Abstract
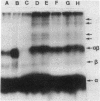
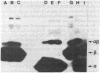
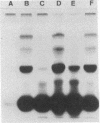
It has been shown that a photoactivable derivative of human choriogonadotropin (hCG) labels the lutropin receptor on porcine granulosa cells [Ji, I. & Ji, T. H. (1980) Proc. Natl. Acad. Sci. USA 77, 7167-7170]. In an attempt to identify which of the hCG subunits labeled the receptor, three sets of different hCG derivatives were prepared. In the first set, hCG was coupled to the N-hydroxysuccinimide ester of 4-azidobenzoylglycine and radioiodinated. In the second set, only one of the subunits was radioiodinated, but both subunits were allowed to react with the reagent. In the third set, both the reagent and [125I]iodine were coupled to only one of the subunits. The binding activity of each hormone derivative was comparable to that of 125I-labeled hCG. After binding of these hormone derivatives to the granulosa cell surface, they were photolyzed. After solubilization, autoradiographs of sodium dodecyl sulfate/polyacrylamide gels of each sample revealed a number of labeled bands; the hCG derivatives containing 125I-labeled alpha subunit produced four bands (molecular weights 120,000 +/- 6,000, 96,000 +/- 5,000, 76,000 +/- 4,000, and 73,000 +/- 4,000) and those containing 125I-labeled beta subunit produced three bands (molecular weights 106,000 +/- 6,000, 88,000 +/- 5,000, and 83,000 +/- 4,000). Results were the same when the hormone-receptor complexes were solubilized in 0.5% Triton X-100 and then photolyzed or when the hormone was derivatized with a family of reagents having arms of various lengths. We conclude that both the alpha subunit and the beta subunit of hCG photoaffinity labeled certain membrane polypeptides and that these polypeptides are related to the hormone receptor.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canfield R. E., Morgan F. J., Kammerman S., Bell J. J., Agosto G. M. Studies of human chorionic gonadotropin. Recent Prog Horm Res. 1971;27:121–164. doi: 10.1016/b978-0-12-571127-2.50028-6. [DOI] [PubMed] [Google Scholar]
- Catt K. J., Dufau M. L., Tsuruhara T. Absence of intrinsic biological activity in LH and hCG subunits. J Clin Endocrinol Metab. 1973 Jan;36(1):73–80. doi: 10.1210/jcem-36-1-73. [DOI] [PubMed] [Google Scholar]
- Combarnous Y., Maghuin-Rogister G. Luteinizing hormone. 2. Relative reactivities of tyrosyl residues of the porcine hormone towards iodination. Eur J Biochem. 1974 Feb 15;42(1):13–19. doi: 10.1111/j.1432-1033.1974.tb03308.x. [DOI] [PubMed] [Google Scholar]
- Dufau M. L., Ryan D. W., Baukal A. J., Catt K. J. Gonadotropin receptors. Solubilization and purification by affinity chromatography. J Biol Chem. 1975 Jun 25;250(12):4822–4824. [PubMed] [Google Scholar]
- Elliott J., Dunn S. M., Blanchard S. G., Raftery M. A. Specific binding of perhydrohistrionicotoxin to Torpedo acetylcholine receptor. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2576–2579. doi: 10.1073/pnas.76.6.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice L. C., Pierce J. G. Studies on the disulfide bonds of glycoprotein hormones. Formation and properties of 11,35-bis(S-alkyl) derivatives of the alpha subunit. J Biol Chem. 1979 Feb 25;254(4):1164–1169. [PubMed] [Google Scholar]
- Hofmann C., Ji T. H., Miller B., Steiner D. F. Photoaffinity labeling of the insulin receptor in H4 hepatoma cells: lack of cellular receptor processing. J Supramol Struct Cell Biochem. 1981;15(1):1–13. doi: 10.1002/jsscb.1981.380150102. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Hazum E., Cuatrecasas P. The subunit structure of rat liver insulin receptor. Antibodies directed against the insulin-binding subunit. J Biol Chem. 1980 Jul 25;255(14):6937–6940. [PubMed] [Google Scholar]
- Ji I., Ji T. H. Macromolecular photoaffinity labeling of the lutropin receptor on granulosa cells. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7167–7170. doi: 10.1073/pnas.77.12.7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji T. H. A novel approach to the identification of surface receptors. The use of photosensitive hetero-bifunctional cross-linking reagent. J Biol Chem. 1977 Mar 10;252(5):1566–1570. [PubMed] [Google Scholar]
- Ji T. H., Kiehm D. J., Middaugh C. R. Presence of spectrin tetramer on the erythrocyte membrane. J Biol Chem. 1980 Apr 10;255(7):2990–2993. [PubMed] [Google Scholar]
- Ji T. H. The application of chemical crosslinking for studies on cell membranes and the identification of surface reporters. Biochim Biophys Acta. 1979 Apr 23;559(1):39–69. doi: 10.1016/0304-4157(79)90007-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Massague J., Pilch P. F., Czech M. P. Electrophoretic resolution of three major insulin receptor structures with unique subunit stoichiometries. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7137–7141. doi: 10.1073/pnas.77.12.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maturo J. M., 3rd, Hollenberg M. D. Insulin receptor: interaction with nonreceptor glycoprotein from liver cell membranes. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3070–3074. doi: 10.1073/pnas.75.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons T. F., Pierce J. G. Biologically active covalently cross-linked glycoprotein hormones and the effects of modification of the COOH-terminal region of their alpha subunits. J Biol Chem. 1979 Jul 10;254(13):6010–6015. [PubMed] [Google Scholar]
- Pierce J. G. Eli Lilly lecture. The subunits of pituitary thyrotropin--their relationship to other glycoprotein hormones. Endocrinology. 1971 Dec;89(6):1331–1344. doi: 10.1210/endo-89-6-1331. [DOI] [PubMed] [Google Scholar]
- Pilch P. F., Czech M. P. The subunit structure of the high affinity insulin receptor. Evidence for a disulfide-linked receptor complex in fat cell and liver plasma membranes. J Biol Chem. 1980 Feb 25;255(4):1722–1731. [PubMed] [Google Scholar]
- Weare J. A., Reichert L. E., Jr Studies with carbodiimide-cross-linked derivatives of bovine lutropin. II. Location of the cross-link and implication for interaction with the receptors in testes. J Biol Chem. 1979 Aug 10;254(15):6972–6979. [PubMed] [Google Scholar]