. Int J Hyg Environ Health. 2002;205(6):479–491 [DOI] [PubMed] [Google Scholar]
- 60.Coral-Alvarado P, Pardo AL, Castano-Rodriguez Net al. Systemic sclerosis: a world-wide global analysis. Clin Rheumatol. 2009;28(7):757–765 [DOI] [PubMed] [Google Scholar]
- 61.Mora GF. Systemic sclerosis: environmental factors. J Rheumatol. 2009;36(11):2383–2396 [DOI] [PubMed] [Google Scholar]
- 62.Sinokki M, Hinkka K, Ahola Ket al. The association between team climate at work and mental health in the Finnish Health 2000 Study. Occup Environ Med. 2009;66(8):523–528 [DOI] [PubMed] [Google Scholar]
- 63.Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum. 2008;37(4):223–235 [DOI] [PubMed] [Google Scholar]
- 64.Smith V, Vanthuyne M, Cruyssen BVet al. Over-representation of construction-related occupations in male patients with systemic sclerosis. Ann Rheum Dis. 2008;67(10):1448–1450 [DOI] [PubMed] [Google Scholar]
- 65.Bovenzi M, Barbone F, Pisa FEet al. A case-control study of occupational exposures and systemic sclerosis. Int Arch Occup Environ Health. 2004;77(1):10–16 [DOI] [PubMed] [Google Scholar]
- 66.Bovenzi M, Barbone F, Pisa FE, Betta A, Romeo L. Scleroderma and occupational exposure to hand-transmitted vibration. Int Arch Occup Environ Health. 2001;74(8):579–582 [DOI] [PubMed] [Google Scholar]
- 67.Sanne B, Mykletun A, Dahl AA, Moen BE, Tell GS. Occupational differences in levels of anxiety and depression: the Hordaland Health Study. J Occup Environ Med. 2003;45(6):628–638 [DOI] [PubMed] [Google Scholar]
- 68.Nietert PJ, Silver RM. Systemic sclerosis: environmental and occupational risk factors. Curr Opin Rheumatol. 2000;12(6):520–526 [DOI] [PubMed] [Google Scholar]
- 69.Piccinelli M, Wilkinson G. Gender differences in depression—critical review. Br J Psychiatry. 2000;177:486–492 [DOI] [PubMed] [Google Scholar]
- 70.Silverstein B, Fan ZJ, Smith CKet al. Gender adjustment or stratification in discerning upper extremity musculoskeletal disorder risk? Scand J Work Environ Health. 2009;35(2):113–126 [DOI] [PubMed] [Google Scholar]
- 71.Amr S, Wolpert B, Loffredo CAet al. Occupation, gender, race, and lung cancer. J Occup Environ Med. 2008;50(10):1167–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lombardi DA, Sorock GS, Holander L, Mittleman MA. A case-crossover study of transient risk factors for occupational hand trauma by gender. J Occup Environ Hyg. 2007;4(10):790–797 [DOI] [PubMed] [Google Scholar]
- 73.Dimich-Ward H, Camp PG, Kennedy SM. Gender differences in respiratory symptoms—does occupation matter? Environ Res. 2006;101(2):175–183 [DOI] [PubMed] [Google Scholar]
- 74.Coughlin SS, Ekwueme DU. Breast cancer as a global health concern. Cancer Epidemiol. 2009;33(5):315–318 [DOI] [PubMed] [Google Scholar]
- 75.Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, Rudel RA. Environmental pollutants and breast cancer—epidemiologic studies. Cancer. 2007;109(12):2667–2711 [DOI] [PubMed] [Google Scholar]
- 76.Cohn BA, Wolff MS, Cirillo PM, Sholtz RI. DDT and breast cancer in young women: new data on the significance of age at exposure. Environ Health Perspect. 2007;115(10):1406–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Teitelbaum SL, Gammon MD, Britton JA, Neugut AI, Levin B, Stellman SD. Reported residential pesticide use and breast cancer risk on Long Island, New York. Am J Epidemiol. 2007;165(6):643–651 [DOI] [PubMed] [Google Scholar]
- 78.Engel LS, Hill DA, Hoppin JAet al. Pesticide use and breast cancer risk among farmers—wives in the Agricultural Health Study. Am J Epidemiol. 2005;161(2):121–135 [DOI] [PubMed] [Google Scholar]
- 79.Boersma K, Linton SJ. Psychological processes underlying the development of chronic pain problems: a prospective study of the relationship between profiles of psychological variables in the fear-avoidance model and disability. Clin J Pain. 2006;22(2):160–166 [DOI] [PubMed] [Google Scholar]
- 80.Dersh J, Gatchel RJ, Polatin P, Mayer T. Prevalence of psychiatric disorders in patients with chronic work-related musculoskeletal pain disability. J Occup Environ Med. 2002;44(5):459–468 [DOI] [PubMed] [Google Scholar]
- 81.Institute of Medicine Musculoskeletal Disorders and the Workplace: Low Back and Upper Extremities. Washington, DC: National Academy Press; 2001 [PubMed] [Google Scholar]
- 82.Bugajska J, Michalak JM, Jedryka-Goral A, Sagan A, Konarska M. Coronary heart disease risk factors and cardiovascular risk in physical workers and managers. Int J Occup Saf Ergon. 2009;15(1):35–43 [DOI] [PubMed] [Google Scholar]
- 83.Crawford MH. Chronic ischemic heart disease. : Crawford MH, Current Diagnosis and Treatment in Cardiology. 3rd ed New York, NY: McGraw-Hill; 2009:25–37 [Google Scholar]
- 84.Humblet O, Birnbaum L, Rimm E, Mittleman MA, Hauser R. Dioxins and cardiovascular mortality: a review. Environ Health Perspect. 2008;116(11):1443–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen CJ, Shih TS, Chang HYet al. The total body burden of chromium associated with skin disease and smoking among cement workers. Sci Total Environ. 2008;391(1):76–81 [DOI] [PubMed] [Google Scholar]
- 86.Chou TC, Chang HY, Chen CJet al. Effect of hand dermatitis on the total body burden of chromium after ferrous sulfate application in cement among cement workers. Contact Dermat. 2008;59(3):151–156 [DOI] [PubMed] [Google Scholar]
- 87.Gautrin D, Cartier A, Howse Det al. Occupational asthma and allergy in snow crab processing in Newfoundland and Labrador. Occup Environ Med. 2010;67(1):17–23 [DOI] [PubMed] [Google Scholar]
- 88.Rimac D, Macan J, Varnai VMet al. Exposure to poultry dust and health effects in poultry workers: impact of mould and mite allergens. Int Arch Occup Environ Health. 2010;83(1):9–19 [DOI] [PubMed] [Google Scholar]
- 89.Smit LAM, Heederik D, Doekes G, Wouters IM. Exhaled nitric oxide in endotoxin-exposed adults: effect modification by smoking and atopy. Occup Environ Med. 2009;66(4):251–255 [DOI] [PubMed] [Google Scholar]
- 90.Jeebhay MF, Robins TG, Miller MEet al. Occupational allergy and asthma among salt water fish processing workers. Am J Ind Med. 2008;51(12):899–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Milkovic-Kraus S, Macan J, Kanceljak-Macan B. Occupational allergic contact dermatitis from azithromycin in pharmaceutical workers: a case series. Contact Dermatitis. 2007;56(2):99–102 [DOI] [PubMed] [Google Scholar]
- 92.de Meer G, Kerkhof M, Kromhout H, Schouten JP, Heederik D. Interaction of atopy and smoking on respiratory effects of occupational dust exposure: a general population-based study. Environ Health. 2004;3(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miller BG, MacCalman L. Cause-specific mortality in British coal workers and exposure to respirable dust and quartz. Occup Environ Med. 2010;67(4):270–276 [DOI] [PubMed] [Google Scholar]
- 94.Kline JN, Doekes G, Bonlokke J, Hoffman HJ, Von Essen S, Zhai RH. Sensitivity to organic dusts—atopy and gene polymorphisms. Am J Ind Med. 2004;46(4):416–418 [DOI] [PubMed] [Google Scholar]
- 95.Kitch BT, Levy BD, Fanta CH. Late onset asthma—epidemiology, diagnosis and treatment. Drugs Aging. 2000;17(5):385–397 [DOI] [PubMed] [Google Scholar]
- 96.Lombardo LJ, Balmes JR. Occupational asthma: a review. Environ Health Perspect. 2000;108(suppl 4):697–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Trinkoff AM, Le R, Geiger-Brown J, Lipscomb J, Lang G. Longitudinal relationship of work hours, mandatory overtime, and on-call to musculoskeletal problems in nurses. Am J Ind Med. 2006;49(11):964–971 [DOI] [PubMed] [Google Scholar]
- 98.Moghtaderi A, Izadi S, Sharafadinzadeh N. An evaluation of gender, body mass index, wrist circumference and wrist ratio as independent risk factors for carpal tunnel syndrome. Acta Neurol Scand. 2005;112(6):375–379 [DOI] [PubMed] [Google Scholar]
- 99.Lam N, Thurston A. Association of obesity, gender, age and occupation with carpal tunnel syndrome. Aust N Z J Surg. 1998;68(3):190–193 [DOI] [PubMed] [Google Scholar]
- 100.Jensen LK. Knee osteoarthritis: influence of work involving heavy lifting, kneeling, climbing stairs or ladders, or kneeling/squatting combined with heavy lifting. Occup Environ Med. 2008;65(2):72–89 [DOI] [PubMed] [Google Scholar]
- 101.Coggon D, Croft P, Kellingray Set al. Occupational physical activities and osteoarthritis of the knee. Arthritis Rheum. 2000;43(7):1443–1449 [DOI] [PubMed] [Google Scholar]
- 102.Suarthana E, Heederik D, Ghezzo Het al. Risks for the development of outcomes related to occupational allergies: an application of the asthma-specific job exposure matrix compared with self-reports and investigator scores on job-training-related exposure. Occup Environ Med. 2009;66(4):256–263 [DOI] [PubMed] [Google Scholar]
- 103.Marabini A, Siracusa A, Stopponi Ret al. Outcome of occupational asthma in patients with continuous exposure—a 3-year longitudinal study during pharmacologic treatment. Chest. 2003;124(6):2372–2376 [DOI] [PubMed] [Google Scholar]
- 104.Kortt M, Baldry J. The association between musculoskeletal disorders and obesity. Aust Health Rev. 2002;25(6):207–214 [DOI] [PubMed] [Google Scholar]
- 105.Toren K, Brisman J, Olin AC, Blanc PD. Asthma on the job: work-related factors in new-onset asthma and in exacerbations of pre-existing asthma. Respir Med. 2000;94(6):529–535 [DOI] [PubMed] [Google Scholar]
- 106.Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137(3):711–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tanne F, Gagnadoux F, Chazouilleres Oet al. Chronic liver injury during obstructive sleep apnea. Hepatology. 2005;41(6):1290–1296 [DOI] [PubMed] [Google Scholar]
- 108.Lundqvist G, Flodin U, Axelson O. A case-control study of fatty liver disease and organic solvent exposure. Am J Ind Med. 1999;35(2):132–136 [DOI] [PubMed] [Google Scholar]
- 109.Alonso A, Logroscino G, Jick SS, Hernan MA. Association of smoking with amyotrophic lateral sclerosis risk and survival in men and women: a prospective study. BMC Neurol. 2010;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Paris C, Clement-Duchene C, Vignaud JMet al. Relationships between lung adenocarcinoma and gender, age, smoking and occupational risk factors: A case-case study. Lung Cancer. 2010;68(2):146–153 [DOI] [PubMed] [Google Scholar]
- 111.Binazzi A, Belli S, Uccelli Ret al. An exploratory case-control study on spinal and bulbar forms of amyotrophic lateral sclerosis in the province of Rome. Amyotroph Lateral Scler. 2009;10(5–6):361–369 [DOI] [PubMed] [Google Scholar]
- 112.Blanc PD, Eisner MD, Earnest Get al. Further exploration of the links between occupational exposure and chronic obstructive pulmonary disease. J Occup Environ Med. 2009;51(7):804–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Blanc PD, Iribarren C, Trupin Let al. Occupational exposures and the risk of COPD: dusty trades revisited. Thorax. 2009;64(1):6–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fang F, Quinlan P, Ye Wet al. Workplace exposures and the risk of amyotrophic lateral sclerosis. Environ Health Perspect. 2009;117(9):1387–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Boggia B, Farinaro E, Grieco L, Lucariello A, Carbone U. Burden of smoking and occupational exposure on etiology of chronic obstructive pulmonary disease in workers of southern Italy. J Occup Environ Med. 2008;50(3):366–370 [DOI] [PubMed] [Google Scholar]
- 116.Ebbehoj NE, Hein HO, Suadicani P, Gyntelberg F. Occupational organic solvent exposure, smoking, and prevalence of chronic bronchitis—an epidemiological study of 3387 men. J Occup Environ Med. 2008;50(7):730–735 [DOI] [PubMed] [Google Scholar]
- 117.Gallo V, Bueno-De-Mesquita H, Vermeulen Ret al. Smoking and risk for amyotrophic lateral sclerosis: analysis of the EPIC cohort. Ann Neurol. 2009;65(4):378–385 [DOI] [PubMed] [Google Scholar]
- 118.Rodriguez E, Ferrer J, Marti S, Zock JP, Plana E, Morell F. Impact of occupational exposure on severity of COPD. Chest. 2008;134(6):1237–1243 [DOI] [PubMed] [Google Scholar]
- 119.Siew SS, Kauppinen T, Kyyronen P, Heikkila P, Pukkala E. Exposure to iron and welding fumes and the risk of lung cancer. Scand J Work Environ Health. 2008;34(6):444–450 [DOI] [PubMed] [Google Scholar]
- 120.Weinmann S, Vollmer WM, Breen Vet al. COPD and occupational exposures: a case-control study. J Occup Environ Med. 2008;50(5):561–569 [DOI] [PubMed] [Google Scholar]
- 121.Sutedja NA, Veldink JH, Fischer Ket al. Lifetime occupation, education, smoking, and risk of ALS. Neurology. 2007;69(15):1508–1514 [DOI] [PubMed] [Google Scholar]
- 122.Weisskopf MG, McCullough ML, Calle EEet al. Prospective study of cigarette smoking and amyotrophic lateral sclerosis. Am J Epidemiol. 2004;160(1):26–33 [DOI] [PubMed] [Google Scholar]
- 123.Steenland K. Ten-year update on mortality among mild-steel welders. Scand J Work Environ Health. 2002;28(3):163–167 [DOI] [PubMed] [Google Scholar]
- 124.Bruske-Hohlfeld I, Mohner M, Pohlabeln Het al. Occupational lung cancer risk for men in Germany: results from a pooled case-control study. Am J Epidemiol. 2000;151(4):384–395 [DOI] [PubMed] [Google Scholar]
- 125.Danielsen TE, Langard S, Andersen A. Incidence of cancer among welders and other shipyard workers with information on previous work history. J Occup Environ Med. 2000;42(1):101–109 [DOI] [PubMed] [Google Scholar]
- 126.Jahn I, Ahrens W, Bruske-Hohlfeld Iet al. Occupational risk factors for lung cancer in women: results of a case-control study in Germany. Am J Ind Med. 1999;36(1):90–100 [DOI] [PubMed] [Google Scholar]
- 127.Merlo F, Costantini M, Doria M. Cause specific mortality among workers exposed to welding fumes and gases: a historical prospective study. J UOEH. 1989;11(suppl):302–315 [PubMed] [Google Scholar]
- 128.Dykewicz MS. Occupational asthma: current concepts in pathogenesis, diagnosis, and management. J Allergy Clin Immunol. 2009;123(3):519–528 [DOI] [PubMed] [Google Scholar]
- 129.Hsu PC, Chang HY, Guo YL, Liu YC, Shih TS. Effect of smoking on blood lead levels in workers and role of reactive oxygen species in lead-induced sperm chromatin DNA damage. Fertil Steril. 2009;91(4):1096–1103 [DOI] [PubMed] [Google Scholar]
- 130.Sousa C, Castro JN, Larsson EJ, Ching TH. Risk factors for presbycusis in a socio-economic middle-class sample. Braz J Otorhinolaryngol. 2009;76(4):530–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boger ME, Barbosa-Branco A, Ottoni AC. The noise spectrum influence on noise-induced hearing loss prevalence in workers. Braz J Otorhinolaryngol. 2009;75(3):328–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.El Zir E, Mansour S, Salameh P, Chahine R. Environmental noise in Beirut, smoking and age are combined risk factors for hearing impairment. East Mediterr Health J. 2008;14(4):888–896 [PubMed] [Google Scholar]
- 133.Kasperczyk A, Kasperczyk S, Horak Set al. Assessment of semen function and lipid peroxidation among lead exposed men. Toxicol Appl Pharmacol. 2008;228(3):378–384 [DOI] [PubMed] [Google Scholar]
- 134.Naha N, Manna B. Mechanism of lead induced effects on human spermatozoa after occupational exposure. Kathmandu Univ Med J. 2007;5(1):85–94 [PubMed] [Google Scholar]
- 135.Pouryaghoub G, Mehrdad R, Mohammadi S. Interaction of smoking and occupational noise exposure on hearing loss: a cross-sectional study. BMC Public Health. 2007;7:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ologe FE, Akande TM, Olajide TG. Occupational noise exposure and sensorineural hearing loss among workers of a steel rolling mill. Eur Arch Otorhinolaryngol. 2006;263(7):618–621 [DOI] [PubMed] [Google Scholar]
- 137.Wild DC, Brewster MJ, Banerjee AR. Noise-induced hearing loss is exacerbated by long-term smoking. Clin Otolaryngol. 2005;30(6):517–520 [DOI] [PubMed] [Google Scholar]
- 138.Prince MM. Distribution of risk factors for hearing loss: implications for evaluating risk of occupational noise-induced hearing loss. J Acoust Soc Am. 2002;112(2):557–567 [DOI] [PubMed] [Google Scholar]
- 139.Cruickshanks KJ, Klein R, Klein BEKet al. Cigarette smoking and hearing loss—the Epidemiology of Hearing Loss Study. JAMA. 1998;279(21):1715–1719 [DOI] [PubMed] [Google Scholar]
- 140.Batty GD, Shipley MJ, Langenberg C, Marmot MG, Smith GD. Adult height in relation to mortality from 14 cancer sites in men in London (UK): evidence from the original Whitehall study. Ann Oncol. 2006;17(1):157–166 [DOI] [PubMed] [Google Scholar]
- 141.van Rossum CTM, Shipley MJ, van de Mheen H, Grobbee DE, Marmot MG. Employment grade differences in cause specific mortality. a 25 year follow up of civil servants from the first Whitehall study. J Epidemiol Community Health. 2000;54(3):178–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.O'Keefe JH, Carter MD, Lavie CJ. Primary and secondary prevention of cardiovascular diseases: a practical evidence-based approach. Mayo Clin Proc. 2009;84(8):741–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.van Kempen EE, Kruize H, Boshuizen HCet al. The association between noise exposure and blood pressure and ischemic heart disease: a meta-analysis. Environ Health Perspect. 2002;110(3):307–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Centers for Disease Control and Prevention Smoking and cardiovascular disease. MMWR Morb Mortal Wkly Rep. 1984;32(52):677–679 [PubMed] [Google Scholar]
- 145.Mastrangelo G, Fedeli U, Fadda Eet al. Increased risk of hepatocellular carcinoma and liver cirrhosis in vinyl chloride workers: synergistic effect of occupational exposure with alcohol intake. Environ Health Perspect. 2004;112(11):1188–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schnall PL, Schwartz JE, Landsbergis PAet al. Relation between job strain, alcohol, and ambulatory blood pressure. Hypertension. 1992;19(5):488–494 [DOI] [PubMed] [Google Scholar]
- 147.Kriebel D, Zeka A, Eisen EA, Wegman DH. Quantitative evaluation of the effects of uncontrolled confounding by alcohol and tobacco in occupational cancer studies. Int J Epidemiol. 2004;33(5):1040–1045 [DOI] [PubMed] [Google Scholar]
- 148.Taylor BC, Yuan JM, Shamliyan TA, Shaukat A, Kane RL, Wilt TJ. Clinical outcomes in adults with chronic hepatitis B in association with patient and viral characteristics: a systematic review of evidence. Hepatology. 2009;49(suppl 5):S85–S95 [DOI] [PubMed] [Google Scholar]
- 149.Luckhaupt SE, Calvert GM. Deaths due to bloodborne infections and their sequelae among health-care workers. Am J Ind Med. 2008;51(11):812–824 [DOI] [PubMed] [Google Scholar]
- 150.Shin BM, Yoo HM, Lee AS, Park SK. Seroprevalence of hepatitis B virus among health care workers in Korea. J Korean Med Sci. 2006;21(1):58–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tarantola A, Abiteboul D, Rachline A. Infection risks following accidental exposure to blood or body fluids in health care workers: a review of pathogens transmitted in published cases. Am J Infect Control. 2006;34(6):367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dement JM, Epling C, Ostbye T, Pompeii LA, Hunt DL. Blood and body fluid exposure risks among health care workers: results from the Duke Health and Safety Surveillance System. Am J Ind Med. 2004;46(6):637–648 [DOI] [PubMed] [Google Scholar]
- 153.Centers for Disease Control and Prevention Exposure to blood: what health care personnel need to know. Available at: http://www.cdc.gov/ncidod/dhqp/pdf/bbp/Exp_to_Blood.pdf. Accessed August 20, 2011
- 154.Palmer KT, Harris EC, Coggon D. Chronic health problems and risk of accidental injury in the workplace: a systematic literature review. Occup Environ Med. 2008;65(11):757–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fireman P. Treatment of allergic rhinitis: effect on occupation productivity and work force costs. Allergy Asthma Proc. 1997;18(2):63–67 [DOI] [PubMed] [Google Scholar]
- 156.Vietri NJ, Purcell BK, Lawler JVet al. Short-course postexposure antibiotic prophylaxis combined with vaccination protects against experimental inhalational anthrax. Proc Natl Acad Sci U S A. 2006;103(20):7813–7816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fowler RA, Sanders GD, Bravata DMet al. Cost-effectiveness of defending against bioterrorism: a comparison of vaccination and antibiotic prophylaxis against anthrax. Ann Intern Med. 2005;142(8):601–610 [DOI] [PubMed] [Google Scholar]
- 158.Martin SW, Tierney BC, Aranas Aet al. An overview of adverse events reported by participants in CDC's Anthrax Vaccine and Antimicrobial Availability Program. Pharmacoepidemiol Drug Saf. 2005;14(6):393–401 [DOI] [PubMed] [Google Scholar]
- 159.Rusnak JM, Kortepeter MG, Aldis J, Boudreau E. Experience in the medical management of potential laboratory exposures to agents of bioterrorism on the basis of risk assessment at the United States Army Medical Research Institute of Infectious Diseases (USAMRIID). J Occup Environ Med. 2004;46(8):801–811 [DOI] [PubMed] [Google Scholar]
- 160.Rusnak JM, Kortepeter MG, Hawley RJet al. Risk of occupationally acquired illnesses from biological threat agents in unvaccinated laboratory workers. Biosecur Bioterror. 2004;2(4):281–293 [DOI] [PubMed] [Google Scholar]
- 161.Rusnak JM, Kortepeter MG, Hawley RJet al. Management guidelines for laboratory exposures to agents of bioterrorism. J Occup Environ Med. 2004;46(8):791–800 [DOI] [PubMed] [Google Scholar]
- 162.Caruso CC, Lusk SL, Gillespie BW. Relationship of work schedules to gastrointestinal diagnoses, symptoms, and medication use in auto factory workers. Am J Ind Med. 2004;46(6):586–598 [DOI] [PubMed] [Google Scholar]
- 163.Verma S, Kaplowitz N. Diagnosis, management and prevention of drug-induced liver injury. Gut. 2009;58(11):1555–1564 [DOI] [PubMed] [Google Scholar]
- 164.Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov. 2005;4(6):489–499 [DOI] [PubMed] [Google Scholar]
- 165.Neumann DA, Kimmel CA. Human variability in susceptibility and response: implications for risk assessment. : Neumann DA, Kimmel CA, Human Variability in Response to Chemical Exposures: Measures, Modeling, and Risk Assessment. Boca Raton, FL: CRC Press; 1999:219–241 [Google Scholar]
- 166.Vineis P, d'Errico A, Malats N, Boffetta P. Overall evaluation and research perspectives. : Vineis P, Malats N, Lang Met al. Metabolic Polymorphisms and Susceptibility to Cancer. Lyon, France: International Agency for Research on Cancer; 1999:403–408 [Google Scholar]
- 167.Yong LC, Schulte PA, Wiencke JKet al. Hemoglobin adducts and sister chromatid exchanges in hospital workers exposed to ethylene oxide: effects of glutathione S-transferase T1 and M1 genotypes. Cancer Epidemiol Biomarkers Prev. 2001;10(5):539–550 [PubMed] [Google Scholar]
- 168.McCanlies E, Landsittel DP, Yucesoy Bet al. Significance of genetic information in risk assessment and individual classification using silicosis as a case model. Ann Occup Hyg. 2002;46(4):375–381 [DOI] [PubMed] [Google Scholar]
- 169.Talmud PJ. How to identify gene-environment interactions in a multifactorial disease: CHD as an example. Proc Nutr Soc. 2004;63(1):5–10 [DOI] [PubMed] [Google Scholar]
- 170.Wang Z, Neuburg D, Li Cet al. Global gene expression profiling in whole-blood samples from individuals exposed to metal fumes. Environ Health Perspect. 2005;113(2):233–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Christiani DC, Mehta AJ, Yu C- L. Genetic susceptibility to occupational exposures. Occup Environ Med. 2008;65:430–436 PubMed doi:10.1136/oem.2007.033977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Carreon T, Ruder AM, Schulte PAet al. NAT2 slow acetylation and bladder cancer in workers exposed to benzidine. Int J Cancer. 2006;118(1):161–168 [DOI] [PubMed] [Google Scholar]
- 173.Puca AA, Daly MJ, Brewster SJet al. A genome-wide scan for linkage to human exceptional longevity identifies a locus on chromosome 4. Proc Natl Acad Sci U S A. 2001;98(18):10505–10508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.McKay JD, Hung RJ, Gaborieau Vet al. Lung cancer susceptibility locus at 5p15.33. Nat Genet. 2008;40(12):1404–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Faragher RGA, Sheerin AN, Ostler EL. Can we intervene in human ageing? Expert Rev Mol Med. 2009;11:e27 [DOI] [PubMed] [Google Scholar]
- 176.Schock NS, Greulich RC, Anders Ret al. Normal Human Aging: The Baltimore Longitudinal Study of Aging. Washington, DC: US Government Printing Office; 1984 [Google Scholar]
- 177.Kennedy SM, Chambers R, Weiwei D, Dimich-Ward H. Environmental and occupational exposures: do they affect chronic obstructive pulmonary disease differently in women and men? Proc Am Thorac Soc. 2007;4(8):692–694 [DOI] [PubMed] [Google Scholar]
- 178.Zahm SH, Blair A. Occupational cancer among women: where have we been and where are we going? Am J Ind Med. 2003;44(6):565–575 [DOI] [PubMed] [Google Scholar]
- 179.Artazcoz L, Borrell C, Cortes I, Escriba-Aguir V, Cascant L. Occupational epidemiology and work related inequalities in health: a gender perspective for two complementary approaches to work and health research. J Epidemiol Community Health. 2007;61(1):39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Prins MH, Smits KM, Smits LJ. Methodologic ramifications of paying attention to sex and gender differences in clinical research. Gend Med. 2007;4(suppl 2):S106–S110 [DOI] [PubMed] [Google Scholar]
- 181a.Munir F, Khan HTA, Yarker Jet al. Self-management of health behaviors among older and younger workers with chronic illness. Patient Educ Couns. 2009;77(1):109–115 [DOI] [PubMed] [Google Scholar]
- 181b.Hymel PA, Loeppke RR, Baase CMet al. Workplace health protection and promotion: a new pathway for a healthier—and safer—workforce. J Occup Environ Med. 2011;53(6):695–702 [DOI] [PubMed] [Google Scholar]
- 182.Cooper GS, Miller FW, Germolec DR. Occupational exposures and autoimmune diseases. Int Immunopharmacol. 2002;2(2–3):303–313 [DOI] [PubMed] [Google Scholar]
- 183.Arrighi HM, Hertz-Picciotto I. The evolving concept of the healthy worker survivor effect. Epidemiology. 1994;5(2):189–196 [DOI] [PubMed] [Google Scholar]
- 184.Li CY, Sung FC. A review of the healthy worker effect in occupational epidemiology. Occup Med (Lond). 1999;49(4):225–229 [DOI] [PubMed] [Google Scholar]
- 185.Le Moual N, Kauffmann F, Eisen EA, Kennedy SM. The healthy worker effect in asthma—work may cause asthma, but asthma may also influence work. Am J Respir Crit Care Med. 2008;177(1):4–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hertz-Picciotto I, Arrighi HM, Hu SW. Does arsenic exposure increase the risk for circulatory disease? Am J Epidemiol. 2000;151(2):174–181 [DOI] [PubMed] [Google Scholar]
- 187.Tucker LA, Friedman GM. Obesity and absenteeism: an epidemiologic study of 10,825 employed adults. Am J Health Promot. 1998;12(3):202–207 [DOI] [PubMed] [Google Scholar]
- 188.Rodbard HW, Fox KM, Grandy S. Impact of obesity on work productivity and role disability in individuals with and at risk for diabetes mellitus. Am J Health Promot. 2009;23(5):353–360 [DOI] [PubMed] [Google Scholar]
- 189.Goetzel RZ, Gibson TB, Short MEet al. A multi-worksite analysis of the relationships among body mass index, medical utilization, and worker productivity. J Occup Environ Med. 2010;52(suppl 1):S52–S58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hetherington MM, Cecil JE. Gene-environment interactions in obesity. Forum Nutr. 2010;63:195–203 [DOI] [PubMed] [Google Scholar]
- 191.Yamada Y, Kameda M, Noborisaka Yet al. Excessive fatigue and weight gain among cleanroom workers after changing from an 8-hour to a 12-hour shift. Scand J Work Environ Health. 2001;27(5):318–326 [DOI] [PubMed] [Google Scholar]
- 192.Brisson C, Larocque B, Moisan Jet al. Psychosocial factors at work, smoking, sedentary behavior, and body mass index: a prevalence study among 6995 white collar workers. J Occup Environ Med. 2000;42(1):40–46 [DOI] [PubMed] [Google Scholar]
- 193.Ostry AS, Radi S, Louie AM, LaMontagne AD. Psychosocial and other working conditions in relation to body mass index in a representative sample of Australian workers. BMC Public Health. 2006;6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tunceli K, Li KM, Williams LK. Long-term effects of obesity on employment and work limitations among US adults, 1986 to 1999. Obesity (Silver Spring). 2006;14(9):1637–1646 [DOI] [PubMed] [Google Scholar]
- 195.Lubin JH, Caporaso N, Wichmann HEet al. Cigarette smoking and lung cancer—modeling effect modification of total exposure and intensity. Epidemiology. 2007;18(5):639–648 [DOI] [PubMed] [Google Scholar]
- 196.Nicholson PJ, D'Auria DAP. Shift work, health, the working time regulations and health assessments. Occup Med (Lond). 1999;49(3):127–137 [DOI] [PubMed] [Google Scholar]
- 197.Cunradi CB, Moore RS, Ames G. Contribution of occupational factors to current smoking among active-duty US Navy careerists. Nicotine Tob Res. 2008;10(3):429–437 [DOI] [PubMed] [Google Scholar]
- 198.Smith DR. Tobacco smoking by occupation in Australia and the United States: a review of national surveys conducted between 1970 and 2005. Ind Health. 2008;46(1):77–89 [DOI] [PubMed] [Google Scholar]
- 199.Cunradi CB, Lipton R, Banerjee A. Occupational correlates of smoking among urban transit operators: a prospective study. Subst Abuse Treat Prev Policy. 2007;2:36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Frost G, Darnton A, Harding AH. The effect of smoking on the risk of lung cancer mortality for asbestos workers in Great Britain (1971–2005). Ann Occup Hyg. 2011;55(3):239–247 [DOI] [PubMed] [Google Scholar]
- 201.Schubauer-Berigan MK, Daniels RD, Pinkerton LE. Radon exposure and mortality among white and American Indian uranium miners: an update of the Colorado Plateau cohort. Am J Epidemiol. 2009;169(6):718–730 [DOI] [PubMed] [Google Scholar]
- 202.Centers for Disease Control and Prevention Alcohol and public health. Available at: http://cdc.gov/alcohol/index.htm. Accessed August 20, 2011
- 203.Dawson DA, Li TK, Grant BF. A prospective study of risk drinking: at risk for what? Drug Alcohol Depend. 2008;95(1–2):62–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Romeri E, Baker A, Griffiths C. Alcohol-related deaths by occupation, England and Wales, 2001–05. Health Stat Q. 2007;35:6–12 [PubMed] [Google Scholar]
- 205.Marchand A. Alcohol use and misuse: what are the contributions of occupation and work organization conditions? BMC Public Health. 2008;8:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Frone MR. Prevalence and distribution of alcohol use and impairment in the workplace: a US national survey. J Stud Alcohol. 2006;67(1):147–156 [DOI] [PubMed] [Google Scholar]
- 207.National Center for Health Statistics Health, United States, 2009: with special feature on medical technology. Available at: http://www.cdc.gov/nchs/data/hus/hus09.pdf. Accessed August 20, 2011 [PubMed]
- 208.Dillon CF, Rasch EK, Gu QP, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. J Rheumatol. 2006;33(11):2271–2279 [PubMed] [Google Scholar]
- 209.Moscato G, Galdi E, Scibilia Jet al. Occupational asthma, rhinitis and urticaria due to piperacillin sodium in a pharmaceutical worker. Eur Respir J. 1995;8(3):467–469 [DOI] [PubMed] [Google Scholar]
- 210.Morissette P, Dedobbeleer N. Is work a risk factor in the prescribed psychotropic drug consumption of female white-collar workers and professionals? Women Health. 1997;25(4):105–121 [DOI] [PubMed] [Google Scholar]
- 211.Boeuf-Cazou O, Lapeyre-Mestre M, Niezborala M, Montastruc JL. Evolution of drug consumption in a sample of French workers since 1986: the ‘Drugs and Work’ study. Pharmacoepidemiol Drug Saf. 2009;18(4):335–343 [DOI] [PubMed] [Google Scholar]
- 212.Croog SH, Sudilovsky A, Levine S, Testa MA. Work performance, absenteeism and antihypertensive medications. J Hypertens. 1987;5(1):S47–S54 [PubMed] [Google Scholar]
- 213.Baeck M, Goossens A. Patients with airborne sensitization/contact dermatitis from budesonide-containing aerosols ‘by proxy.’ Contact Dermat. 2009;61(1):1–8 [DOI] [PubMed] [Google Scholar]
- 214.Rappaport SM, Smith TM. Environment and disease risks. Science. 2010;330(6003):460–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Blair A, Stewart P, Lubin JH, Forastiere F. Methodological issues regarding confounding and exposure misclassification in epidemiological studies of occupational exposures. Am J Ind Med. 2007;50(3):199–207 [DOI] [PubMed] [Google Scholar]
- 216.US Dept of Health and Human Services 45 CFR Part 170, health information technology: initial set of standards, implementation specifications, and certification criteria for electronic health record technology; interim final rule. Available at: http://edocket.access.gpo.gov/2010/pdf/2010-17210.pdf. Accessed August 20, 2011 [PubMed]
- 217.Clapp R. Industrial carcinogens: a need for action. Rev Environ Health. 2009;24(4):257–262 [DOI] [PubMed] [Google Scholar]
- 218. 124 Stat 119–124, §§1201, 2705 (2010)
- 219.Diez-Roux AV. Bringing context back into epidemiology: variables and fallacies in multilevel analysis. Am J Public Health. 1998;88(2):216–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Marmot M. Multi-level approaches to understanding social determinants. : Berkman L, Kawachi I, Social Epidemiology. Oxford, England: Oxford University Press; 1999:349–367 [Google Scholar]
- 221.Ilies R, Schwind KM, Heller D. Employee well-being: a multilevel model linking work and nonwork domains. Eur J Work Organ Psychol. 2007;16(3):326–341 [Google Scholar]
- 222.Braveman P, Egerter S, Williams DR. The social determinants of health: coming of age. Annu Rev Public Health. 2011;32:381–398 [DOI] [PubMed] [Google Scholar]
- 223.Enander RT, Gute DM, Cohen HJ. The concordance of pollution prevention and occupational health and safety: a perspective on U.S. policy. Am J Ind Med. 2003;44(3):312–320 [DOI] [PubMed] [Google Scholar]
- 224.Cherniack M, Henning R, Merchant JA, Punnet L, Sorensen GR, Wagner G. Statement on national worklife priorities. Am J Ind Med. 2011;54(1):10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Barbeau EM, Krieger N, Soobader M. Working class matters: socioeconomic disadvantage, race/ethnicity, gender and smoking in the National Health Interview Survey, 2000. Am J Public Health. 2004;94(2):269–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Barbeau EM, Leavy-Sperounis A, Balbach E. Smoking, social class, and gender: what can public health learn from the tobacco industry about disparities in smoking? Tob Control. 2004;13(2):115–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Barbeau E, Roelofs C, Youngstrom R, Stoddard A, Sorensen G, LaMontagne A. Assessment of occupational safety and health programs in small businesses. Am J Ind Med. 2004;45(4):371–379 [DOI] [PubMed] [Google Scholar]
- 228.Sorensen G, Barbeau E, Hunt MK, Emmons K. A social-contextual model for reducing tobacco use among blue-collar workers. Am J Public Health. 2004;94(2):230–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Egerter S, Dekker M, An J, Grossman-Kahn R, Braveman P. Work matters for health. Available at: http://www.commissiononhealth.org/PDF/0e8ca13d-6fb8-451d-bac8-7d15343aacff/Issue%20Brief%204%20Dec%2008%20-%20Work%20and%20Health.pdf. Accessed August 20, 2011
- 230.National Institute for Occupational Safety and Health Total Worker Health. Available at: http://www.cdc.gov/niosh/TWH. Accessed August 20, 2011