Abstract
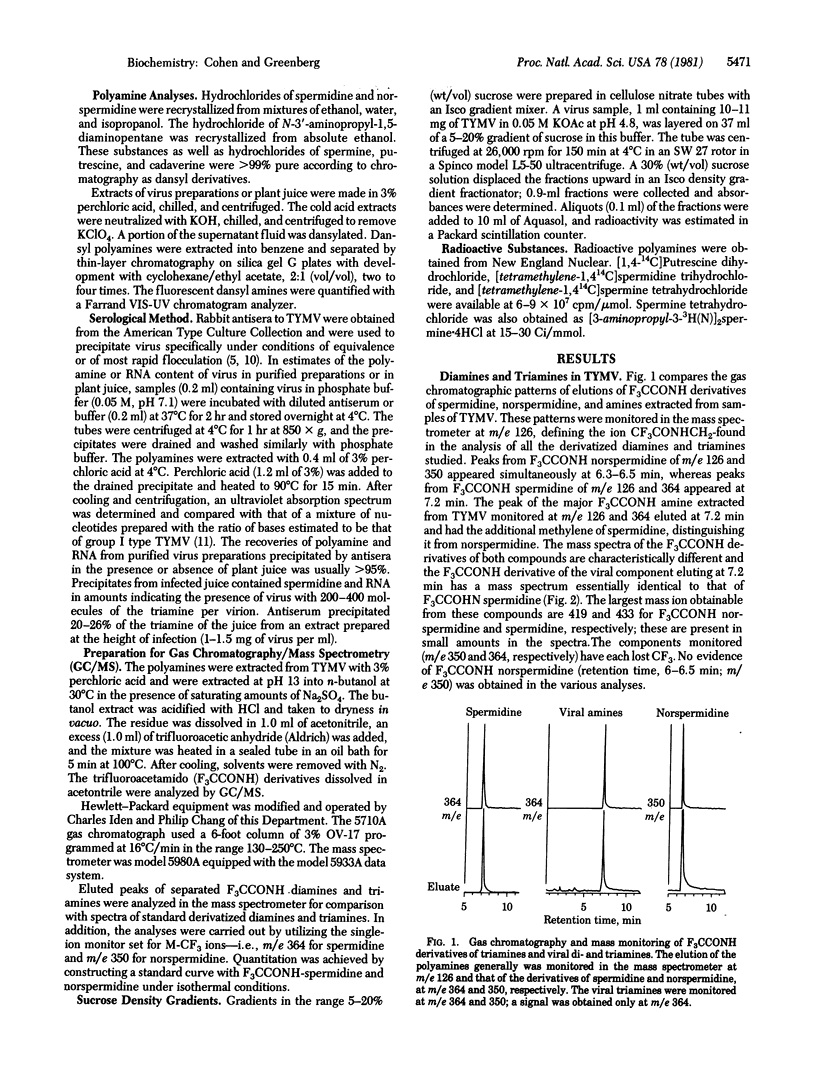
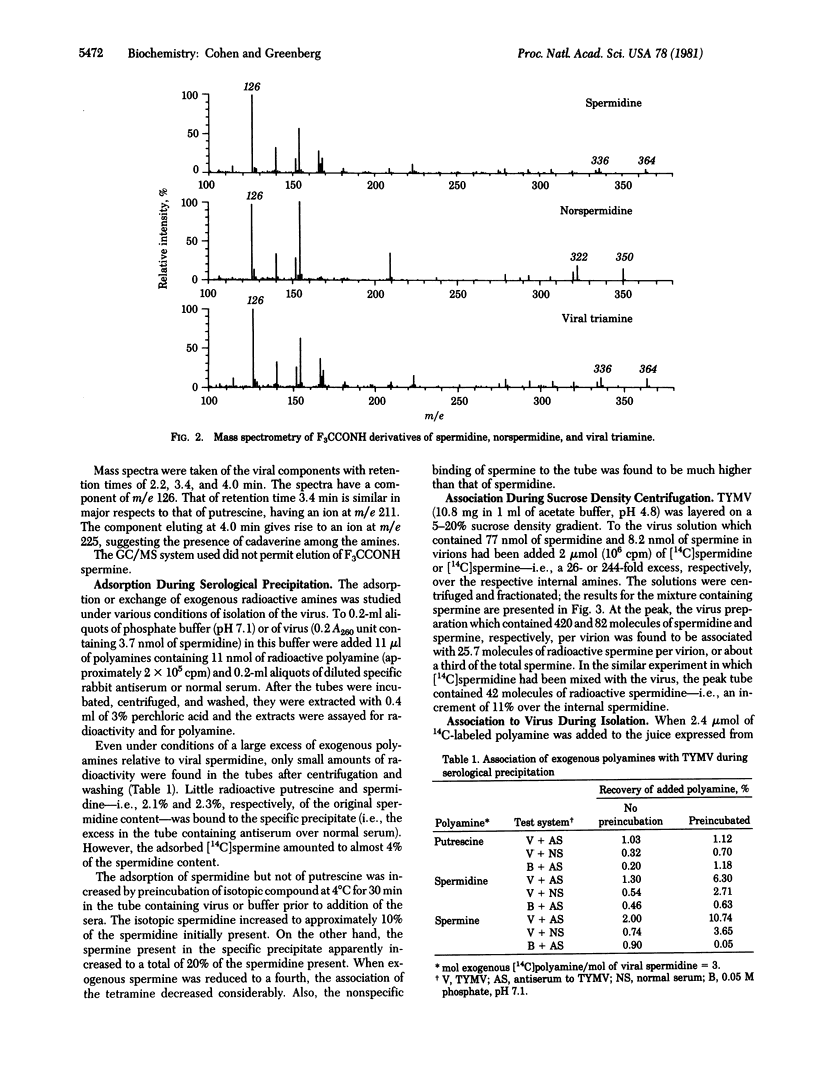
The major polyamine of turnip yellow mosaic virus has been identified as spermidine by gas chromatography and mass spectrometry of the trifluoroacetamido derivative. Very small amounts of putrescine and cadaverine, but not norspermidine, have been detected in the virus. The spermidine contents of numerous virus preparations were in the range 200-700 molecules per virion and were considerably in excess of those of spermine. The RNA and spermidine contents of small amounts of the virus were determined after serological precipitation in purified preparations and in the juice of infected plants. Under conditions of the precipitation or purification from juice by differential centrifugation, only small amounts of exogenous radioactive spermidine and spermine became bound to virus. Although adsorbed radioactive spermidine could be removed almost quantitatively by washing the virus in dilute buffers, only a small part of adsorbed spermine was removed by such treatment. However, greater than 95% of the newly attached spermine was separated from the virus without loss of the original spermidine by sedimentation in buffers containing 0.5 M NaCl or 0.06 M MgCl2. Crystallization of virus in 40% saturated MgSO4 or 7.5% heparin or dialysis did not decrease viral spermidine. Although the virus coat may adsorb small amounts of the polyamines reversibly, the virus is impermeable to exogenous spermidine and spermine and does not exchange or leak internal spermidine. The spermidine present in purified virus is associated with viral RNA at the time of packaging and formation of intact virions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Bartos D., Bartos F., Campbell R. A., Grettie D. P., Smejtek P. Antibody to spermine: a natural biological constituent. Science. 1980 Jun 6;208(4448):1178–1181. doi: 10.1126/science.7375929. [DOI] [PubMed] [Google Scholar]
- Beer S. V., Kosuge T. Spermidine and spermine--polyamine components of turnip yellow mosaic virus. Virology. 1970 Apr;40(4):930–938. doi: 10.1016/0042-6822(70)90139-x. [DOI] [PubMed] [Google Scholar]
- COHEN S. S., SCHACHMAN H. K. Physical studies on the ribonucleic acid of turnip yellow mosaic virus. Virology. 1957 Jun;3(3):575–586. doi: 10.1016/0042-6822(57)90011-9. [DOI] [PubMed] [Google Scholar]
- Furuichi K., Ezoe H., Obara T., Oka T. Evidence for a naturally occurring anti-spermine antibody in normal rabbit serum. Proc Natl Acad Sci U S A. 1980 May;77(5):2904–2908. doi: 10.1073/pnas.77.5.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON M. W., MARKHAM R. Nature of the polyamine in plant viruses. Virology. 1962 Jun;17:276–281. doi: 10.1016/0042-6822(62)90117-4. [DOI] [PubMed] [Google Scholar]
- Mezzetti G., Loor R., Liao S. Androgen-sensitive spermine-binding protein of rat ventral prostate. Purification of the protein and characterization of the hormonal effect. Biochem J. 1979 Nov 15;184(2):431–440. doi: 10.1042/bj1840431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roch A. M., Quash G., Huppert J. Mise en évidence dans les sérums humains d'anticorps (IgG) réagissant spécifiquement avec les polyamines. C R Acad Sci Hebd Seances Acad Sci D. 1978 Oct 30;287(11):1071–1074. [PubMed] [Google Scholar]
- Sakai T. T., Cohen S. S. Effects of polyamines on the structure and reactivity of tRNA. Prog Nucleic Acid Res Mol Biol. 1976;17:15–42. doi: 10.1016/s0079-6603(08)60064-1. [DOI] [PubMed] [Google Scholar]
- Torget R., Lapi L., Cohen S. S. Synthesis and accumulation of polyamines and S-adenosylmethionine in Chinese cabbage infected by turnip yellow mosaic virus. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1132–1139. doi: 10.1016/s0006-291x(79)80025-x. [DOI] [PubMed] [Google Scholar]



