Abstract
We argue that cluster-randomized trials are an important methodology, essential to the evaluation of many public health interventions.
However, in the case of at least some cluster-randomized trials, it is not possible, or is incompatible with the aims of the study, to obtain individual informed consent. This should not necessarily be seen as an impediment to ethical approval, providing that sufficient justification is given for this omission.
We further argue that it should be the institutional review board’s task to evaluate whether the protocol is sufficiently justified to proceed without consent and that this is preferable to any reliance on community consent or other means of proxy consent.
THE PARADIGM CASE FOR REsearch testing the effectiveness of medical interventions is the randomized controlled trial (RCT), in which individual patients are randomly allocated to 1 of 2 or more different interventions to test their relative effect on a predetermined outcome variable. The randomization process, together with other features of the design of RCTs, controls for extraneous factors that could plausibly influence the outcome variable and thereby lead to confounding.1 This focus on RCTs and the ethical issues that they create has also influenced the development of research ethics, in which the RCT seems well established as the assumed methodology of choice. However, other methods are often used, and we focus on the cluster-randomized trial.
A cluster-randomized trial randomizes at the social group level (e.g., village, hospital, school)—hence “cluster”—rather than at the level of individual patients. Cluster-randomized trials are popular in the assessment of organizational change and social, behavioral, and community-level interventions in public health.2 They retain the element of randomization and thus have many of the benefits of RCTs in terms of seeking to avoid confounding, and this is why many hold them to be superior to the obvious alternative method of cohort studies. Cluster-randomized trials are not merely an alternative to RCTs but are used when an RCT is inappropriate or impossible. This alternative design might be indicated for the following reasons.
First is the nature of the intervention. Some interventions that we wish to assess are delivered at the cluster rather than the individual level, such that it is not possible to randomly assign individual patients to interventions. Kumar et al.3 studied 3 different approaches to behavior change management relating to childbirth, delivered through a community education approach; the unit of randomization was the village rather than individual community members.
Second is the nature of the delivery of the intervention. For example, on some occasions, the target of the intervention is not the patient directly but the care provider. If the intervention involves the education of practitioners or a change in their practice, then the results of such an intervention cannot be applied selectively to certain patients in that practitioner’s caseload. Hence Figueiras et al.4 used a cluster-randomized trial design when testing educational outreach visits relating to Portuguese physicians’ reporting of adverse drug reactions.
Third is obtaining a clear and consistent answer to the chosen research question. For example, some treatments (e.g., certain behavioral or educational interventions) are susceptible to contamination, whereby individuals who have not been allocated to the intervention in question may nonetheless be exposed to it through interaction with those who have been allocated to it. Therefore, Peri et al.5 adopted a cluster-randomized trial design when evaluating functional activity in residential care facilities. Although activity programs were individually designed, there would be a risk in an individually randomized trial that residents allocated to the control intervention might observe and thereby come to adopt the activities in these programs. Similarly, Smith et al.6 used a cluster design to evaluate the use of antibiotics and dry mopping for otitis media among Kenyan schoolchildren.
The studies cited as examples illustrate a basic distinction that can be drawn between “cluster-cluster” and “individual-cluster” cluster-randomized trials,7 depending on where the intervention is delivered and where randomization occurs. In cluster-cluster trials, intervention is delivered and the units are randomized at the cluster level.3,4 In individual-cluster trials, the intervention is delivered at the individual level, whereas units are randomized at the cluster level.5,6 This is an important distinction, as shown in the next section.
The cluster-randomized trial presents several methodological challenges, at the level of both design and statistical analysis.8 It also has been recognized that cluster-randomized trials pose certain ethical challenges.7,9–11 We consider the specific ethical issues raised by such trials in relation to informed consent. We argue that a different perspective needs to be taken on this issue from that commonly taken in respect of the conventional RCT12 and that research ethics guidelines and regulations should allow sufficient flexibility to permit the use of cluster-randomized trials when appropriate.
ETHICS AND METHODOLOGY
Two fundamental assumptions form the basis of this discussion. First, specific ethical requirements (e.g., informed consent, confidentiality, avoidance of harm) should be fulfilled, unless they are overridden by other morally relevant considerations. No such requirements are immune from being overridden in this way; that is, no ethical requirements are absolute, but for some, the threshold for their being overridden may be very high. The stringency of a particular ethical concern is not predetermined but will vary from situation to situation; the requirement not to cause harm will, for example, vary in relation to the magnitude of the potential harm in question.13
Second, a generally accepted claim is that a study being methodologically sound constitutes a necessary condition but not a sufficient condition for its being ethically sound. Because only sound design can produce valid findings, and, in general, only valid findings can bring about therapeutic benefits, methodological demands carry moral weight.14 Therefore, all else being equal, the more methodologically rigorous means of investigating a phenomenon is the more ethically desirable. An initial ethical requirement is therefore to ensure that the use of a cluster-randomized trial design is a methodologically sound decision, but cluster-randomized trials should not be used merely as a means of avoiding inconvenient ethical requirements such as gaining informed consent.
PROBLEMS ASSOCIATED WITH INFORMED CONSENT
Informed consent is widely seen as an essential ethical requirement in clinical research, as can be seen from a brief glance at research guidelines and regulations.15–17 At its simplest, informed consent constitutes the giving of permission to act. It requires that participation is freely given, or withheld, by a competent person, based on an adequate understanding of relevant information related to that decision. Informed consent is most often supported by appeal to the idea of respect for an individual’s autonomy.
We have good reason to doubt that informed consent, thus understood, can be an essential requirement in clinical research, given the empirical evidence that it is extraordinarily difficult to achieve because of problems of comprehension, recall, and other circumstantial barriers to patients’ understanding.18,19 However, let us assume here that we should aim at gaining informed consent. The problem is that gaining informed consent within at least some cluster-randomized trials either is impossible or would seriously undermine the ability of the cluster-randomized trial to answer the relevant research question. Are we to take the requirement to gain an informed consent so seriously that cluster-randomized trials in such cases are to be judged unethical? Any decision is not helped by the silence of all the major ethical guidelines and regulations in relation to cluster-randomized trials. Indeed, even the Council for International Organizations of Medical Sciences epidemiological guidelines20 do not mention cluster-randomized trials.
In the RCT, consent is generally viewed in terms of a dyadic relationship and is construed in terms of the agreement, or otherwise, of the individual patient or participant. In cluster-randomized trials, however, consent potentially occurs at 2 levels, reflecting the stages of recruitment within a cluster-randomized trial.21
Consent to the trial occurring—for example, agreement that a particular village or other collective unit can be randomized within the trial.
Consent to receiving an intervention within the trial—for example, taking a particular drug.
Accordingly, the consent process and its implications become more complex. In a cluster-cluster trial, individual consent is problematic. Because the trial occurs at the level of the community unit, agreement to this taking place cannot occur at an individual level. Equally, because the intervention in a cluster-cluster trial is delivered at a community rather than an individual level, there is little or no scope for any individual community member to opt out (although individual consent may feasibly be given or withheld for outcome assessment or access to health records). For example, individuals cannot realistically exclude themselves from a community-based environmental management program aimed at reducing the incidence of dengue fever,22 unless such individual refusal of consent were deemed to constitute some sort of veto on the program as a whole. Moreover, in practical terms, individual consent on the part of patients is likely to be impossible in the case of very large clusters23 or when the practitioner, rather than his or her patients, is the immediate target of the intervention.24 On both logical and logistical grounds, individual informed consent is difficult if not impossible in a cluster-cluster cluster-randomized trial.
In the individual-cluster trial, the individual is similarly not well placed to give consent to the trial taking place because, just as in the case of cluster-cluster trials, this is a community rather than an individual decision. By contrast, given that the intervention occurs at the level of the particular patient, individual consent to that intervention (within the trial) is possible. For example, in an assessment of a new colorectal cancer–screening program, the focus of the cluster-randomized trial would be on the real-world acceptability of the program as a whole, not on the actual screening tool (which might already have been assessed with an RCT). In this case, any individual may opt out by choosing not to use the screening tool, but the nature of the cluster-randomized trial design may mean that alternative tests are unavailable. A further problem is that if consent is sought from individuals in control clusters, then the problem of contamination that the trial was likely designed to circumvent may reemerge. Individual consent is therefore possible in the individual-cluster cluster-randomized trial but risks being either inert or methodologically problematic.
POSSIBLE SOLUTIONS
Given the problems associated with achieving individual consent in cluster-randomized trials outlined in the previous section, one option would be to regard such cluster-randomized trials as unethical. However, we provide a series of reasons that cluster-randomized trials may nonetheless be ethically justifiable in the absence of such consent.
In the cluster-cluster cluster-randomized trial, it was argued that individual consent—in relation to both the study and the interventions tested within it—is not feasible, and this may be seen as a moral barrier to such a trial taking place. A possible counter to this objection would be to point out that in the everyday health care arena, individual consent is not normally considered necessary in relation to health care interventions that are implemented at a community or practice level and that, for this reason, a cluster-cluster cluster-randomized trial does not necessarily require individual consent. A change in a general treatment policy across a clinician’s practice, or a public health measure implemented within a community, is not generally subject to the agreement of individual patients or citizens. This is not just a question of the practicality of securing such individual agreement. It reflects an acceptance that such measures are legitimately decided at a higher level and that the same degree of consent is not expected for community-level interventions as it is for individual treatment decisions. This may reflect a greater willingness to delegate decision making to professionals in respect of community-level interventions and a tacit acknowledgment on the part of patients that they lack the same central role in such decisions that they possess in relation to individual care.
In the individual-cluster cluster-randomized trial, a somewhat different response is required to the objection that individual consent is an ethical sine qua non. It must be argued that individual consent in principle is often feasible but is nonetheless undesirable, or at least dispensable. One such argument rests on the methodological implications of individual consent. If such consent induces the very contamination that a cluster-randomized trial seeks to avoid, or leads to the recruitment of an unrepresentative sample,24 then the methodological integrity of the study may be undermined. Given our initial claims that methodological considerations carry moral weight and that no specific ethical requirement is absolute, we may argue that individual consent can be overridden in an individual-cluster cluster-randomized trial (assuming the research meets other moral requirements). This argument will depend on the relative moral weight given to consent and the goals of the study in the case in question. If a cluster-randomized trial seeks to address a major public health concern while representing minimal risk of harm to, or interference with, the lives of potential participants, and consent is deemed incompatible with the methodological integrity of the study, then it is reasonable to think that the need for consent can be waived.25 Of course, in the case of an individual-cluster cluster-randomized trial in which the risk of harm is markedly greater than minimal, the argument for individual informed consent may become stronger, to the point that it may not appropriately be overridden by the value of the goals of the study. This might mandate an alternative design to the cluster-randomized trial in which individual informed consent can be preserved. However, this serves to reinforce our central argument that no moral consideration can be regarded as absolute; neither the value attached to the goals of the study nor that attached to informed consent should be immune from being overridden.
The advocate of informed consent might concede all this but argue that rather than trying to dispense with consent entirely, we should find an alternative to the traditional individual model. Several alternative strategies are available, but each is problematic.
First, the most common proposal in the literature is that the community constituting the cluster could be represented by someone—a “guardian”7 or some other “cluster representation mechanism.”26 However, choosing an appropriately representative guardian (either a person or a body) for a particular cluster is not straightforward. It requires a high level of confidence that the guardian can represent the interests of all (or at least most) members of the cluster—and it is doubtful whether such a level of confidence is achievable. For example, Hutton10 argued that some such guardians (e.g., village elders or community leaders) may have conflicts of interest. Even if the guardian is an elected representative, there may be good grounds not to assume that such a guardian can speak for the relevant cluster.
Many ingenious proposals have been made to try and bridge this legitimacy deficit for cluster-randomized trials, including Hutton’s10 proposal for the use of multiple guardians and the range of suggestions by Edwards et al.7 covering opinion polls, through focus groups and citizens’ juries, to referenda. However, even if we accept any of these as sufficient to permit research to proceed, they do not really constitute a form of consent parallel to the individual informed consent presumed to be required for participation in an RCT. Moreover, in relation to the 2 types of consent required for a cluster-randomized trial, even if it is reasonable to seek guardian consent to a trial taking place in relation to a particular cluster-cluster cluster-randomized trial, it is perhaps less reasonable to use such consent in relation to the specific interventions being appraised in an individual-cluster cluster-randomized trial.
Second, it might be argued that consent to participate in a cluster-randomized trial can be presumed—for example, by invoking some sort of social contract whereby citizens can be assumed to be obligated to contribute to the public good through research participation. We should note, however, that a presumed consent is not an actual consent, and the idea of any such obligation is controversial.27
Third, we might appeal to the idea of subsequent consent, in that permission given after participation in the cluster-randomized trial might be sufficient to provide legitimacy. However, this seems to miss the point because consent given after the fact hardly counts as consent at all because the decision about participation that is central to the notion of consent has already been taken on the individual’s behalf.
Fourth, we might invoke the idea of hypothetical consent. This can take 2 forms. The first appeals to what an actual person would consent to if he or she were able to do so. This is problematic because it is doubtful that we would ever have sufficient information to reach the relevant evidential standard to ensure that the judgment really was what the person wanted. Thus, it seems unreasonable to use this as a means of opting out of (or, for that matter, into) a cluster-randomized trial. The second form appeals to a hypothetical about what any rational person would (or perhaps should) believe—but if this is to be the standard, why is it better than just asking any rational person or the relevant experts for their opinion?
All of these “technical fixes” seem misguided given that they do not solve 2 key problems. First, they provide no solution to the difficulty of fulfilling the disclosure and comprehension requirements for informed consent, which as previously argued are already problematic in the traditional consent situation. Second, and more important, such strategies raise a more general concern. Invoking the consent of 1 or more third parties, or appealing to some theoretical and unsatisfactory notion of “pseudoconsent,” takes us away from what consent really is. Such solutions are best seen not as varieties of consent but as substitutes for it. Thus, in the clinical situation, third-party consent from relatives or others is sought precisely because consent at the level of the individual is not possible (normally for reasons of incompetence). A parallel argument has been made in areas such as emergency medicine.28 In a similar way, we should accept that individual consent is either impossible or undesirable within many cluster-randomized trials but that cluster-randomized trials are too valuable to give up.
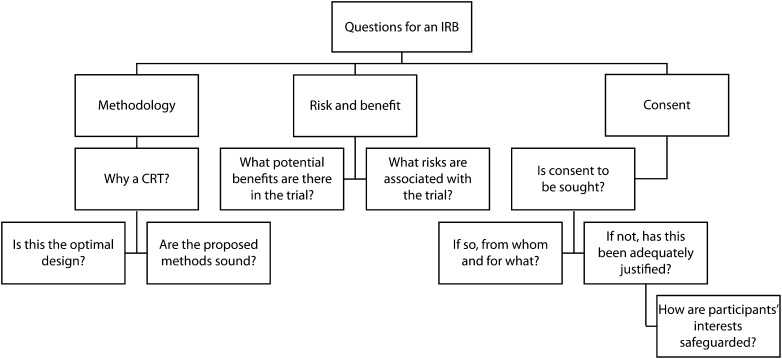
If the role of informed consent is limited in the context of cluster-randomized trials, what mechanisms can we use to ensure that such studies are ethically acceptable? The best practical option is to see institutional review boards (IRBs) as being in the preeminent position to act as guarantors that a cluster-randomized trial is ethical. The advantage of IRBs (over guardians or other cluster representation mechanisms) is that they do not purport to give a form of consent as a permission on behalf of a community; rather, they provide overall ethical scrutiny of a study from perspectives that include but extend beyond those of the participants in the study. In more moral terms, IRBs can focus on those ethical concerns that consent is designed to protect—the welfare of the participant, the privacy or confidentiality of his or her medical data, and so forth—and weigh these against the moral (and scientific) value of the study. The greater the moral importance of these considerations, and the more they are perceived to be central to individuals’ personal values and goals, the more weight they can be given in such calculations. Thus, although informed consent may not be sought in a cluster-randomized trial, the goals that it seeks to achieve may nonetheless be protected by other means. A starting point is for the IRB to ask a series of questions of researchers proposing a cluster-randomized trial if they have not provided such information in a protocol (Figure 1).
FIGURE 1—
Questions for those proposing a cluster-randomized trial (CRT) and an institutional review board (IRB) reviewing a proposal.
CONCLUSIONS
IRBs ought to be the key decision-making body in evaluating whether a particular cluster-randomized trial should go ahead; they are well positioned to synthesize arguments relating to both the ethical and the scientific aspects of a cluster-randomized trial protocol. The proposal to use other means of consent proposed in the literature are either misleadingly labeled as such or inadequate for other moral reasons. This, of course, does not mean that consultation with interested communities relevant to the proposed research cannot and should not occur.
Given the arguments presented earlier, no IRB should treat gaining informed consent as an absolute ethical requirement and therefore must not judge a cluster-randomized trial unethical if the proposed study protocol presents sound reasons that seeking individual informed consent is inappropriate. Nonetheless, the usual criteria used by the IRB will still apply. Researchers must articulate and defend their reasons for not obtaining informed consent in those particular circumstances. Sound reasons may include the impossibility of obtaining consent and damage to the possibility of answering an important proposed research question. To ensure that the use of cluster-randomized trials is ethically justifiable, future revisions of research ethics guidelines and regulations should not assume that the RCT is the paradigm method of research and should make it clear that other research methods are not just permissible but also may actually be preferable.
Human Participant Protection
No protocol approval was necessary because no human participants participated in this study.
References
- 1.Piantadosi S. Clinical Trials: A Methodologic Perspective. Hoboken, NJ: Wiley-Interscience; 2005 [Google Scholar]
- 2.Osrin D, Azad K, Fernandez Aet al. Ethical challenges in cluster randomized controlled trials: experiences from public health interventions in Africa and Asia. Bull World Health Organ. 2009;87:772–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar V, Mohanty S, Kumar Aet al. Effect of community-based behaviour change management on neonatal mortality in Shivgarh, Uttar Pradesh, India: a cluster-randomised controlled trial. Lancet. 2008;372:1151–1162 [DOI] [PubMed] [Google Scholar]
- 4.Figueiras A, Herdeiro MT, Polónia J, Gestal-Otero JJ. An educational intervention to improve physician reporting of adverse drug reactions: a cluster-randomized controlled trial. JAMA. 2006;296:1086–1093 [DOI] [PubMed] [Google Scholar]
- 5.Peri K, Kerse N, Robinson E, Parsons M, Parsons J, Latham N. Does functionally based activity make a difference to health status and mobility? A randomised controlled trial in residential care facilities (The Promoting Independent Living Study; PILS). Age Ageing. 2008;37:57–63 [DOI] [PubMed] [Google Scholar]
- 6.Smith AW, Hatcher J, Mackenzie IJet al. Randomised controlled trial of treatment of chronic suppurative otitis media in Kenyan schoolchildren. Lancet. 1996;348:1128–1133 [DOI] [PubMed] [Google Scholar]
- 7.Edwards SJL, Braunholtz DA, Lilford RJ, Stevens AJ. Ethical issues in the design and conduct of cluster randomised controlled trials. BMJ. 1999;318:1407–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donner A, Klar N. Design and Analysis of Cluster Randomisation Trials in Health Research. London, UK: Arnold; 2000 [Google Scholar]
- 9.Campbell MJ. Cluster randomized trials in general (family) practice research. Stat Methods Med Res. 2000;9:81–94 [DOI] [PubMed] [Google Scholar]
- 10.Hutton JL. Are distinctive ethical principles required for cluster randomized controlled trials? Stat Med. 2001;20:473–488 [DOI] [PubMed] [Google Scholar]
- 11.Sabin JE, Mazor K, Meterko V, Goff SL, Platt R. Comparing drug effectiveness at health plans: the ethics of cluster randomized trials. Hastings Cent Rep. 2008;38(5):39–48 [DOI] [PubMed] [Google Scholar]
- 12.Verweij M, Dawson A. Public health research ethics: a research agenda. Public Health Ethics. 2009;2:1–6 [Google Scholar]
- 13.Dawson A, Garrard E. In defence of moral imperialism: four equal and universal prima facie duties. J Med Ethics. 2006;32:200–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawson AJ, Yentis SM. Contesting the science/ethics distinction in the review of clinical research. J Med Ethics. 2007;33:165–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Council for International Organizations of Medical Sciences (CIOMS) International Ethical Guidelines for Biomedical Research Involving Human Subjects. Geneva, Switzerland: CIOMS; 2002. Available at: http://www.cioms.ch/publications/guidelines/guidelines_nov_2002_blurb.htm. Accessed August 8, 2010 [PubMed] [Google Scholar]
- 16.Office for Human Research Protection (OHRP) Protection of Human Subjects. Federal Regulations, Title 45, Part 46. Washington, DC: US Dept of Health and Human Services; 2009. Available at: http://ohsr.od.nih.gov/guidelines/45cfr46.html. Accessed August 8, 2010 [Google Scholar]
- 17.World Medical Association (WMA) Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects. 2008. Available at: http://www.wma.net/en/30publications/10policies/b3/index.html. Accessed August 8, 2010 [Google Scholar]
- 18.Sugarman J, McCrory D, Powell D, Krasny A, Ball E, Cassell C. Empirical research on informed consent. Hastings Cent Rep. 1999;29(suppl):S1–S42 [PubMed] [Google Scholar]
- 19.Dawson A. The normative status of the requirement to gain an informed consent in clinical trials: comprehension, obligations and empirical evidence. : Corrigan O, Liddell K, McMillan J, Richards M, Weijer C, The Limits of Consent: A Socio-Legal Approach to Human Subject Research in Medicine. Oxford, UK: Oxford University Press; 2009:99–113 [Google Scholar]
- 20.Council for International Organizations of Medical Sciences (CIOMS) International Ethical Guidelines for Epidemiological Research. Geneva, Switzerland: CIOMS; 2009 [Google Scholar]
- 21.Donner A. Some aspects of the design and analysis of cluster randomization trials. J R Stat Soc Ser C Appl Stat. 1998;47:95–113 [Google Scholar]
- 22.Vanlerberghe V, Toledo ME, Rodríguez M, et al. Community involvement in dengue vector control: cluster randomised trial. BMJ. 2009;338:b1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donner A, Klar N. Pitfalls of and controversies in cluster randomized trials. Am J Public Health. 2004;94:416–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutton JL, Eccles MP, Grimshaw JM. Ethical issues in implementation research: a discussion of the problems in achieving informed consent. Implement Sci. 2008;3:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sim J. Addressing conflicts in research ethics: consent and risk of harm. Physiother Res Int. 2010;15:80–87 [DOI] [PubMed] [Google Scholar]
- 26.Medical Research Council (MRC) Cluster Randomised Trials: Methodological and Ethical Considerations. London, England: Medical Research Council; 2002 [Google Scholar]
- 27.John S. Is there an obligation to participate in medical research? : Corrigan O, McMillan J, Liddell K, Richards M, Weijer C, The Limits of Consent: a Socio-Ethical Approach to Human Subject Research in Medicine. Oxford, UK: Oxford University Press; 2009:115–132 [Google Scholar]
- 28.Largent EA, Wendler D, Emanuel E, Miller FG. Is emergency research without initial consent justified? The consent substitute model. Arch Intern Med. 2010;170:668–674 [DOI] [PubMed] [Google Scholar]