Abstract
The National Human Genome Research Institute recommends pursuing “genomic information to improve behavior change interventions” as part of its strategic vision for genomics. The limited effectiveness of current behavior change strategies may be explained, in part, by their insensitivity to individual variation in adherence responses.
The first step in evaluating whether genomics can inform customization of behavioral recommendations is evidence reviews to identify adherence macrophenotypes common across behaviors and individuals that have genetic underpinnings. Conceptual models of how biological, psychological, and environmental factors influence adherence also are needed.
Researchers could routinely collect biospecimens and standardized adherence measurements of intervention participants to enable understanding of genetic and environmental influences on adherence, to guide intervention customization and prospective comparative effectiveness studies.
IN ITS RECENTLY PUBLISHED Nature article, “Charting a Course for Genomic Medicine: From Base Pairs to Bedside,” the National Human Genome Research Institute describes research priorities for the coming decade, with the overarching aim of using a genomic understanding of disease to inform improvements in medical care.1 The breadth of territory covered is impressive, but it precludes precise delineation of the many and diverse directions that could be taken to achieve this vision.
Noteworthy for the field of public health is the recommendation for research to pursue applications of “genomic information to improve behavior change interventions.”1(p210) Indeed, the fundamental importance of behavior change in any efforts to improve health outcomes is indisputable. Behavioral risk factors (e.g., tobacco use, poor diet, and physical inactivity) are major contributors to the incidence of chronic diseases worldwide.2 Recognition is increasing that reducing the burden of chronic disease will require researchers to acknowledge the complex interrelationships among behavior, environment (both social and physical), and genetics3: the genomic perspective.
Nascent research suggests elemental questions and initial hypotheses about how genomic discovery may lead to more effective behavior change interventions. Certain crucial tenets should guide this exploration: (1) current intervention strategies have had limited effect on long-term behavior change and need innovation, (2) standard behavior change recommendations are insensitive to individual variation in intervention response, and (3) understanding the genomics that underlie individual variation could suggest methods for customizing behavior change recommendations to be evaluated in comparative effectiveness trials.
REALISTIC EXPECTATIONS
Posing the research question, Can genomic knowledge be used to improve behavioral interventions? is different from suggesting that genomics is the holy grail for effective interventions. Indeed, any innovation derived from understanding genomic influences on behavioral adherence will be implemented and evaluated in the context of ubiquitous and competing environmental influences. Numerous reviews document the powerful negative influence that pervasive marketing of unhealthy products and misleading advertisements for prescription drugs have on health behaviors.4–8 Public policy interventions have also had powerful positive influences on health behaviors, for example, in prompting a steady decline in per capita consumption of cigarettes in the United States over the past 35 years.9
Three decades of community-based and public policy research also demonstrate that environmental interventions, while necessary, likely are not sufficient for promoting lasting behavior change.3 Indeed, rapidly emerging information about epigenetics is demonstrating that social and physical environments (e.g., stress, pollutants) may modify our genome to produce new phenotypes.10 The converging forces of genomic discovery, technological advances, decreasing cost of generating genomic information, and growing evidence about joint gene–environment influences on health oblige intervention researchers to consider whether these developments offer any guidance for improving behavior change interventions.
Any benefits derived from new genomic knowledge must be augmented by a broader armamentarium of strategies that, in combination, can be targeted optimally toward promoting improvements in population health.
INNOVATION NEEDED
This is a good time to examine how genomics research might bring innovation to behavior change interventions and produce robust and durable improvements in tobacco and alcohol use, dietary behaviors, and physical activity. The National Science Foundation recently reported leading scholars’ perceptions of the “most pressing questions” facing social scientists, that is, to identify “grand challenge questions that are both foundational and transformative.”11(p18) How to induce people to take care of their health turned out to be the number one priority on this top-10 list. Despite widespread awareness of the importance of health behaviors for preventing common diseases, national surveys document the public’s poor adherence to recommendations for diet, physical activity, and alcohol use; adherence levels in these areas have declined steadily since 1988.12 A Cochrane review evaluating adherence to medical prescriptions for chronic health conditions yielded similar findings.13
A recent survey assessing behavioral risk factors among a sample of American adults offers insight into these adherence declines. In this population-based sample of managed care enrollees, respondents with the most behavioral risk factors were the least interested in seeking behavioral risk information.14 Possibly these respondents previously sought and applied standard behavior change advice without success. Such failure experiences could create message cynicism or fatigue, phenomena associated with long-term and repeated exposure to public health messages.15,16
Whether behavior change interventions have reached their maximum effectiveness is a matter of perspective. Several systematic reviews of controlled trials show that interventions are just as likely to be ineffective as effective,17 achieve low-to-moderate changes in diet and exercise,18,19 and produce only short-lived behavior change for most participants. Despite progress with tobacco control, 46 million Americans still smoke.20 Yet publicly available interventions, such as state tobacco quitlines intended to broadly disseminate effective smoking cessation interventions, are underused by target groups.21 With prevalence exceeding 30%, obesity now rivals tobacco use as a leading cause of preventable morbidity and mortality.22 Although current approaches work for some, it is hard to dispute that better behavior change interventions are urgently needed.
Individual Variability
Improving the effectiveness of behavior change interventions requires increasing adherence. Adherence describes the complement of actions taken to comply with intervention recommendations (e.g., exercising at 60% of maximum oxygen uptake for 30 minutes, maintaining optimal daily calories, or routinely using pharmacotherapy, within prescribed limits, to reduce nicotine cravings); these are distinct from gold-standard postintervention behavior change outcomes (e.g., cardiovascular fitness, weight loss, abstinence from cigarettes). Individuals vary in how they respond both physically and emotionally to behavioral recommendations: this is the adherence response.
Increasing evidence indicates that genetic variation accounts for some of the differences in physiological responses to caloric restriction, dietary composition, and engagement in moderate or intensive physical activity regimens.23–31 These first-generation studies were small, retrospective, and targeted at select groups (e.g., perimenopausal women, college students, and professional athletes), but they represent an intriguing starting point.
Individualizing Recommendations
Use of genomics to individualize treatment has been embraced enthusiastically as a means to reduce unwanted and often dangerous side effects of pharmacological agents. The potential contribution of genomics to customizing behavior change recommendations has yet to be rigorously investigated.
Why pursue such a line of research? The fanfare surrounding the Human Genome Project raised public expectations that genomic discoveries would help individuals reduce their risk for disease; indeed, direct-to-consumer genetic testing companies are capitalizing on these expectations. Recent experiences with the Multiplex Initiative,32 Scripps–Navigenics Health Compass cohort,33 and numerous studies evaluating the use of genomic risk communication to motivate behavior change34 reveal that genetic test seekers are knowledgeable about risk factors for common diseases and amply motivated to improve health behaviors. Unfortunately, our limited current genomic knowledge offers these engaged individuals generic behavior change recommendations that provide little or no new information. Whether and how enhanced genomic knowledge can inform the customization of behavior change interventions, particularly in ways that improve adherence and intervention effectiveness are scientific questions worth exploring.
FROM SUPPOSITION TO SCIENCE
We propose 4 steps to initiate research on genomics-informed customization of behavior change interventions: (1) review scientific literature to identify areas with potential to influence behavioral adherence (e.g., phenotypes that are likely to have strong genetic underpinnings and are associated with the relevant health condition), especially those that are common across behaviors (e.g., dopaminergic rewards associated with eating, such as DRD4 genes,35 and brain-derived neurotrophic factors involved in energy metabolism, such as BDNF genes36); (2) develop conceptual models to map the interrelationships of relevant biological, psychological, and macro-level factors that influence adherence; (3) analyze data from existing intervention studies with collected biospecimens to jump-start discovery studies; and (4) conduct prospective comparative effectiveness studies to evaluate whether genotype-informed customization adds value to current behavior change interventions.
Evidence Reviews
Systematic literature reviews could characterize categories of physiological and subjective adherence responses that are common across behavioral interventions, such as stress tolerance, reward and pleasure, mood, and metabolism. The rationale for this effort is to examine whether adherence response macrophenotypes share common biological mechanisms and genetic underpinnings (e.g., linking affective response to dopamine receptor genes).
A comprehensive literature review also would help to map the evidence along the temporal continuum of adherence from initial attempts (initiation) through early habituation (engagement) and relapse–recovery responses (reengagement). Such systematic reviews could pave the way to characterizing biobehavioral conceptual models that are needed to guide hypothesis development and testing.
Generating Hypotheses
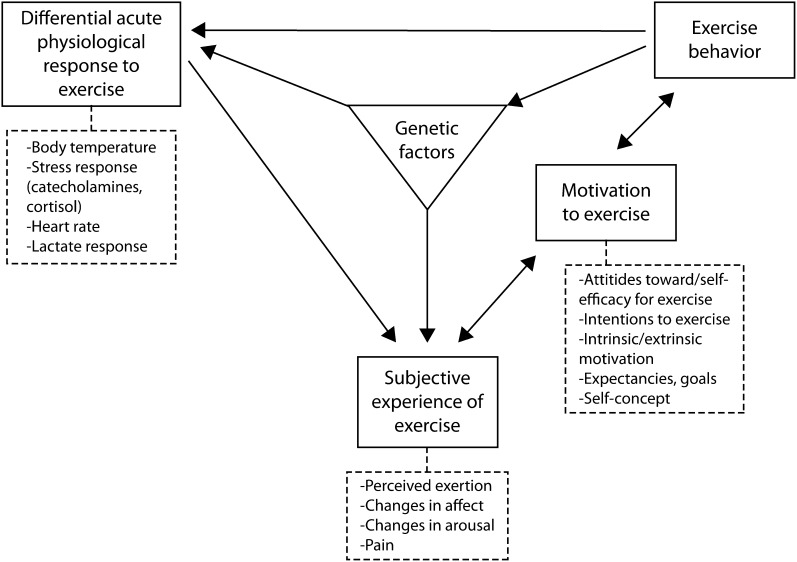
Conceptual models will be essential in guiding systematic consideration of the multiple pathways that influence adherence responses. Bryan et al.37 propose a model that suggests possible points in the pathway to improved physical activity at which to introduce interventions (Figure 1); this model could be applied to other types of behavior change. The model posits that individual variability, influenced by genetics, in physiological response (e.g., thermoregulation) and in subjective experience (e.g., improved mood state) may explain individual reports of limited tolerance for some or all aspects of intervention recommendations. Building conceptual models for a range of health behaviors could guide discovery studies in exploring whether genetically influenced macrophenotypes that inhibit intervention adherence are common across behaviors and along the adherence continuum. Such information could prompt innovation by moving beyond behavior-specific interventions to target these macrophenotypes (e.g., tolerance for negative mood or other physiological responses prompted by adherence).
FIGURE 1—
Computer rendering of a handgun designed for elderly and disabled persons.
Source. Constitution Arms.1
Exploring pathways involved in behavioral reward and reinforcement is another worthwhile research pursuit. Adherence to any behavior change effort will be influenced by the extent to which it is rewarding, as experienced through the application of various schedules of reinforcement provided by the behavior change program. Determining the extent to which genomic variation is related to the variation in human response to different schedules of reinforcement could lend insight into contingency management (and the use of different schedules) in the design of behavior change programs personalized to varying genetic makeups.38
Whether and how genomics may contribute to choices regarding alternative behaviors (healthy and unhealthy) are questions as yet largely unexplored.39 Research is needed to elucidate the underlying neurobiology of responses to various schedules of reinforcement and behavioral economic choices within the context of behavior change interventions. Early research suggests that reward pathways and dopaminergic functioning could play a central role in maximizing adherence to health behavior change programs and that innovative strategies may be found to encourage new behaviors to stimulate this pathway.40,41
Discovery and Comparative Effectiveness Studies
Literature review and conceptual model development may point to candidate genes associated with adherence responses at specific junctures along the adherence continuum. Such approaches have been suggested for understanding tobacco use and dependence phenotypes.42 To our knowledge, with the exception of the Training Interventions and Genetics of Exercise Response study,43 no research has characterized adherence responses for specific behavioral interventions or explored common responses across behavioral interventions. Ongoing large intervention programs (e.g., state quitlines and Weight Watchers) that encourage self-reported adherence (e.g., daily food and activity diaries) could be enlisted to add objective monitoring (e.g., personal digital assistants, pedometers, heart rate monitors) and biospecimens (e.g., buccal samples for DNA analysis), thereby enabling the testing of hypotheses derived from the previous steps in large, heterogeneous samples.
Identifying conceptual pathways, candidate genes, and their associations with adherence macrophenotypes could pave the way for prospective intervention trials that put customization based on genomic information to the ultimate test: Do such approaches add value to current behavior change interventions, and are they cost effective? Socioenvironmental contexts and target populations for which intervention adherence presents the biggest challenge are the best places to start with such comparative effectiveness studies because they hold the greatest potential for public health impact. However, in any efforts to innovate, the interests of those who benefit from current interventions must be protected. Clearly, intervention reach to these populations must be maintained.44
COLLABORATION
Transdisciplinary collaborations will be essential to building an evidence base to reveal whether and how genomics can contribute to behavior change interventions.3 Engaging public health researchers—including those in the social, communication, and behavioral sciences—will be key in any translational research efforts to consider whether genomic knowledge can inform intervention innovation and to integrate best practices with that innovation. The deepening appreciation that new genomic findings can engender considerable public controversy and concern about social impact means that experts in community-based participatory research also will have a central role in these endeavors.45,46
It will be important to build collaboration readiness across multiple disciplines to encourage full participation and leadership in shaping behavioral genomics research questions and methods. Building competencies in disciplinary cross talk, scientific advocacy, and the team process will be needed for these collaborations to flourish and achieve their full potential.
These efforts could expand the collective scientific imagination for efforts that move beyond the current and limited focus on genetic risk communications to motivate behavior change and to begin considering whether genomic knowledge can inform customized recommendations that more successfully help individuals modify behaviors. Considering whether genomic discoveries can catalyze such innovations is warranted in light of the crucial importance of improving health behaviors for redressing public health epidemics (e.g., obesity) and the soaring health care costs generated by chronic disease.
Acknowledgments
We gratefully acknowledge participants in a working group organized by the National Institutes of Health for their input to the ideas presented in this article: Tanya Agurs-Collins and Erik Augustson, National Cancer Institute; Lawrence Brody, Maximilian Muenke, Ryan Paquin, and Emi Watanabe, National Human Genome Research Institute; Kevin Conway, National Institute of Drug Abuse; Deborah Olster and Kay Wanke, Office of Behavioral and Social Sciences Research; Marcia Scott, National Institute of Alcohol Abuse and Alcoholism.
References
- 1.Green ED. Guyer MS, National Human Genome Research Institute. Charting a course for genomic medicine from base pairs to bedside. Nature. 2011;470(7333):204–213 [DOI] [PubMed] [Google Scholar]
- 2. 3FOUR50 Connect-Collaborate-Create. Available at: http://www.3four50.com. Accessed December 19, 2011.
- 3.Mabry PL, Olster DH, Morgan GD, Abrams DB. Interdisciplinarity and systems science to improve population health: a view from the NIH Office of Behavioral and Social Sciences Research. Am J Prev Med. 2008;35(2 Suppl):S211–S224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brezis M, Wiist WH. Vulnerability of health to market forces. Med Care. 2011;49(3):232–239 [DOI] [PubMed] [Google Scholar]
- 5.Wellman RJ, Sugarman DB, DiFranza JR, Winickoff JP. The extent to which tobacco marketing and tobacco use in films contribute to children’s use of tobacco: a meta-analysis. Arch Pediatr Adolesc Med. 2006;160(12):1285–1296 [DOI] [PubMed] [Google Scholar]
- 6.Cohen EL, Caburnay CA, Rodgers S. Alcohol and tobacco advertising in Black and general audience newspapers: targeting with message cues? J Health Commun. 2011;16(6):566–582 [DOI] [PubMed] [Google Scholar]
- 7.Harris JL, Pomeranz JL, Lobstein T, Brownell KD. A crisis in the marketplace: how food marketing contributes to childhood obesity and what can be done. Annu Rev Public Health. 2009;30211–225 [DOI] [PubMed] [Google Scholar]
- 8.Frosch DL, Grande D, Tarn DM, Kravitz RL. A decade of controversy: balancing policy with evidence in the regulation of prescription drug advertising. Am J Public Health. 2010;100(1):24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention. Cigarette smoking among adults—United States, 2001. MMWR Morb Mortal Wkly Rep. 2003;52(40):953–956. [PubMed]
- 10.Petronis A. Epigenetics as a unifying principle in the aetiology of complex traits and diseases. Nature. 2010;465(7299):721–727 [DOI] [PubMed] [Google Scholar]
- 11.Giles J. Social science lines up its biggest challenges. Nature. 2011;470(7332):18–19 [DOI] [PubMed] [Google Scholar]
- 12.King DE, Mainous AG, 3rd, Carnemolla M, Everett CJ. Adherence to healthy lifestyle habits in US adults, 1988–2006. Am J Med. 2009;122(6):528–534 [DOI] [PubMed] [Google Scholar]
- 13.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;16(2):CD000011. [DOI] [PubMed] [Google Scholar]
- 14.O’Neill SC, McBride CM, Alford SH, Kaphingst KA. Preferences for genetic and behavioral health information: the impact of risk factors and disease attributions. Ann Behav Med. 2010;40(2):127–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson T, Mayer J, Weaver F. Prevention message fatigue as an influence on condom use among urban MSM [abstract]. Paper presented at: 131st Annual Meeting of the American Public Health Association, November 17, 2003. Available at: http://apha.confex.com/apha/131am/techprogram/paper57839.htm. Accessed December 12, 2009.
- 16.Goldberg JP. Nutrition and health communication: the message and the media over half a century. Nutr Rev. 1992;50(3):71–77 [DOI] [PubMed] [Google Scholar]
- 17.Michie S, Jochelson K, Markham WA, Bridle C. Low-income groups and behavior change interventions: a review of intervention content, effectiveness and theoretical frameworks. J Epidemiol Community Health. 2009;63(8):610–622 [DOI] [PubMed] [Google Scholar]
- 18.Johnson BT, Scott-Sheldon LA, Carey MP. Meta-synthesis of health behavior change meta-analyses. Am J Public Health. 2010;100(11):2193–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krebs P, Prochaska JO, Rossi JS. A meta-analysis of computer-tailored interventions for health behavior change. Prev Med. 2010;51(3–4):214–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiore MC, Jaen CR, Baker TBet al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: Public Health Service; 2008 [Google Scholar]
- 21.Kaufman A, Augustson E, Davis K, Finney Rutten LJ. Awareness and use of tobacco quitlines: evidence from the Health Information National Trends Survey. J Health Commun. 2010;15(Suppl 3):264–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen DM, El-Serag HB. The epidemiology of obesity. Gastroenterol Clin North Am. 2010;39(1):1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiwaku K, Nogi A, Anuurad Eet al. Difficulty in losing weight by behavioral intervention for women with Trp64Arg polymorphism of the b3-adrenergic receptor gene. Int J Obes. 2003;271028–1036 [DOI] [PubMed] [Google Scholar]
- 24.Hutchison KE, Allen DL, Filbey FMet al. CHRNA4 and tobacco dependence: from gene regulation to treatment outcome. Arch Gen Psychiatry. 2007;64(9):1078–1086 [DOI] [PubMed] [Google Scholar]
- 25.Jenkins NT, McKenzie JA, Damcott CM, Witkowski S, Hagberg JM. Endurance exercise training effects on body fatness V02max, HDL-C subfractions, and glucose tolerance are influenced by PLIN hapolotype in older caucasions. J Appl Physiol. 2010;108(3):498–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bryan A, Hutchison KE, Seals DR, Allen DL. A transdisciplinary model integrating genetic, physiological and psychological correlates of voluntary exercise. Health Psychol. 2007;26(1):30–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonestedt E, Roos C, Gullberg B, Ericson U, Wirfalt E, Orho-Melander M. Fat and carbohydrate intake modify the association between genetic variation in the FTO genotype and obesity. Am J Clin Nutr. 2009;90(5):1418–1425 [DOI] [PubMed] [Google Scholar]
- 28.Arkadianos I, Valdes AM, Marinos E, Florou A, Gill RD, Grimaldi KA. Improved weight management using genetic information to personalize a calorie controlled diet. Nutr J. 2007;629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho SW, Kang JY, Park YK, Paek YM, Choi TI. A 12-week worksite health promotion program reduces cardiovascular risk factors in male workers with the apolipoprotein E2 and apolipoprotein E3 genotypes, but not in apolipoprotein E4 genotype. Nutr Res. 2009;29(8):542–550 [DOI] [PubMed] [Google Scholar]
- 30.Rethorst CD, Landers DM, Nagoshi CT, Ross JTD. Efficacy of exercise in reducing depressive symptoms across 5-HTTLPR genotypes. Med Sci Sports Exerc. 2010;42(11):2141–2147 [DOI] [PubMed] [Google Scholar]
- 31.Matsuo T, Nakata Y, Katayama Yet al. PPARG genotype accounts for part of individual variation in body weight reduction in response to calorie restriction. Obesity (Silver Spring). 2009;17(10):1924–1931 [DOI] [PubMed] [Google Scholar]
- 32.McBride CM, Alford SH, Reid RJ, Larson EB, Baxevanis AD, Brody LC. Characteristics of users of online personalized genomic risk assessments: implications for physician-patient interactions. Genet Med. 2009;11(8):582–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bloss CS, Schork NJ, Topol EJ. Effect of direct-to-consumer genomewide profiling to assess disease risk. N Engl J Med. 2011;346(6):524–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McBride CM, Koehly LM, Sanderson SC, Kaphingst KA. The behavioral response to personalized genetic information: will genetic risk profiles motivate individuals and families to choose more healthful behaviors? Annu Rev Public Health. 2010;3189–103 [DOI] [PubMed] [Google Scholar]
- 35.Stice E, Dagher A. Genetic variation in dopaminergic reward in humans. Forum Nutr. 2010;63176–185 [DOI] [PubMed] [Google Scholar]
- 36.Noble EE, Billington C, Kotz CM, Wang C. The lighter side of BDNF. Am J Physiol Regul Integr Comp Physiol. 2011;300(5):R1053–R1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bryan AD, Nilsson R, Tompkins SA, Magnan RE, Marcus BH, Hutchison KE. The big picture of individual differences in physical activity behavior change: a transdisciplinary approach. Psychol Sport Exerc. 2011;12(1):20–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dallery J, Raiff BR. Contingency management in the 21st century: technological innovations to promote smoking cessation. Subst Use Misuse. 2011;46(1):10–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson MW, Bickel WK, Kirshenbaum AP. Substitutes for tobacco smoking: a behavioral economic analysis of nicotine gum, denicotinized cigarettes, and nicotine-containing cigarettes. Drug Alcohol Depend. 2004;74(3):253–264 [DOI] [PubMed] [Google Scholar]
- 40.Filbey FM, Claus ED, Morgan M, Forester GR, Hutchison K. Dopaminergic genes modulate response inhibition in alcohol abusing adults. Addict Biol. 2011;Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hutchison KE. Substance use disorders: realizing the promise of pharmacogenomics and personalized medicine. Annu Rev Clin Psychol. 2010;6577–589 [DOI] [PubMed] [Google Scholar]
- 42.Swan GE, Hudmon KS, Jack LMet al. Environmental and genetic determinants of tobacco use: methodology for a multidisciplinary, longitudinal family-based investigation. Cancer Epidemiol Biomarkers Prev. 2003;12(10):994–1005 [PMC free article] [PubMed] [Google Scholar]
- 43.Sailors MH, Jackson AS, McFarlin BKet al. Exposing college students to exercise: the Training Interventions and Genetics of Exercise Response (TIGER) study. J Am Coll Health. 2010;59(1):13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Emmons KM, Viswanath K, Colditz GA. The role of transdisciplinary collaboration in translating and disseminating health research: lessons learned and exemplars of success. Am J Prev Med. 2008;35(suppl 2):S204–S210 [DOI] [PubMed] [Google Scholar]
- 45.Oliver JM, McGuire AL. Exploring the ELSI universe: critical issues in the evolution of human genomic research. Genome Med. 2011;3(6):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hebert JR, Brandt HM, Armstead CA, Adams SA, Steck SE. Interdisciplinary, translational, and community-based participatory research: finding a common language to improve cancer research. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1213–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]