Abstract
The basal ganglia are a chain of subcortical nuclei that facilitate action selection. Striatal direct and indirect pathways form the functional backbone of the basal ganglia circuit. Twenty years ago it was proposed that the ability of the striatum to use the rise and fall of dopamine (DA) to control action selection was due to the segregation of D1 and D2 DA receptors in direct and indirect pathway spiny projection neurons. Although this sparked a debate, the evidence accumulated since clearly supports this model. In particular, recent advances in the means of marking neural circuits with optical or molecular reporters has revealed a clear-cut dichotomy between these two cell types at the molecular, anatomical and physiological levels. The contrast provided by these studies has provided new insights into how the striatum responds to fluctuations in DA signaling and how diseases that alter this signaling change striatal function.
Keywords: Basal ganglia, synaptic plasticity, movement disorders, Parkinson’s Disease, motor learning
Introduction
The basal ganglia are part of the forebrain circuitry involved in the selection of actions (Mink, 1996; Redgrave et al., 1999; Wichmann and DeLong, 2003; Cisek and Kalaska, 2010). Diseases of the basal ganglia result in a spectrum of movement disorders from those characterized by hypokinesia, such as Parkinson’s disease (PD) to those producing hyperkinesia, such as chorea and dystonia (Mink, 2003). A cardinal feature of the striatum – the principal of cortical and thalamic information reaching the basal ganglia –is its dense DA innervation, which arises from the midbrain nigrostriatal pathway (Bolam et al., 2000). This input is critical to normal functioning of the striatum and basal ganglia. One of the major targets of this innervation is the principal neuron of the striatum: GABAergic spiny projection neurons (SPNs). SPNs constitute as much as 90% of the striatal neuron population. SPNs can be divided into two approximately equally-sized populations based on axonal projections. One population – direct pathway SPNs – projects axons to the nuclei at the interface between the basal ganglia and the rest of the brain, whereas the other population – indirect pathway SPNs – projects only to an intermediate basal ganglia nucleus, connecting only indirectly to the interface nuclei.
A second distinguishing feature of direct and indirect pathway SPNs is their differential expression of DA receptors. The D1 DA receptor (Drd1a) is expressed selectively by direct pathway SPNs, whereas the D2 (Drd2) is expressed by indirect pathway SPNs. These two G-protein coupled receptors (GPCRs) receptors have distinctive linkages to intracellular signaling cascades and targets, leading to fundamentally different cellular responses to extracellular DA. The dichotomous effect of DA on the expression of gene expression in direct and indirect pathway SPNs was demonstrated some 20 years ago (Gerfen et al., 1990). However, electrophysiological studies did not yield as clean a picture, leading to a debate about the extent to which these receptors were co-localized in SPNs. Subsequently, the murkiness of the physiological picture has been shown to be largely a consequence of the complexity of the striatal circuitry, not co-localization of D1 and D2 receptors. The most compelling evidence for this conclusion has come from work with bacterial artificial chromosome (BAC) transgenic mice in which expression of a fluorescent reporter molecule, most commonly enhanced green fluorescent protein (eGFP), is driven by the promoter region of either the D1 or D2 receptor (Gong et al., 2003). In these mice, there is a precise alignment of D1 and D2 receptor expression with direct and indirect SPN pathways. This review summarizes work in the last few years, primarily with these new mice, that has advanced our understanding of how DA modulates the structure and function of direct and indirect pathway SPNs in health and in disease states, like PD.
Organization of the basal GANGLIA
The largest nucleus of the basal ganglia is the striatum, which comprises the caudate, putamen and ventral striatum, including the nucleus accumbens. Essentially all cortical areas – sensory, motor and associational – project to the striatum (). The other major input to the striatum comes from the thalamus, particularly the intralaminar thalamic nuclei (Smith et al., 2004). Both projections are glutamatergic, forming excitatory synaptic connections with SPNs and four classes of interneuron.
Striatal information flows to the rest of the basal ganglia through GABAergic SPNs of the direct and indirect pathways (Gerfen and Wilson, 1996). Direct pathway SPNs extend axonal projections to the GABAergic output nuclei of the basal ganglia, the internal segment of the globus pallidus (GPi) and substantia nigra pars reticulata (SNr). These nuclei may be considered as one somatotopically organized structure, with the GPi involved in axial and limb movements and the SNr involved in head and eye movements. Direct pathway neurons also extend an axon collateral to the GABAergic neurons of the external segment of the globus pallidus (GPe). Indirect pathway SPNs extend axonal projections only to the GPe. GPe neurons project to the glutamatergic neurons of the subthalamic nucleus (STN) and to the output nuclei (GPi/SNr). STN neurons also project to both the GPi and SNr, forming a parallel pathway to the output nuclei. Thus, the indirect pathway forms a multisynaptic circuit between the striatum and the basal ganglia output nuclei. Activity within this indirect pathway circuit is also modulated by excitatory cortical input to the STN. All of the neurons in the GPe, GPi, STN and SNr are autonomous pacemakers. This creates a means by which direct and indirect pathway GABAergic neurons can bidirectionally modulate the spiking of GPi and SNr neurons projecting to the thalamus, superior colliculus and pedunculopontine nucleus (PPN). Thus, their neuroanatomical connections endow direct and indirect pathway SPNs with distinct effects on the activity of the inhibitory output interface of the basal ganglia. Indirect pathway provides excitatory activity whereas the direct pathway provides inhibition of activity in this interface. Additionally, some neurons in the globus pallidus provide collaterals to the substantia nigra, which provide an additional source of inhibition to the output nuclei from the indirect pathway circuit.
Striatal distribution of DA receptors
Five G-protein coupled receptors mediate DA signaling (D1–D5). These receptors are grouped into two classes based on the G-protein to which they couple: D1, D5 (D1-like) receptors stimulate Gs and Golf proteins, whereas D2, D3, and D4 (D2-like) receptors stimulate Go and Gi proteins (Neve et al., 2004). Gs and Golf proteins stimulate adenylyl cyclase, elevating intracellular levels of cyclic adenosine monophosphate (cAMP) and activating protein kinase A (PKA). PKA has a broad array of cellular targets, including transcription factors, voltage-dependent ion channels and glutamate receptors (Svenningsson et al., 2004). Gi/o proteins target voltage-dependent ion channels through a membrane-delimited mechanism, as well as enzymes like phospholipase C (PLC) isoforms (e.g. Hernandez-Lopez et al., 2000). Gi proteins also inhibit adenylyl cyclase (Stoof and Kebabian, 1984). Another important feature of the D2-like pathway is its potent modulation by RGS (regulators of G-protein signaling) proteins that are robustly expressed in striatal neurons (Geurts et al., 2003).
All five DA receptor are expressed in the striatum, but D1 and D2 receptors are far and away the most abundant. These two receptors are segregated in direct and indirect pathway SPNs: D1 receptors are expressed by direct pathway SPNs, whereas D2 receptors are expressed by indirect pathway SPNs (Gerfen et al., 1990; Surmeier et al., 1996). This dichotomy was first inferred from the effects of DA depletion on gene expression. In the mid-eighties it was discovered that direct pathway SPNs express high levels of substance P (SP) and dynorphin (DYN), whereas indirect pathway SPNs express enkephalin (ENK). Subsequently, it was found that after DA-depleting lesions, striatal ENK levels rose and SP levels fell, suggesting that DA was differentially modulating these two populations (Young et al., 1986). D1 receptor agonists restored striatal SP levels and D2 receptor agonists restored ENK levels (Gerfen et al., 1990). In situ hybridization studies confirmed that D1 receptor mRNA and SP colocalized in direct pathway SPNs and D2 receptor mRNA and ENK colocalized in indirect pathway SPNs. As elegant and simple as this story was, it didn’t align perfectly with electrophysiological studies showing that D1 and D2 receptor agonists seemed both to have effects on individual striatal neurons(Surmeier et al., 1992). The simplest explanation for the physiological studies was that D1 and D2 receptors were co-localized in SPNs. Although parsimonious, this explanation was wrong. The absence of any significant degree of D1 and D2 receptor co-localization was evident in single cell RT-PCR studies (Surmeier et al., 1996). Because these studies were not quantitative, the very modest extent of co-localization found in these studies could be attributable to the detection of low abundance transcripts that had little or no functional impact. This point has been driven home more recently by the development of BAC transgenics in which eGFP or Cre-recombinase are expressed under control of the D1 or D2 promoter (Gong et al., 2003; 2007). Examination of these mice has confirmed the stark segregation of D1 and D2 receptor expression in direct and indirect pathway SPNs (Gertler et al., 2008; Valjent et al., 2009), confirming the original hypothesis advanced by Gerfen (Gerfen et al., 1990).
What then explains the physiological data? There are two obvious alternatives to colocalization. First, because the pharmacological tools are crude and only allow the D1- and D2-like receptors to be discriminated, it could be that SPNs express the less abundant DA receptors (D5, D3, D4). Single cell RT-PCR profiles of SPNs clearly support this view, showing that direct pathway SPNs expressed low levels of D3 receptor mRNA and indirect pathway SPNs expressed low levels of D5 mRNA (Surmeier et al., 1996). However, the functional roles of these receptors have been difficult to demonstrate.
Another possibility that has gained more support in recent years is that DA receptor expressing striatal interneurons play an important role in regulating both direct and indirect pathway SPNs, leading to indirect responses to a global DA signal. There are four well-characterized classes of striatal interneuron: cholinergic interneurons, parvalbumin-expressing GABAergic interneurons, calretinin-expressing GABAergic interneurons and NPY/nitric oxide-expressing GABAergic interneurons (Tepper et al., 2008). Together, these interneurons constitute about 5–10% of all striatal neurons. One of them, the cholinergic interneuron, co-express D2 and D5 receptors (Bergson et al., 1995; Hersch et al., 1995; Yan et al., 1997) and modulate both SPN populations through muscarinic receptors (Bernard et al., 1992; Yan and Surmeier, 1996). Two other prominent interneurons, the somatostatin/neuropeptide Y (NPY) expressing GABAergic interneuron and the parvalbumin (PV) GABAergic interneuron, express D5 DA receptors (Centonze et al., 2002; Centonze et al., 2003). To complicate matters, the PV interneuron is strongly innervated by globus pallidus neurons that express D2 receptors (Bevan et al., 1998), creating a microcircuit that is influenced by both D1- and D2-class receptors (Wiltschko et al., 2010). As outlined below, there are good reasons to believe that these interneurons play important roles in regulating direct and indirect pathway activity, providing a mechanism by which a ligand for a single receptor class can have broad effects.
DA modulation of canonical striatal microcircuits
The striatal circuitry controlling direct and indirect pathways is complex and the impact of DA on these circuits even more so. Nevertheless, in the last decade, considerable progress has been made toward understanding both. To make the review of this work more tractable, five canonical striatal microcircuits will be considered. These basic circuits are found throughout the striatum.
The corticostriatal circuit
The most basic striatal microcircuit is the one formed by glutamatergic cortical pyramidal neurons and SPNs (Bolam et al., 2000). The synapses formed by cortical pyramidal neurons are exclusively on dendritic spines of SPNs. These spines are absent from soma and the most proximal dendrites, rising to a peak density (1–2 per micron) 50–60 microns from the soma and then falling off very gradually in density to the tips of the sparsely branching dendrites (250–400 μm) (Wilson, 1994). Individual cortical axons are sparsely connected to any one SPN, typically making one or two en passant synapses (Parent and Parent, 2006). There is no obvious organization to the cortical synapses on the dendritic tree of SPNs, but this could simply be that this organization is difficult to see, as the striatum lacks the lamination characteristic of other regions where this is apparent (e.g., cerebral cortex).
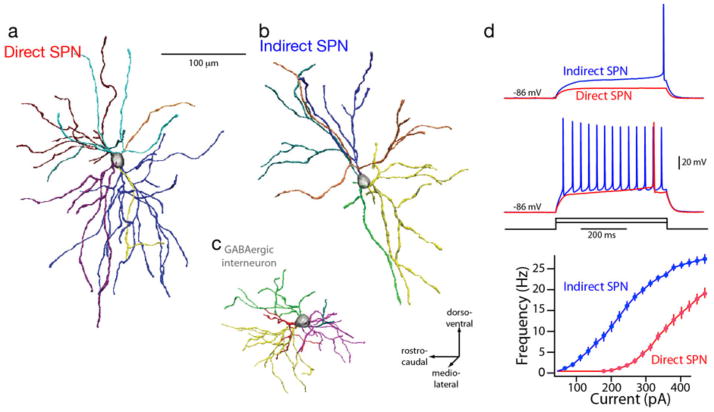
New techniques are revealing the extent of differences between direct and indirect SPNs (Lobo et al., 2006; Heiman et al., 2008; Meurers et al., 2009). The functional implications of this dichotomy are only beginning to be understood. One dichotomy that is readily interpretable is in the somatodendritic anatomy (Gertler et al., 2008). In rodents, the total dendritic length of indirect pathway SPNs is significantly less that that of direct pathway SPNs (Fig. 2). This difference is due to indirect pathway SPNs having on average two fewer primary dendrites that direct pathway SPNs. This difference in surface area, in the absence of any obvious difference in spine density, suggests that direct pathway SPNs receive roughly 50% more glutamatergic input than indirect pathway SPNs. This also makes indirect pathway SPNs more excitable, as judged by somatic current injection. Thus, although SPNs have long been thought to a single morphologic type in terms of dendritic organization, there are distinct differences between direct and indirect SPNs.
FIGURE 2.
Dichotomous anatomy and physiology of direct and indirect pathway SPNs. Reconstructions of biocytin-filled direct (a) and indirect pathway (b) SPNs from P35–P45 BAC transgenic mice; a GABAergic interneuron (c) is included for comparison. Intrasomatic current injection consistently revealed that indirect pathway SPNs were more excitable (d). Summary of the responses of direct and indirect pathway SPNs to a range of intrasomatic current steps; indirect pathway SPNs were more excitable over a broad range of injected currents. From Gertler et al., 2008.
The neuromodulatory effects of DA on direct and indirect pathway SPNs parallel this dichotomy in intrinsic excitability. Both SPNs have a similar core physiological phenotype. At rest, SPNs are dominated by inwardly rectifying, Kir2 K+ channels that hold the membrane potential near the K+ equilibrium potential (~−90 mV), far from spike threshold (Wilson, 1993; Shen et al., 2007). This is the so-called ‘down-state’. In response to excitatory glutamatergic synaptic input from the cerebral cortex, SPNs depolarize. If this input lacks spatial or temporal convergence, the constitutively open Kir2 K+ channels shunt this synaptic current, minimizing the cellular response. However, if this input is highly convergent, the glutamatergic synapses can overwhelm the Kir2 channels and promote their closure (Wilson and Kawaguchi, 1996). Closure of dendritic Kir2 K+ channels and inactivation of neighboring Kv4 (A-type) K+ channels leads to a dramatic elevation of the input impedance of SPN dendrites and a reduction in their electrotonic length (Wilson, 1993; Day et al., 2008). With this, the SPN somatic membrane reaches a potential near spike threshold. This ‘up-state’ can last hundreds of milliseconds. It is during this up-state that SPNs spike. These spikes are typically not correlated with the transition to the up-state, suggesting that they are driven by an independent synaptic input (Stern et al., 1998).
DA modulates the glutamatergic synapses responsible for the transition to the up-state and the ion channels controlling spiking. The qualitative features of the modulation depend upon which DA receptor is being stimulated. As originally hypothesized on the basis of changes in gene expression induced by DA depletion (Gerfen et al., 1990), D2 receptor signaling impedes the up-state transition and diminishes up-state spiking in indirect pathways SPNs, whereas D1 receptor signaling does precisely the opposite in direct pathways SPNs (Surmeier et al., 2007). How this happens is still being unraveled, but some elements of the formula have been defined. In the somatic region where spikes are generated, activation of D2 receptor signaling reduces inward, depolarizing currents through Cav1 (L-type) Ca2+ channels and Nav1 Na+ channels, while increasing outward, hyperpolarizing K+ channel currents (Surmeier et al., 1992; Kitai and Surmeier, 1993; Greif et al., 1995; Schiffmann et al., 1998; Hernandez-Lopez et al., 2000; Olson et al., 2005). D2 receptor stimulation also decreases dendritic Ca2+ entry through voltage-dependent channels (Day et al.; Higley and Sabatini). Complementing these alterations in the gating of voltage-dependent ion channels is a reduction in glutamatergic signaling. Although it is not clear whether they are located pre- or postsynaptically (Yin and Lovinger, 2006), D2 receptor stimulation diminishes presynaptic release of glutamate (Bamford et al., 2004). Activation of D2 receptors also has been reported to decrease AMPA receptor currents of SPNs (Cepeda et al., 1993; Hernandez-Echeagaray et al., 2004). This could be accomplished by D2 receptor triggered dephosphorylation of GluR1 subunits, which should promote trafficking of AMPA receptors out of the synaptic membrane (Hakansson et al., 2006). However, this modulation might be specific to a particular type of synapse, as recent work using glutamate uncaging on proximal dendritic spines failed to find any acute effect of D2 receptor stimulation on AMPA receptor currents (Higley and Sabatini, 2010). What they did find was that D2 receptor signaling decrease Ca2+ entry through NMDA receptors, keeping with the effect on voltage-dependent Ca2+ channels.
D1 receptor signaling has almost diametrically opposing effects on direct pathway SPNs. D1 receptor stimulation and PKA activation increases Cav1 L-type Ca2+ channel currents and decreases somatic K+ currents (Kitai and Surmeier, 1993; Surmeier et al., 1995; Galarraga et al., 1997; Gao et al., 1997). In addition, D1 receptor signaling decreases the opening of Cav2 Ca2+ channels that control activation of Ca2+-dependent, small conductance K+ (SK) channels that slow repetitive spiking in SPNs (Surmeier et al., 1995; Vilchis et al., 2000). All three of these effects serve to increase spiking of direct pathway SPNs with somatic depolarization. The only apparently incongruent modulation is the one that was described first. Confirming inferences drawn from earlier work in tissue slices (Calabresi et al., 1987), voltage clamp work has shown that D1 receptor signaling reduces Na+ channel availability without altering the voltage-dependence of fast activation or inactivation (Surmeier et al., 1992). Subsequent work has shown that PKA phosphorylation of the pore-forming subunit of the Na+ channel promotes activity dependent entry into a non-conducting, slow inactivated state that can be reversed only by membrane hyperpolarization (Carr et al., 2003)(Chen et al., 2006). It is likely that the D1 receptor modulation is mediated by phosphorylation of somatic Nav1.1 channels, as axon initial segment Nav1.6 channels are not efficiently phosphorylated by PKA (Scheuer and Catterall, 2006) and Na+ channels do not extend any significant distance into the dendrites of SPNs (Day et al., 2008). Given this restricted site of action and its time and voltage-dependence, it’s reasonable to hypothesize that this effect simply acts as a brake on the spike-promoting effects of D1 receptors on Ca2+ and K+ channels (as well as glutamergic signaling described below).
A number of studies suggest that D1 receptor signaling has positive effects on AMPA and NMDA receptor function and trafficking, in contrast to D2 receptors. For example, D1 receptor activation of PKA enhances surface expression of both AMPA and NMDA receptors (Snyder et al., 2000; Sun et al., 2005; Hallett et al., 2006). The precise mechanisms underlying the trafficking are still being pursued but the tyrosine kinase Fyn and the protein phosphatase STEP (striatal-enriched-phosphatase) appear to be important regulators of surface expression of glutamate receptors (Braithwaite et al., 2006). Trafficking and localization might also be affected by a direct interaction between D1 and NMDA receptors (Dunah et al., 2000; Lee et al., 2002; Scott et al., 2006). Rapid D1 receptor effects on glutamate receptor gating have been more difficult to see. Although PKA phosphorylation of the NR1 subunit is capable of enhancing NMDA receptor currents (Blank et al., 1997), the presence of this modulation in SPNs is controversial. In neurons where the engagement of dendritic voltage-dependent ion channels has been minimized by dialyzing the cytoplasm with cesium ions, D1 receptor agonists have little or no discernible effect on AMPA or NMDA receptor mediated currents (Nicola and Malenka, 1998). However, in SPNs where this has not been done, D1 receptor stimulation rapidly enhances currents evoked by NMDA receptor stimulation (Cepeda et al., 1993). The difference between these results suggests that the effect of D1 receptors on NMDA receptor currents is largely indirect and mediated by voltage-dependent dendritic conductances that are taken out of play by blocking K+ channels and clamping dendritic voltage. Indeed, blocking L-type Ca2+ channels, which open in the same voltage range as NMDA receptors (Mg2+ unblock) attenuates the D1 receptor mediated enhancement of NMDA receptor currents (Liu et al., 2004).
Taken together, this body of work paints a relatively simple picture of the divergent modulation of direct and indirect pathway SPN excitability by DA. However there are two important caveats to the simplicity of this model. DA receptor expressing striatal interneurons are important regulators of activity in the striatal circuit. In most of the studies of SPNs, their actions have been blocked, to reduce the complexity of the preparation. The other caveat is that virtually nothing is known about the integrative mechanisms of SPN dendrites. This is where the majority of D1 and D2 receptors are located.
Although DA has an important role in modulating the moment-to-moment activity of the corticostriatal network, it also has important part to play in regulating long-term changes in synaptic strength. There are several recent reviews that cover this ground (Surmeier et al., 2007; Wickens, 2009), so only the major points bearing on the dichotomy between direct and indirect pathways will be discussed here. At the outset, it must be acknowledged that nearly all of what has been described as corticostriatal synaptic plasticity is truly glutamatergic synaptic plasticity, as corticostriatal and thalamostriatal glutamatergic synapses have not been reliably distinguished. With that caveat in mind, we’ll follow the convention and assume that what has been described applies to the corticostriatal synapse formed on a dendritic spine.
The best characterized form of SPN synaptic plasticity is long-term depression (LTD). Unlike the situation at many other synapses, striatal LTD induction requires pairing of postsynaptic depolarization with moderate to high frequency afferent stimulation at physiological temperatures (Lovinger et al., 1993; Kreitzer and Malenka, 2005). Typically for the induction to be successful, postsynaptic L-type calcium channels and mGluR5 receptors need to be co-activated. Both L-type calcium channels and mGluR5 receptors found near glutamatergic synapses on MSN spines, making them capable of responding to local synaptic events (Testa et al., 1994; Carter and Sabatini, 2004; Day et al., 2006; Carter et al., 2007). The induction of LTD requires the postsynaptic generation of endocannabinoids (ECs) (Gerdeman et al., 2002). ECs diffuse retrogradely to activate presynaptic CB1 receptors and decrease glutamate release probability. Ongoing work suggests that both of the abundant striatal ECs: anandamide and 2-arachidonylglycerol (2-AG) are involved in the metabolic mechanisms responsible for EC production (Giuffrida et al., 1999; Gao et al., 2010; Lerner et al., 2010; Tanimura et al., 2010). A key question about the induction of striatal LTD is whether activation of D2 receptors is necessary. Activation of D2 receptors is a potent stimulus for anandamide production (Giuffrida et al., 1999). However, recent work showing the sufficiency of L-type channel opening in EC-dependent LTD (Adermark and Lovinger, 2007), makes it clear that D2 receptors play a modulatory – not obligatory – role. The real issue is the role of D2 receptors in LTD induction using synaptic stimulation. Studies have consistently found that in indirect pathway SPNs, D2 receptor activation is necessary (Wang et al., 2006; Kreitzer and Malenka, 2007; Shen et al., 2008). This could be due to the need to suppress A2a adenosine receptor signaling that could impede efficient EC synthesis and LTD induction (Fuxe et al., 2007; Shen et al., 2008). Indeed, Lerner et al. demonstrate quite convincingly that antagonism of A2a receptors promotes EC-dependent LTD induction in indirect pathway SPNs (Lerner et al., 2010).
The question then is can EC-dependent LTD be induced in direct pathway SPNs that do not express D2 receptors? When a minimal local stimulation paradigm is used, LTD does not appear to be induced in these SPNs (Kreitzer and Malenka, 2007; Shen et al., 2008). However, using macroelectrode stimulation, EC-dependent LTD is readily inducible in identified direct pathway SPNs (Wang et al., 2006), consistent with the high probability of SPN induction seen in previous work (Calabresi et al., 2007). In this circumstance, LTD induction was dependent upon D2 receptors. But how? There are a couple of possibilities. One is that D2 receptor stimulation reduces DA release through a presynaptic mechanism, preferentially reducing stimulation of D1 receptors that oppose the induction of LTD in direct pathway neurons (Shen et al., 2008). The other possibility is that for LTD to be induced in direct pathway SPNs, acetylcholine release and postsynaptic M1 muscarinic receptor signaling must fall (Wang et al., 2006).
Long-term potentiation (LTP) at glutamatergic synapses is less well characterized because it is more difficult to induce in the in vitro preparations typically used to study plasticity. Most of the work describing LTP at glutamatergic synapses has been done with sharp electrodes (either in vivo or in vitro), not with patch clamp electrodes in brain slices that afford greater experimental control and definition of the cellular and molecular determinants of induction. Previous studies have argued that LTP induced in SPNs by pairing HFS of glutamatergic inputs and postsynaptic depolarization depends upon co-activation of D1 and NMDA receptors (Calabresi et al., 2007). The involvement of NMDA receptors in LTP induction is clear. The necessity of D1 receptors is another matter. If this were the case, there would be no LTP in indirect pathway SPNs – an unlikely situation. Again, the advent of BAC transgenic mice has provided an invaluable for unraveling this puzzle. In direct pathway SPNs, the induction of LTP at glutamatergic synapses is dependent upon D1 DA receptors (Pawlak and Kerr, 2008; Shen et al., 2008). However, this is not the case in indirect pathway SPNs (Flajolet et al., 2008; Shen et al., 2008). In them, LTP induction required activation of A2a adenosine receptors. These receptors are robustly expressed in the indirect pathway and have a very similar intracellular signaling linkage to that of D1 receptors; that is, they positively couple to adenylyl cyclase and protein kinase A (PKA). Acting through PKA, D1 and A2a receptor activation leads to the phosphorylation of DARPP-32 and a variety of other signaling molecules, including MAPKs, linked to synaptic plasticity (Svenningsson et al., 2004; Sweatt, 2004).
Although most of the induction protocols for synaptic plasticity that have been used to study striatal plasticity are decidedly unphysiological, involving sustained, strong depolarization and/or high frequency synaptic stimulation that induces dendritic depolarization, they do make the necessity of postsynaptic depolarization clear. In a physiological setting, what types of depolarization are likely to gate induction? One possibility is that spikes generated in the axon initial segment (AIS) propagate into dendritic regions where synapses are formed. Recent work has shown that STDP is present in SPNs (Fino et al., 2005; Pawlak and Kerr, 2008; Shen et al., 2008). But there are reasons to believe that this type of plasticity is relevant for only a subset of the synapses formed on SPNs. SPN dendrites are several hundred microns long, thin and modestly branched. Their initial 20–30 microns are largely devoid of spines and glutamatergic synapses. Glutamatergic synapse and spine density peaks near 50 microns from the soma and then modestly declines with distance (Wilson, 2004). Because of their geometry and ion channel expression, AIS generated spikes rapidly decline in amplitude as they invade SPN dendrites (as judged by their ability to open voltage-dependent calcium channels), producing only a modest depolarization 80–100 microns from the soma. This is less than half the way to the dendritic tips (Day et al., 2008), arguing that a large portion of the synaptic surface area is not normally accessible to somatic feedback about the outcome of aggregate synaptic activity. High frequency, repetitive somatic spiking improves dendritic invasion, but distal (>100 microns) synapses remain relatively inaccessible, particularly in direct pathway SPNs (Day et al., 2008).
In the more distal dendritic regions, what controls plasticity? The situation in SPNs might be very similar to that found in deep layer pyramidal neurons where somatically generated bAPs do not invade the apical dendritic tuft (Golding et al., 2002). In this region, convergent synaptic stimulation is capable of producing a local calcium spike or plateau potential that produces a strong enough depolarization to open NMDA receptors and promote plasticity. Calcium imaging using 2PLSM has shown that there is robust expression of both low threshold Cav3 and Cav1 Ca2+ channels – in addition to NMDA receptors – in SPN dendrites (Carter and Sabatini, 2004; Day et al., 2006; Carter et al., 2007), a result that has been confirmed using cell-type specific gene profiling (Day et al., 2006) (unpublished observations). If distal dendrites are capable of regenerative activity, they could also be important to the induction of plasticity in SPNs. SPN dendrites do appear to be active in some circumstances (Kerr and Plenz, 2002; Vergara et al., 2003; Carter and Sabatini, 2004; Kerr and Plenz, 2004; Flores-Barrera et al., 2010) and this activity does not appear to arise from the somatic and proximal dendrites of adult SPNs (Wilson and Kawaguchi, 1996). One possible scenario is that distal SPN dendrites are bistable when NMDA receptors are active during synaptic stimulation. If this is the case, spatial convergence of glutamatergic inputs onto a distal dendrite could induce a local plateau potential capable of pulling the rest of the cell into depolarized state – like an up-state. Doing so would collapse the electrotonic structure of SPNs (Wilson, 1992), enhancing the impact of excitatory input anywhere on the dendrite. The lack of temporal correlation between up-state transitions and EPSP-driven spike generation is consistent with a scenario like this one (Stern et al., 1998). If this were how SPNs operate, it would significantly increase their pattern recognition capacity (e.g., Poirazi and Mel, 2001) and create a new means of inducing plasticity. Dendritic D1 and D2 receptors should play complementary roles in modulating these dendritic events and the induction of synaptic plasticity there.
In vivo studies of striatal synaptic plasticity have provided an important counterpoint to the perspectives based upon reduced in vitro preparations. The pioneering work of Charpier and Deniau (Charpier and Deniau, 1997; Charpier et al., 1999) demonstrated that with more intact input, LTP was readily inducible in SPNs. More recently, it has been shown that the sign of synaptic plasticity in SPNs is influenced by anesthetic and presumably the degree of cortical synchronization in corticostriatal projections (Stoetzner et al., 2010). In particular, in barbiturate-anesthetized rats, 5Hz stimulation of motor cortex evokes LTP in the striatum, but in awake animals the same stimulation induced LTD. A challenge facing the field is how to bridge these observations. Because glutamatergic connections are sparse, it is virtually impossible to reliably stimulate a collection of synapses onto a particular SPN dendrite with an electrode in a brain slice. Optogenetic techniques might provide a feasible alternative strategy. Another strategy would be to employ two-photon laser uncaging (2PLU) of glutamate at visualized synaptic sites (Carter and Sabatini, 2004; Higley and Sabatini, 2010). These tools are becoming more widely available and should allow the regenerative capacity of SPN dendrites to be tested soon.
The feedforward corticostriatal circuit
Fast-spiking (FS), PV GABAergic interneurons receive a prominent glutamatergic input from cortical pyramidal neurons and, in turn, convey this activity through perisomatic synapses to both direct and indirect pathway SPNs (Kita, 1993; Bennett and Bolam, 1994; Koos and Tepper, 1999; Gittis et al., 2010; Planert et al., 2010). This feed-forward inhibition is thought to contribute to action selection by suppressing SPN activity in circuits associated with unwanted actions (Kita et al., 1990; Parthasarathy and Graybiel, 1997; Gage et al., 2010). Although both types of SPN are targeted in this circuit, paired recordings in BAC mice have found some preferential connectivity of FS interneurons with direct pathway SPNs (Gittis et al., 2010). More importantly, the dichotomy between direct and indirect pathway SPNs contributes to the regulation of this network. One of the major projections to FS interneurons originates from GPe neurons that are preferentially controlled by indirect pathway SPNs (Rajakumar et al., 1994; Spooren et al., 1996; Bevan et al., 1998). This feedback loop complements the one formed by collateral projections of SPNs. D2 receptor agonists depress the GABAergic inputs to FS interneurons (Bracci et al., 2002; Centonze et al., 2003; Sciamanna et al., 2009; Gage et al., 2010), which are presumably derived in large measure from GPe neurons (which express D2 receptors (Hoover and Marshall, 2004)). This dual control of the feedback by D2 receptors in indirect pathway and GPe neurons suggests that its function is attenuated by basal DA, but capable of rapid facilitation if DA levels fall. This suppression of the GABAergic feedback circuit complements the elevation in FS interneuron excitability mediated by postsynaptic D5 receptors.
SOM/NPY GABAergic interneurons also form another, less well studied, part of the feedforward corticostriatal circuit (Tepper et al.). If these interneurons are like the SOM expressing, Martinotti interneurons of cortex (Wang et al., 2004), their innervation of distal dendrites could make it difficult to accurately judge their importance (Gittis et al., 2010), as with SPN recurrent collaterals. Whether this component of feedforward circuit differentially controls direct and indirect pathway SPNs remains to be determined, but their expression of D5 receptors certainly creates a situation in which D1 class agonists might influence indirect pathway SPNs (Centonze et al., 2002).
The feedback striatal circuit
SPNs have a richly branching recurrent axon collateral that arborizes in the neighborhood of its parent cell body (Kawaguchi et al., 1989). This feedback could provide the substrate for lateral inhibition (Groves, 1983) and has figured prominently in several models of striatal processing (Beiser et al., 1997). However, the functional significance of this feedback circuit has been controversial. In large measure, this is because the synapses formed by recurrent collaterals are largely onto distal dendrites of other SPNs (Wilson and Groves, 1980; Bolam et al., 1983), making their physiological effects difficult to see with a somatic electrode (Jaeger et al., 1994). Using paired patch clamp recordings from neighboring SPNs, it has been possible to more reliably see the effects of collateral activation (Czubayko and Plenz, 2002; Tunstall et al., 2002; Koos et al., 2004; Taverna et al., 2008), but the percentage of synaptically connected neighbors has been small (~10–15%) in randomly selected SPNs in brain slices. Using D1 and D2 BAC transgenic mice to direct sampling, it was found that although indirect pathway SPNs project to both themselves and direct pathway SPNs, direct pathway SPNs connect essentially only with other direct pathway SPNs (Taverna et al., 2008). The percentage of SPNs showing demonstrable connectivity doubled when sampling was not random. As at their extrastriatal terminals, the release of GABA at recurrent collateral synapses is regulated by DA (Guzman et al., 2003). D2 receptor stimulation decreases GABA release, whereas D1 receptor stimulation increases release. Given the differences in the DA affinity in native membrane (see above), at normal extrastriatal DA levels, the feedback circuit of the indirect pathway should be depressed, disinhibiting dendritic integration. A transient elevation in striatal DA should preferentially activate D1 receptors, leading to enhanced GABA release at collaterals formed between direct pathway SPNs, enhancing intra-pathway lateral inhibition of dendritic integration. Because D1 receptor stimulation enhances the somatodendritic excitability of direct pathway SPNs, the collateral modulation should in principle promote the type of population sculpting typically envisioned for lateral inhibition (Beiser et al., 1997). Conversely, a transient drop in striatal DA should enhance GABA release at collaterals formed by the indirect pathway SPNs. Because this drop elevates the somatodendritic excitability of indirect pathways SPNs, the collateral modulation should promote a similar type of population sculpting, but one favoring a subset of strongly activated indirect pathway SPNs. The critical gap in this scenario is the lack of compelling data about how dendritic GABAergic synapses shape synaptic integration in SPNs. There are suggestions from recent work that these processes might differ in direct and indirect pathway SPNs (Flores-Barrera et al., 2010).
The feedforward thalamostriatal circuit
The other major glutamatergic projection to the striatum originates in the thalamus (Smith et al.). The synapses formed by this projection are found both on dendritic shafts and spine heads, in the same regions as those formed by the corticostriatal projection. In contrast to the corticostriatal synapses, those formed by thalamic axons have a high release probability, making them well-suited to signaling transient events (Ding et al., 2008). Another major target of this projection is the cholinergic interneuron. Like the corticostriatal feedforward circuit involving FS interneurons, the thalamostriatal projection makes a feedforward connection to SPNs through cholinergic interneurons (Ding et al., 2010). With a burst of thalamic activity like that seen after presentation of a salient stimulus, cholinergic interneurons exhibit a burst-pause pattern of activity that enhances the somatic excitability of both SPNs (Perez-Rosello et al., 2005; Pisani et al., 2007), but preferentially enhances the dendritic excitability of indirect pathway SPNs by decreasing Kir2 K+ channel opening (Shen et al., 2007). As noted above, reducing Kir2 K+ channel opening increases dendritic input resistance and the ability to sum corticostriatal inputs. The role of DA in regulating the thalamostriatal projection to SPNs has not been systematically explored. However, DA potently modulates the activity of cholinergic interneurons through D2 and D5 receptors. D2 receptors suppress the ongoing pacemaking activity of interneurons (Maurice et al., 2004) and diminished acetylcholine release (DeBoer et al., 1996; Ding et al., 2006). D5 receptors complement this modulation by enhancing GABAergic responsiveness (Yan and Surmeier, 1997; Deng et al., 2007), possibly to FS interneuron inputs (Sullivan et al., 2008). Thus, by modulating cholinergic interneurons, transient changes in striatal DA release differentially modulate direct and indirect pathway SPNs.
Normal function of direct and indirect pathways
According to the ‘classical’ model of basal ganglia function, movements occur during pauses in the tonic inhibitory activity of the basal ganglia output interface, generated by activity in the direct pathway. This model had its origins in neurophysiologic studies showing that corticostriatal activation of the direct striatonigral pathway resulted in pauses of the tonic activity of GABAergic neurons in the substantia nigra pars reticulata (Chevalier et al., 1985; Deniau and Chevalier, 1985). The demonstration that saccadic eye movements occur during these pauses clearly implicated the direct pathway in movement control (Hikosaka and Wurtz, 1983). The opposing role of the indirect pathway in suppressing movements originally was proposed based on studies in animal models of PD (see below). Recent work using optogenetic or genetic approaches have provided support for the general tenets of the model (Hikida et al., 2010; Kravitz et al., 2010). Although this model has proven to be of considerable clinical value, it fails to account for the great diversity and complexity of the decision-making process in action selection (Cisek and Kalaska, 2010).
Mink (Mink, 2003) elaborated the classic model, proposing that the activity of direct and indirect pathway is coordinated to select particular motor programs, and to inhibit ‘competing’ motor programs. This model predicts that during ongoing behavior, there will be increased activity in neuronal ensembles that are part of direct and indirect pathway circuits, rather than one or the other. The execution of a sequence of movements would then generate a complex pattern of activity in specific neuronal ensembles. Physiologic studies of limb reaching movements in primates have confirmed this prediction (Turner and Anderson, 1997; 2005).
Consistent with such data, current models propose that the striatum performs a computation on sensorimotor, cognitive and emotional/motivational information provided by the cerebral cortex to facilitate the selection of an appropriate action out of a collections of possibilities (Balleine et al., 2007; Nambu, 2008; Cisek and Kalaska, 2010).. Distinct cortico-basal ganglia loops are thought to perform different aspects of this computation. Distinct cortico-basal ganglia loops might also play different roles in the acquisition and stabilization of context-dependent action selection. For example, work by Costa’s group suggests that the dorsomedial striatum-associative cortex loop plays an important role in the early phases of skill acquisition, whereas the dorsolateral striatum-sensorimotor cortex loop is more involved once the skill has been established and the action program becomes more automated and inflexible.
More germane to our topic is the role that DA modulation of direct and indirect pathway SPNs might serve in this process. Certainly, DA is thought to play a crucial role in linking the outcome of a particular action choice to the probability it will be chosen in the future. Given the robust DA innervation of the striatum, it’s natural to think that modulation of the striatal circuitry is critical to decision making. How might this happen? As noted above, SNc DA neurons innervating the striatum are autonomous pacemakers, providing a tonic release of DA in the striatum. Rewarding events transiently increase the activity of SNc DA neurons, whereas aversive events transiently decrease it, providing bidirectional signaling to the striatum (Schultz, 1998; Schultz, 2007; Hikosaka et al., 2008; Brown et al., 2009). Although there is debate about precisely what SNc DA neuron activity codes, this isn’t critical to our discussion. What is important from our standpoint is that the dichotomous modulation of direct and indirect pathways by DA provides a mechanism by which these outcome-driven changes in SNc activity can be translated into changes in corticostriatal networks facilitating action selection. As outlined above, the transient elevation in striatal DA release associated with rewarding events promotes the induction of LTP at corticostriatal synapses formed on direct pathway SPNs and increases their excitability. In contrast, this same change promotes LTD induction at synapses formed on indirect pathway SPNs and decreases their excitability. A reasonable conjecture from this data is that reward promotes the ability of a particular cortical ensemble to turn-on direct pathway SPNs linked to action initiation while at the same time decreasing the ability of that cortical ensemble to activate indirect pathway SPNs linked to action suppression (Cohen and Frank, 2009). Transient depression of DA release associated with aversive events should have exactly the opposite effect on the corticostriatal circuitry – promoting the ability of a cortical ensemble to turn-on indirect pathway SPNs and reducing its ability to turn-on direct pathway SPNs. Thus, the differential expression of DA receptors by striatal efferent pathways appears to be critical to the ability of the striatum to participate in action selection.
Although this model is consistent with most of what we know, we don’t really know that much and a great many questions need to be answered. For example, the role of the striatal microcircuits outlined above are almost completely undefined in this model. Also, it is commonly assumed that direct and indirect pathway SPNs receive the same cortical information. This assumption is being debated in the literature (Parthasarathy and Graybiel, 1997; Lei et al., 2004; Ballion et al., 2008), but will not be easy to resolve given the ‘chaotic’ architecture of the striatum. If this is not true, it would fundamentally change our ideas about what each pathway was doing to control behavior. It is also far from clear how the timing of activity in the direct and indirect pathways shapes the activity in the circuitry controlling actual movement (Nambu, 2008). In diseases of the basal ganglia, like PD, the timing of activity, rather than the overall level of activity, appears to be the most important determinant of movement choice and initiation (Bevan et al., 2002).
Parkinson’s Disease
The striatum and the basal ganglia have been implicating in a wide variety of psychomotor disorders, ranging from PD to schizophrenia and drug abuse. Altered DA regulation of direct and indirect pathway SPNs appears to be a pivotal aspect of most, if not all, of these diseases. The best characterized example of this is in PD (PD) where the DA neurons innervating the basal ganglia degenerate (Hornykiewicz, 1966). Studies of PD models provided the first clear indication of the dichotomy in the regulation of the direct and indirect pathways, suggesting that their excitability shifts in opposite directions following the loss of DA, creating an ‘imbalance’ in the regulation of the motor thalamus favoring suppression of movement (Albin et al., 1989). Specifically, direct pathway SPNs were posited to spike more in the PD state, whereas indirect pathway SPNs were thought to spike less. This conjecture was based upon changes in gene expression following DA depleting lesions (Gerfen et al., 1990). The cellular mechanisms underlying the postulated shift in excitability were not known at the time the model was formulated, but have widely been assumed to reflect changes in intrinsic excitability. In the last two decades, electrophysiological studies have provided strong support for the proposition that following DA depletion, the excitability of direct and indirect pathway SPNs moves in opposite directions (Surmeier et al., 2007). Recent work has added a dimension to the classical model, showing that DA depletion alters the induction of long term plasticity at glutamatergic synapses (Shen et al., 2008). In direct pathway SPNs, the loss of D1 receptor signaling biases glutamatergic synapses toward LTD. In contrast, in indirect pathway SPNs, the loss of D2 receptor signaling promotes the induction of LTP. Thus, activity dependent changes in synaptic strength parallel those of intrinsic excitability following DA depletion. Work in vivo examining the responsiveness of antidromically identified SPNs to cortical stimulation following unilateral lesions of the striatal DA innervation is consistent with this broader model (Mallet et al., 2006). Additional support for this model has recently come from an elegant application of optogenetics to selectively activate direct and indirect pathway SPNs in mouse model of PD (Kravitz et al., 2010).
What this model does not account for is the propensity of neural circuits to adapt to sustained perturbations in activity, like those induced by DA depletion. The fact that changes in striatal gene expression take weeks to stabilize after lesioning DA fibers, suggests that there are a broad array of adaptations taking place. Typically, sustained perturbations in synaptic or intrinsic properties that make neurons spike more or less than their set-point engage homeostatic mechanisms that attempt to bring activity back to the desired level (Turrigiano, 1999; Marder and Goaillard, 2006). One of the most common mechanisms of homeostatic plasticity is to alter synaptic strength or to scale synapses. In indirect pathway SPNs, the elevation in activity following DA depletion triggers a dramatic down-regulation of glutamatergic synapses formed on spines (Day et al., 2006; Deutch et al., 2007). Like scaling seen in other cell types, the synaptic modification depends upon calcium entry through voltage-dependent L-type channels that activates the protein phosphatase calcineurin, which dephosphorylates myocyte enhancer factor 2 (MEF2) (Flavell et al., 2006), increasing its transcriptional activity. In indirect pathway SPNs, MEF2 up-regulation increased the expression of at least two genes linked to synaptic remodeling – Nur77 and Arc (Tian et al., 2010).
Although studies are in their very early stages, it is clear that DA depletion induces adaptations in the striatal microcircuits shaping direct and indirect pathway activity. In the feedforward pathway, the activity of SOM/NOS interneurons is elevated, where as that of FS interneurons appears unchanged (Dehorter et al., 2009). The strength of the feedback, recurrent collateral system within the striatum appears to be dramatically down-regulated (Taverna et al., 2008). These adaptations could be a major factor in the attenuation of differences between direct and indirect pathway SPNs to repetitive cortical stimulation in PD models (Flores-Barrera et al., 2010). Complementing these changes, there is an enhancement of cholinergic signaling in the thalamostriatal feedforward circuit following DA depletion (DeBoer et al., 1996; Ding et al., 2006) that could differentially modulate SPNs.
Another example of an asymmetric adaptation in direct and indirect pathway SPNs in PD models comes from study of the effects of treatment with DA receptor agonists. In indirect pathway SPNs, D2 receptor agonists reverse the effects of DA depletion on gene expression (Gerfen et al., 1990). However, in direct pathway SPNs, the responses to DA precursors or D1 receptor agonists fundamentally change, resulting in a loss of both regional and compartmental regulation of gene expression (Gerfen et al., 2002; 2008). The signaling changes underlying this shift remain to be fully elucidated but an important component appears to be the enhanced coupling of D1 receptors to activation of two mitogen-activated protein kinases (MAPKs), extracellular signal–regulated kinases 1/2 and mammalian target of rapamycin (mTOR) (Gerfen et al., 2002; Pavon et al., 2006; Santini et al., 2007; Westin et al., 2007; Santini et al., 2009). This enhancement and the aberrant activation of direct pathway SPNs is widely thought to be a key step in the induction of dyskinesias following the repeated administration of the DA precursor, L-DOPA.
Summary
Direct and indirect striatal efferent pathways form the anatomical and functional backbone of the basal ganglia. These pathways are formed by two types of SPN: one that projects directly to the basal ganglia interface (the internal segment of the globus pallidus and the substantia nigra pars reticulata) and one that indirectly connects with this interface. These GABAergic SPNs integrate information conveyed by the principal neurons of the cerebral cortex and thalamus to facilitate the selection of appropriate actions. DA released by SNc neurons, whose activity is regulated by the outcome of chosen actions, modulates this process. By virtue of the dichotomous expression of D1 and D2 receptors, DA differentially regulates the intrinsic excitability and synaptic connectivity of direct and indirect pathway SPNs. Activation of D1 receptors in direct pathway SPNs increases their excitability and promotes long-term potentiation of excitatory synapses. In contrast, activation of D2 receptors in indirect pathway SPNs decreases their excitability and promotes long-term depression of excitatory synapses. In addition, DA modulates striatal microcircuits formed by either SPN recurrent collaterals or GABAergic interneurons to modulate SPN activity. This dichotomous modulation of SPNs is consistent with their hypothesized roles in action selection. However, many questions remain unanswered. The development of genetic tools that allow the selective expression of transgenes in these two populations of SPN promises to put many of these questions within our experimental reach in the near future.
FIGURE 1.
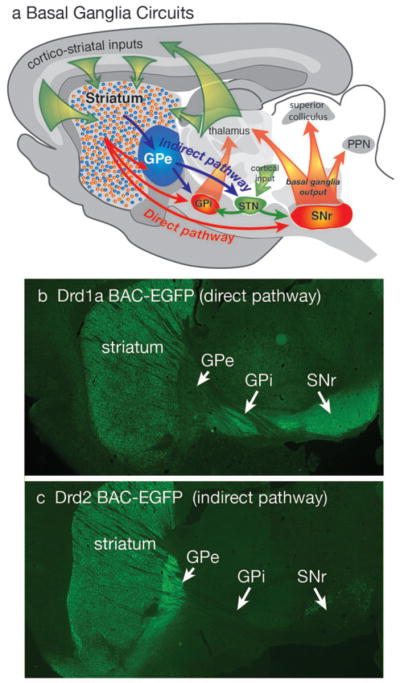
Diagram of basal ganglia circuits (a). The striatum receives excitatory corticostriatal and thalamic inputs. Outputs of the basal ganglia arise from the internal segment of the globus pallidus (GPi) and substantia nigra pars reticulata (SNr), which are directed to the thalamus, superior colliculus and pendunculopontine nucleus (PPN). The direct pathway originates from Drd1a expressing SPNs that project to the GPi and SNr output nuclei. The indirect pathway originates from Drd2 expressing SPNs that project only to the external segment of the globus pallidus (GPe), which together with the subthalamic nucleus (STN) contains transynaptic circuits connecting to the basal output nuclei. The direct and indirect pathways provide opponent regulation of the basal ganglia output interface. (b) Fluorescent imaging of a brain section from a mouse expressing EGFP under regulation of the Drd1a promoter shows Drd1a expressing SPNs in the striatum that project axons through the GPe, which terminate in the GPi and GPe. (c) Fluorescent of imaging of a Drd2-EGFP mouse shows that labeled SPNs provide axonal projections that terminate in the GPe, but do not extend to the GPi or SNr.
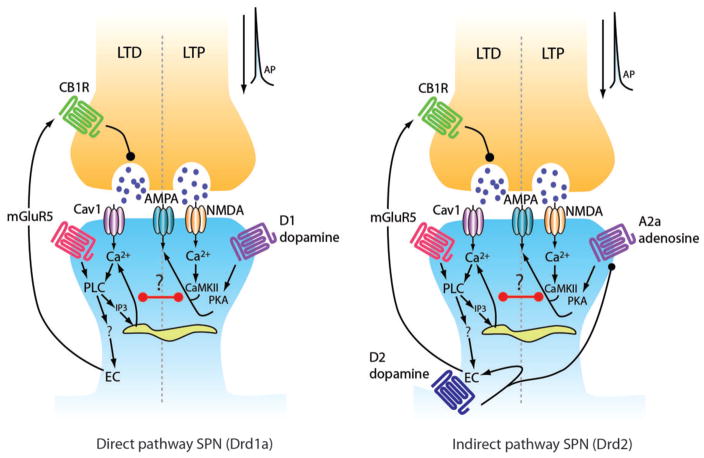
FIGURE 3.
Synaptic plasticity in direct and indirect SPNs. The presynaptic glutamatergic terminal is shown above (orange) and the postsynaptic spine shown below (blue). Black arrowheads depict positive regulation and black circles depict negative regulation. Purple circles in the synaptic cleft represent glutamate. Non-standard abbreviations: CB1R: cannabinoid receptor type 1; AMPAR: α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptor; N-methyl-D-aspartic acid (NMDA) receptor; Cav1C: Cav1 containing calcium channel (L-type); PLC: phospholipase C; PKA: protein kinase A; CaMKII: calcium/calmodulin-dependent protein kinase type II; IP3: inositol trisphosphate; D1R: D1 dopamine receptor; D2R: D2 dopamine receptor; EC: endocannabinoid; mGluR5: metabotropic glutamate receptor type 5; LTD: long-term depression; LTP: long-term potentiation. From Surmeier et al., 2009.
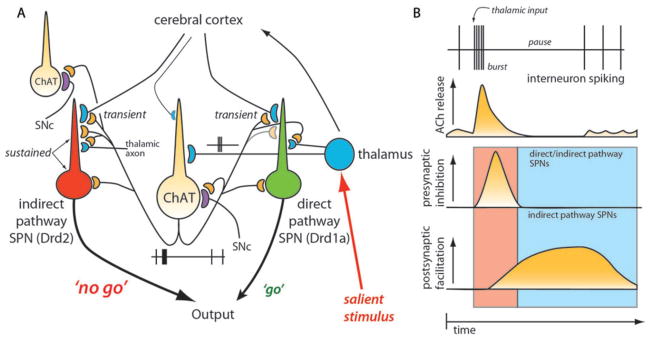
FIGURE 4.
Thalamic Gating of Glutamatergic Signaling in the Striatum (A) Schematic illustration of cortico- and thalamostriatal glutamatergic projections. Both direct and indirect pathway SPNs receive glutamatergic afferents from the cortex and the thalamus. However, cholinergic interneuron receives glutamatergic inputs primarily from the thalamus. (B) Thalamic inputs efficiently drive cholinergic interneuron and generate a burst-pause firing pattern. By acting at presynaptic M2-class receptors, acetylcholine release transiently suppresses release probability at corticostriatal synapses formed on both direct and indirect pathway SPNs. By acting at postsynaptic M1 receptors, acetylcholine release primarily enhances the responsiveness of D2 SPNs to corticostriatal input for about a second. The pause in cholinergic interneuron activity ensures that there is not a concomitant presynaptic suppression in this window. Thalamic stimulation should activate neighboring cholinergic interneurons as well. The pause is generated in part by recurrent collateral or neighboring interneuron activation of nicotinic receptors on dopaminergic terminals. In this way, the burst of thalamic spikes engages cholinergic interneurons to transiently suppress cortical drive of striatal circuits and then create a second long period in which the striatal network is strongly biased toward cortical activation of D2 SPNs. From Ding et al. (2010).
Acronyms
- BAC
bacterial artificial chromosome
- DA
dopamine
- GPCR
G-protein coupled receptor
- GPe
globus pallidus external segment
- GPi
globus pallidus internal segment
- LTD
long term depression
- LTP
long term potentiation
- PD
Parkinson’s Disease
- SPN
spiny projection neuron
- SNR
substantia nigra pars reticulata
References
- Adermark L, Lovinger DM. Combined activation of L-type Ca2+ channels and synaptic transmission is sufficient to induce striatal long-term depression. J Neurosci. 2007;27:6781–6787. doi: 10.1523/JNEUROSCI.0280-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballion B, Mallet N, Bezard E, Lanciego JL, Gonon F. Intratelencephalic corticostriatal neurons equally excite striatonigral and striatopallidal neurons and their discharge activity is selectively reduced in experimental parkinsonism. Eur J Neurosci. 2008;27:2313–2321. doi: 10.1111/j.1460-9568.2008.06192.x. [DOI] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42:653–663. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- Beiser DG, Hua SE, Houk JC. Network models of the basal ganglia. Curr Opin Neurobiol. 1997;7:185–190. doi: 10.1016/s0959-4388(97)80006-2. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Bolam JP. Synaptic input and output of parvalbumin-immunoreactive neurons in the neostriatum of the rat. Neuroscience. 1994;62:707–719. doi: 10.1016/0306-4522(94)90471-5. [DOI] [PubMed] [Google Scholar]
- Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci. 1995;15:7821–7836. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard V, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing muscarinic receptor genes. J Neurosci. 1992;12:3591–3600. doi: 10.1523/JNEUROSCI.12-09-03591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MD, Booth PA, Eaton SA, Bolam JP. Selective innervation of neostriatal interneurons by a subclass of neuron in the globus pallidus of the rat. J Neurosci. 1998;18:9438–9452. doi: 10.1523/JNEUROSCI.18-22-09438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MD, Magill PJ, Terman D, Bolam JP, Wilson CJ. Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network. Trends Neurosci. 2002;25:525–531. doi: 10.1016/s0166-2236(02)02235-x. [DOI] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Teichert U, Kugler H, Behrsing H, Fienberg A, Greengard P, Spiess J. The phosphoprotein DARPP-32 mediates cAMP-dependent potentiation of striatal N-methyl-D-aspartate responses. Proc Natl Acad Sci U S A. 1997;94:14859–14864. doi: 10.1073/pnas.94.26.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organisation of the basal ganglia. J Anat. 2000;196 (Pt 4):527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Somogyi P, Takagi H, Fodor I, Smith AD. Localization of substance P-like immunoreactivity in neurons and nerve terminals in the neostriatum of the rat: a correlated light and electron microscopic study. Journal of neurocytology. 1983;12:325–344. doi: 10.1007/BF01148468. [DOI] [PubMed] [Google Scholar]
- Bracci E, Centonze D, Bernardi G, Calabresi P. Dopamine excites fast-spiking interneurons in the striatum. J Neurophysiol. 2002;87:2190–2194. doi: 10.1152/jn.00754.2001. [DOI] [PubMed] [Google Scholar]
- Braithwaite SP, Paul S, Nairn AC, Lombroso PJ. Synaptic plasticity: one STEP at a time. Trends Neurosci. 2006;29:452–458. doi: 10.1016/j.tins.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MT, Henny P, Bolam JP, Magill PJ. Activity of neurochemically heterogeneous dopaminergic neurons in the substantia nigra during spontaneous and driven changes in brain state. J Neurosci. 2009;29:2915–2925. doi: 10.1523/JNEUROSCI.4423-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Mercuri N, Stanzione P, Stefani A, Bernardi G. Intracellular studies on the dopamine-induced firing inhibition of neostriatal neurons in vitro: evidence for D1 receptor involvement. Neuroscience. 1987;20:757–771. doi: 10.1016/0306-4522(87)90239-9. [DOI] [PubMed] [Google Scholar]
- Carr DB, Day M, Cantrell AR, Held J, Scheuer T, Catterall WA, Surmeier DJ. Transmitter modulation of slow, activity-dependent alterations in sodium channel availability endows neurons with a novel form of cellular plasticity. Neuron. 2003;39:793–806. doi: 10.1016/s0896-6273(03)00531-2. [DOI] [PubMed] [Google Scholar]
- Carter AG, Sabatini BL. State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron. 2004;44:483–493. doi: 10.1016/j.neuron.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Carter AG, Soler-Llavina GJ, Sabatini BL. Timing and location of synaptic inputs determine modes of subthreshold integration in striatal medium spiny neurons. J Neurosci. 2007;27:8967–8977. doi: 10.1523/JNEUROSCI.2798-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Bracci E, Pisani A, Gubellini P, Bernardi G, Calabresi P. Activation of dopamine D1-like receptors excites LTS interneurons of the striatum. Eur J Neurosci. 2002;15:2049–2052. doi: 10.1046/j.1460-9568.2002.02052.x. [DOI] [PubMed] [Google Scholar]
- Centonze D, Grande C, Usiello A, Gubellini P, Erbs E, Martin AB, Pisani A, Tognazzi N, Bernardi G, Moratalla R, Borrelli E, Calabresi P. Receptor subtypes involved in the presynaptic and postsynaptic actions of dopamine on striatal interneurons. J Neurosci. 2003;23:6245–6254. doi: 10.1523/JNEUROSCI.23-15-06245.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Buchwald NA, Levine MS. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc Natl Acad Sci U S A. 1993;90:9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpier S, Deniau JM. In vivo activity-dependent plasticity at cortico-striatal connections: evidence for physiological long-term potentiation. Proc Natl Acad Sci U S A. 1997;94:7036–7040. doi: 10.1073/pnas.94.13.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpier S, Mahon S, Deniau JM. In vivo induction of striatal long-term potentiation by low-frequency stimulation of the cerebral cortex. Neuroscience. 1999;91:1209–1222. doi: 10.1016/s0306-4522(98)00719-2. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yu FH, Surmeier DJ, Scheuer T, Catterall WA. Neuromodulation of Na+ channel slow inactivation via cAMP-dependent protein kinase and protein kinase C. Neuron. 2006;49:409–420. doi: 10.1016/j.neuron.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Chevalier G, Vacher S, Deniau JM, Desban M. Disinhibition as a basic process in the expression of striatal functions. I. The striato-nigral influence on tecto-spinal/tecto-diencephalic neurons. Brain Res. 1985;334:215–226. doi: 10.1016/0006-8993(85)90213-6. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci. 2010;33:269–298. doi: 10.1146/annurev.neuro.051508.135409. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Frank MJ. Neurocomputational models of basal ganglia function in learning, memory and choice. Behav Brain Res. 2009;199:141–156. doi: 10.1016/j.bbr.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czubayko U, Plenz D. Fast synaptic transmission between striatal spiny projection neurons. Proc Natl Acad Sci U S A. 2002;99:15764–15769. doi: 10.1073/pnas.242428599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Wokosin D, Plotkin JL, Tian X, Surmeier DJ. Differential excitability and modulation of striatal medium spiny neuron dendrites. J Neurosci. 2008;28:11603–11614. doi: 10.1523/JNEUROSCI.1840-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- DeBoer P, Heeringa MJ, Abercrombie ED. Spontaneous release of acetylcholine in striatum is preferentially regulated by inhibitory dopamine D2 receptors. Eur J Pharmacol. 1996;317:257–262. doi: 10.1016/s0014-2999(96)00761-3. [DOI] [PubMed] [Google Scholar]
- Dehorter N, Guigoni C, Lopez C, Hirsch J, Eusebio A, Ben-Ari Y, Hammond C. Dopamine-deprived striatal GABAergic interneurons burst and generate repetitive gigantic IPSCs in medium spiny neurons. J Neurosci. 2009;29:7776–7787. doi: 10.1523/JNEUROSCI.1527-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P, Zhang Y, Xu ZC. Involvement of I(h) in dopamine modulation of tonic firing in striatal cholinergic interneurons. J Neurosci. 2007;27:3148–3156. doi: 10.1523/JNEUROSCI.5535-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniau JM, Chevalier G. Disinhibition as a basic process in the expression of striatal functions. II. The striato-nigral influence on thalamocortical cells of the ventromedial thalamic nucleus. Brain Res. 1985;334:227–233. doi: 10.1016/0006-8993(85)90214-8. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Colbran RJ, Winder DJ. Striatal plasticity and medium spiny neuron dendritic remodeling in parkinsonism. Parkinsonism & Related Disorders. 2007;13:S251–S258. doi: 10.1016/S1353-8020(08)70012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Peterson JD, Surmeier DJ. Corticostriatal and thalamostriatal synapses have distinctive properties. J Neurosci. 2008;28:6483–6492. doi: 10.1523/JNEUROSCI.0435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Guzman JN, Tkatch T, Chen S, Goldberg JA, Ebert PJ, Levitt P, Wilson CJ, Hamm HE, Surmeier DJ. RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nat Neurosci. 2006;9:832–842. doi: 10.1038/nn1700. [DOI] [PubMed] [Google Scholar]
- Ding JB, Guzman JN, Peterson JD, Goldberg JA, Surmeier DJ. Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron. 2010;67:294–307. doi: 10.1016/j.neuron.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Wang Y, Yasuda RP, Kameyama K, Huganir RL, Wolfe BB, Standaert DG. Alterations in subunit expression, composition, and phosphorylation of striatal N-methyl-D-aspartate glutamate receptors in a rat 6-hydroxydopamine model of Parkinson’s disease. Mol Pharmacol. 2000;57:342–352. [PubMed] [Google Scholar]
- Fino E, Glowinski J, Venance L. Bidirectional activity-dependent plasticity at corticostriatal synapses. J Neurosci. 2005;25:11279–11287. doi: 10.1523/JNEUROSCI.4476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flajolet M, Wang Z, Futter M, Shen W, Nuangchamnong N, Bendor J, Wallach I, Nairn AC, Surmeier DJ, Greengard P. FGF acts as a co-transmitter through adenosine A(2A) receptor to regulate synaptic plasticity. Nat Neurosci. 2008;11:1402–1409. doi: 10.1038/nn.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell SW, Cowan CW, Kim TK, Greer PL, Lin Y, Paradis S, Griffith EC, Hu LS, Chen C, Greenberg ME. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science. 2006;311:1008–1012. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- Flores-Barrera E, Vizcarra-Chacon BJ, Tapia D, Bargas J, Galarraga E. Different corticostriatal integration in spiny projection neurons from direct and indirect pathways. Front Syst Neurosci. 2010;4:15. doi: 10.3389/fnsys.2010.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Ferre S, Genedani S, Franco R, Agnati LF. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol Behav. 2007;92:210–217. doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Gage GJ, Stoetzner CR, Wiltschko AB, Berke JD. Selective activation of striatal fast-spiking interneurons during choice execution. Neuron. 2010;67:466–479. doi: 10.1016/j.neuron.2010.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarraga E, Hernandez-Lopez S, Reyes A, Barral J, Bargas J. Dopamine facilitates striatal EPSPs through an L-type Ca2+ conductance. Neuroreport. 1997;8:2183–2186. doi: 10.1097/00001756-199707070-00019. [DOI] [PubMed] [Google Scholar]
- Gao T, Yatani A, Dell’Acqua ML, Sako H, Green SA, Dascal N, Scott JD, Hosey MM. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19:185–196. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- Gao Y, et al. Loss of Retrograde Endocannabinoid Signaling and Reduced Adult Neurogenesis in Diacylglycerol Lipase Knock-out Mice. J Neurosci. 2010;30:2017–2024. doi: 10.1523/JNEUROSCI.5693-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Miyachi S, Paletzki R, Brown P. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. J Neurosci. 2002;22:5042–5054. doi: 10.1523/JNEUROSCI.22-12-05042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Paletzki R, Worley PW. Differences between dorsal and ventral striatum in Drd1a-dopamine receptor coupling of DARPP-32 to activation of extracellular receptor kinase (ERK1/2) J Neurosci. 2008;28:7113–7120. doi: 10.1523/JNEUROSCI.3952-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Wilson CJ. The Basal Ganglia. In: Hokfelt T, Swanson LW, editors. Handbook of Chemical Neuroanatomy. Elsevier; 1996. pp. 365–462. [Google Scholar]
- Gertler TS, Chan CS, Surmeier DJ. Dichotomous anatomical properties of adult striatal medium spiny neurons. J Neurosci. 2008;28:10814–10824. doi: 10.1523/JNEUROSCI.2660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts M, Maloteaux JM, Hermans E. Altered expression of regulators of G-protein signaling (RGS) mRNAs in the striatum of rats undergoing dopamine depletion. Biochem Pharmacol. 2003;66:1163–1170. doi: 10.1016/s0006-2952(03)00447-7. [DOI] [PubMed] [Google Scholar]
- Gittis AH, Nelson AB, Thwin MT, Palop JJ, Kreitzer AC. Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. J Neurosci. 2010;30:2223–2234. doi: 10.1523/JNEUROSCI.4870-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Golding NL, Staff NP, Spruston N. Dendritic spikes as a mechanism for cooperative long-term potentiation. Nature. 2002;418:326–331. doi: 10.1038/nature00854. [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting CRE recombinase to specific neuron populations with Bacterial Artificial Chromosome constructs. J Neurosci. 2007;27:9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greif GJ, Lin YJ, Liu JC, Freedman JE. Dopamine-modulated potassium channels on rat striatal neurons: specific activation and cellular expression. J Neurosci. 1995;15:4533–4544. doi: 10.1523/JNEUROSCI.15-06-04533.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves PM. A theory of the functional organization of the neostriatum and the neostriatal control of voluntary movement. Brain Res. 1983;286:109–132. doi: 10.1016/0165-0173(83)90011-5. [DOI] [PubMed] [Google Scholar]
- Guzman JN, Hernandez A, Galarraga E, Tapia D, Laville A, Vergara R, Aceves J, Bargas J. Dopaminergic modulation of axon collaterals interconnecting spiny neurons of the rat striatum. J Neurosci. 2003;23:8931–8940. doi: 10.1523/JNEUROSCI.23-26-08931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson K, Galdi S, Hendrick J, Snyder G, Greengard P, Fisone G. Regulation of phosphorylation of the GluR1 AMPA receptor by dopamine D2 receptors. J Neurochem. 2006;96:482–488. doi: 10.1111/j.1471-4159.2005.03558.x. [DOI] [PubMed] [Google Scholar]
- Hallett PJ, Spoelgen R, Hyman BT, Standaert DG, Dunah AW. Dopamine D1 activation potentiates striatal NMDA receptors by tyrosine phosphorylation-dependent subunit trafficking. J Neurosci. 2006;26:4690–4700. doi: 10.1523/JNEUROSCI.0792-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, Suarez-Farinas M, Schwarz C, Stephan DA, Surmeier DJ, Greengard P, Heintz N. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–748. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Echeagaray E, Starling AJ, Cepeda C, Levine MS. Modulation of AMPA currents by D2 dopamine receptors in striatal medium-sized spiny neurons: are dendrites necessary? Eur J Neurosci. 2004;19:2455–2463. doi: 10.1111/j.0953-816X.2004.03344.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H, Surmeier DJ. D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC[beta]1-IP3-calcineurin-signaling cascade. J Neurosci. 2000;20:8987–8995. doi: 10.1523/JNEUROSCI.20-24-08987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, Bolam JP, Ince E, Yi H, Levey AI. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J Neurophysiol. 1983;49:1285–301. doi: 10.1152/jn.1983.49.5.1285. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sesack SR, Lecourtier L, Shepard PD. Habenula: crossroad between the basal ganglia and the limbic system. J Neurosci. 2008;28:11825–9. doi: 10.1523/JNEUROSCI.3463-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Sabatini BL. Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci. 2010;13:958–966. doi: 10.1038/nn.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Hoover BR, Marshall JF. Molecular, chemical, and anatomical characterization of globus pallidus dopamine D2 receptor mRNA-containing neurons. Synapse. 2004;52:100–113. doi: 10.1002/syn.20007. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev. 1966;18:925–964. [PubMed] [Google Scholar]
- Jaeger D, Kita H, Wilson CJ. Surround inhibition among projection neurons is weak or nonexistent in the rat neostriatum. J Neurophysiol. 1994;72:2555–2558. doi: 10.1152/jn.1994.72.5.2555. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC. Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparation preserving cortical inputs. J Neurophysiol. 1989;62:1052–1068. doi: 10.1152/jn.1989.62.5.1052. [DOI] [PubMed] [Google Scholar]
- Kerr JN, Plenz D. Dendritic calcium encodes striatal neuron output during up-states. J Neurosci. 2002;22:1499–1512. doi: 10.1523/JNEUROSCI.22-05-01499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JN, Plenz D. Action potential timing determines dendritic calcium during striatal up-states. J Neurosci. 2004;24:877–885. doi: 10.1523/JNEUROSCI.4475-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H. GABAergic circuits of the striatum. Prog Brain Res. 1993;99:51–72. doi: 10.1016/s0079-6123(08)61338-2. [DOI] [PubMed] [Google Scholar]
- Kita H, Kosaka T, Heizmann CW. Parvalbumin-immunoreactive neurons in the rat neostriatum: a light and electron microscopic study. Brain Res. 1990;536:1–15. doi: 10.1016/0006-8993(90)90002-s. [DOI] [PubMed] [Google Scholar]
- Kitai ST, Surmeier DJ. Cholinergic and dopaminergic modulation of potassium conductances in neostriatal neurons. Adv Neurol. 1993;60:40–52. [PubMed] [Google Scholar]
- Koos T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci. 1999;2:467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM, Wilson CJ. Comparison of IPSCs evoked by spiny and fast-spiking neurons in the neostriatum. J Neurosci. 2004;24:7916–7922. doi: 10.1523/JNEUROSCI.2163-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci. 2005;25:10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Xue S, Pei L, Vukusic B, Chery N, Wang Y, Wang YT, Niznik HB, Yu XM, Liu F. Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Cell. 2002;111:219–230. doi: 10.1016/s0092-8674(02)00962-5. [DOI] [PubMed] [Google Scholar]
- Lei W, Jiao Y, Del Mar N, Reiner A. Evidence for differential cortical input to direct pathway versus indirect pathway striatal projection neurons in rats. J Neurosci. 2004;24:8289–8299. doi: 10.1523/JNEUROSCI.1990-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner TN, Horne EA, Stella N, Kreitzer AC. Endocannabinoid signaling mediates psychomotor activation by adenosine A2A antagonists. J Neurosci. 2010;30:2160–2164. doi: 10.1523/JNEUROSCI.5844-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JC, DeFazio RA, Espinosa-Jeffrey A, Cepeda C, de Vellis J, Levine MS. Calcium modulates dopamine potentiation of N-methyl-D-aspartate responses: electrophysiological and imaging evidence. J Neurosci Res. 2004;76:315–322. doi: 10.1002/jnr.20079. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Karsten SL, Gray M, Geschwind DH, Yang XW. FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat Neurosci. 2006;9:443–452. doi: 10.1038/nn1654. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Tyler EC, Merritt A. Short- and long-term synaptic depression in rat neostriatum. J Neurophysiol. 1993;70:1937–1949. doi: 10.1152/jn.1993.70.5.1937. [DOI] [PubMed] [Google Scholar]
- Mallet N, Ballion B, Le Moine C, Gonon F. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J Neurosci. 2006;26:3875–3884. doi: 10.1523/JNEUROSCI.4439-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- Maurice N, Mercer J, Chan CS, Hernandez-Lopez S, Held J, Tkatch T, Surmeier DJ. D2 dopamine receptor-mediated modulation of voltage-dependent Na+ channels reduces autonomous activity in striatal cholinergic interneurons. J Neurosci. 2004;24:10289–10301. doi: 10.1523/JNEUROSCI.2155-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurers BH, Dziewczapolski G, Shi T, Bittner A, Kamme F, Shults CW. Dopamine depletion induces distinct compensatory gene expression changes in DARPP-32 signal transduction cascades of striatonigral and striatopallidal neurons. J Neurosci. 2009;29:6828–6839. doi: 10.1523/JNEUROSCI.5310-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50(4):381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Mink JW. The Basal Ganglia and involuntary movements: impaired inhibition of competing motor patterns. Archives of neurology. 2003;60:1365–1368. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- Nambu A. Seven problems on the basal ganglia. Curr Opin Neurobiol. 2008;18:595–604. doi: 10.1016/j.conb.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Malenka RC. Modulation of synaptic transmission by dopamine and norepinephrine in ventral but not dorsal striatum. J Neurophysiol. 1998;79:1768–1776. doi: 10.1152/jn.1998.79.4.1768. [DOI] [PubMed] [Google Scholar]
- Olson PA, Tkatch T, Hernandez-Lopez S, Ulrich S, Ilijic E, Mugnaini E, Zhang H, Bezprozvanny I, Surmeier DJ. G-protein-coupled receptor modulation of striatal CaV1.3 L-type Ca2+ channels is dependent on a Shank-binding domain. J Neurosci. 2005;25:1050–1062. doi: 10.1523/JNEUROSCI.3327-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent M, Parent A. Single-axon tracing study of corticostriatal projections arising from primary motor cortex in primates. J Comp Neurol. 2006;496:202–213. doi: 10.1002/cne.20925. [DOI] [PubMed] [Google Scholar]
- Parthasarathy HB, Graybiel AM. Cortically driven immediate-early gene expression reflects modular influence of sensorimotor cortex on identified striatal neurons in the squirrel monkey. J Neurosci. 1997;17:2477–2491. doi: 10.1523/JNEUROSCI.17-07-02477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavon N, Martin AB, Mendialdua A, Moratalla R. ERK phosphorylation and FosB expression are associated with L-DOPA-induced dyskinesia in hemiparkinsonian mice. Biol Psychiatry. 2006;59:64–74. doi: 10.1016/j.biopsych.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Pawlak V, Kerr JN. Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. J Neurosci. 2008;28:2435–2446. doi: 10.1523/JNEUROSCI.4402-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Rosello T, Figueroa A, Salgado H, Vilchis C, Tecuapetla F, Guzman JN, Galarraga E, Bargas J. Cholinergic control of firing pattern and neurotransmission in rat neostriatal projection neurons: role of CaV2.1 and CaV2.2 Ca2+ channels. J Neurophysiol. 2005;93:2507–2519. doi: 10.1152/jn.00853.2004. [DOI] [PubMed] [Google Scholar]
- Pisani A, Bernardi G, Ding J, Surmeier DJ. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 2007;30:545–553. doi: 10.1016/j.tins.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Planert H, Szydlowski SN, Hjorth JJ, Grillner S, Silberberg G. Dynamics of synaptic transmission between fast-spiking interneurons and striatal projection neurons of the direct and indirect pathways. J Neurosci. 2010;30:3499–3507. doi: 10.1523/JNEUROSCI.5139-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirazi P, Mel BW. Impact of active dendrites and structural plasticity on the memory capacity of neural tissue. Neuron. 2001;29:779–796. doi: 10.1016/s0896-6273(01)00252-5. [DOI] [PubMed] [Google Scholar]
- Rajakumar N, Elisevich K, Flumerfelt BA. The pallidostriatal projection in the rat: a recurrent inhibitory loop? Brain Res. 1994;651:332–336. doi: 10.1016/0006-8993(94)90714-5. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. The basal ganglia: a vertebrate solution to the selection problem? Neuroscience. 1999;89:1009–1023. doi: 10.1016/s0306-4522(98)00319-4. [DOI] [PubMed] [Google Scholar]
- Santini E, Heiman M, Greengard P, Valjent E, Fisone G. Inhibition of mTOR signaling in Parkinson’s disease prevents L-DOPA-induced dyskinesia. Sci Signal. 2009;2:ra36. doi: 10.1126/scisignal.2000308. [DOI] [PubMed] [Google Scholar]
- Santini E, Valjent E, Usiello A, Carta M, Borgkvist A, Girault JA, Herve D, Greengard P, Fisone G. Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J Neurosci. 2007;27:6995–7005. doi: 10.1523/JNEUROSCI.0852-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuer T, Catterall WA. Control of neuronal excitability by phosphorylation and dephosphorylation of sodium channels. Biochemical Society transactions. 2006;34:1299–1302. doi: 10.1042/BST0341299. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Desdouits F, Menu R, Greengard P, Vincent JD, Vanderhaeghen JJ, Girault JA. Modulation of the voltage-gated sodium current in rat striatal neurons by DARPP-32, an inhibitor of protein phosphatase. Eur J Neurosci. 1998;10:1312–1320. doi: 10.1046/j.1460-9568.1998.00142.x. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of DA neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple DA functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Sciamanna G, Bonsi P, Tassone A, Cuomo D, Tscherter A, Viscomi MT, Martella G, Sharma N, Bernardi G, Standaert DG, Pisani A. Impaired striatal D2 receptor function leads to enhanced GABA transmission in a mouse model of DYT1 dystonia. Neurobiology of disease. 2009;34:133–145. doi: 10.1016/j.nbd.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott L, Zelenin S, Malmersjo S, Kowalewski JM, Markus EZ, Nairn AC, Greengard P, Brismar H, Aperia A. Allosteric changes of the NMDA receptor trap diffusible dopamine 1 receptors in spines. Proc Natl Acad Sci U S A. 2006;103:762–767. doi: 10.1073/pnas.0505557103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Tian X, Day M, Ulrich S, Tkatch T, Nathanson NM, Surmeier DJ. Cholinergic modulation of Kir2 channels selectively elevates dendritic excitability in striatopallidal neurons. Nat Neurosci. 2007;10:1458–1466. doi: 10.1038/nn1972. [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Allen PB, Fienberg AA, Valle CG, Huganir RL, Nairn AC, Greengard P. Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. J Neurosci. 2000;20:4480–4488. doi: 10.1523/JNEUROSCI.20-12-04480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooren WP, Lynd-Balta E, Mitchell S, Haber SN. Ventral pallidostriatal pathway in the monkey: evidence for modulation of basal ganglia circuits. J Comp Neurol. 1996;370:295–312. doi: 10.1002/(SICI)1096-9861(19960701)370:3<295::AID-CNE2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Stern EA, Jaeger D, Wilson CJ. Membrane potential synchrony of simultaneously recorded striatal spiny neurons in vivo. Nature. 1998;394:475–478. doi: 10.1038/28848. [DOI] [PubMed] [Google Scholar]
- Stoetzner CR, Pettibone JR, Berke JD. State-dependent plasticity of the corticostriatal pathway. Neuroscience. 2010;165:1013–1018. doi: 10.1016/j.neuroscience.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoof JC, Kebabian JW. Two dopamine receptors: biochemistry, physiology and pharmacology. Life Sci. 1984;35:2281–2296. doi: 10.1016/0024-3205(84)90519-8. [DOI] [PubMed] [Google Scholar]
- Sullivan MA, Chen H, Morikawa H. Recurrent inhibitory network among striatal cholinergic interneurons. J Neurosci. 2008;28:8682–8690. doi: 10.1523/JNEUROSCI.2411-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J Neurosci. 2005;25:7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Bargas J, Hemmings HC, Jr, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Eberwine J, Wilson CJ, Cao Y, Stefani A, Kitai ST. Dopamine receptor subtypes colocalize in rat striatonigral neurons. Proc Natl Acad Sci U S A. 1992;89:10178–10182. doi: 10.1073/pnas.89.21.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annual review of pharmacology and toxicology. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Tanimura A, Yamazaki M, Hashimotodani Y, Uchigashima M, Kawata S, Abe M, Kita Y, Hashimoto K, Shimizu T, Watanabe M, Sakimura K, Kano M. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron. 2010;65:320–327. doi: 10.1016/j.neuron.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Taverna S, Ilijic E, Surmeier DJ. Recurrent collateral connections of striatal medium spiny neurons are disrupted in models of Parkinson’s disease. J Neurosci. 2008;28:5504–5512. doi: 10.1523/JNEUROSCI.5493-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Koos T, Wilson CJ. GABAergic microcircuits in the neostriatum. Trends Neurosci. 2004;27:662–669. doi: 10.1016/j.tins.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Wilson CJ, Koos T. Feedforward and feedback inhibition in neostriatal GABAergic spiny neurons. Brain Res Rev. 2008;58:272–281. doi: 10.1016/j.brainresrev.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa CM, Standaert DG, Young AB, Penney JB., Jr Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J Neurosci. 1994;14:3005–3018. doi: 10.1523/JNEUROSCI.14-05-03005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Kai L, Hockberger PE, Wokosin DL, Surmeier DJ. MEF-2 regulates activity-dependent spine loss in striatopallidal medium spiny neurons. Mol Cell Neurosci. 2010;44:94–108. doi: 10.1016/j.mcn.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RS, Anderson ME. Pallidal discharge related to the kinematics of reaching movements in two dimensions. J Neurophysiol. 1997;77:1051–1074. doi: 10.1152/jn.1997.77.3.1051. [DOI] [PubMed] [Google Scholar]
- Turner RS, Anderson ME. Context-dependent modulation of movement-related discharge in the primate globus pallidus. J Neurosci. 2005;25:2965–76. doi: 10.1523/JNEUROSCI.4036-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall MJ, Oorschot DE, Kean A, Wickens JR. Inhibitory interactions between spiny projection neurons in the rat striatum. J Neurophysiol. 2002;88:1263–1269. doi: 10.1152/jn.2002.88.3.1263. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci. 1999;22:221–227. doi: 10.1016/s0166-2236(98)01341-1. [DOI] [PubMed] [Google Scholar]
- Valjent E, Bertran-Gonzalez J, Herve D, Fisone G, Girault JA. Looking BAC at striatal signaling: cell-specific analysis in new transgenic mice. Trends Neurosci. 2009;32:538–547. doi: 10.1016/j.tins.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Vergara R, Rick C, Hernandez-Lopez S, Laville JA, Guzman JN, Galarraga E, Surmeier DJ, Bargas J. Spontaneous voltage oscillations in striatal projection neurons in a rat corticostriatal slice. J Physiol. 2003;553:169–182. doi: 10.1113/jphysiol.2003.050799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchis C, Bargas J, Ayala GX, Galvan E, Galarraga E. Ca2+ channels that activate Ca2+-dependent K+ currents in neostriatal neurons. Neuroscience. 2000;95:745–752. doi: 10.1016/s0306-4522(99)00493-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Toledo-Rodriguez M, Gupta A, Wu C, Silberberg G, Luo J, Markram H. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J Physiol. 2004;561:65–90. doi: 10.1113/jphysiol.2004.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Westin JE, Vercammen L, Strome EM, Konradi C, Cenci MA. Spatiotemporal pattern of striatal ERK1/2 phosphorylation in a rat model of L-DOPA-induced dyskinesia and the role of dopamine D1 receptors. Biol Psychiatry. 2007;62:800–810. doi: 10.1016/j.biopsych.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Functional neuroanatomy of the basal ganglia in Parkinson’s disease. Adv Neurol. 2003;91:9–18. [PubMed] [Google Scholar]
- Wickens JR. Synaptic plasticity in the basal ganglia. Behavioural Brain Research. 2009;199:119–128. doi: 10.1016/j.bbr.2008.10.030. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. Dendritic Morphology, Inward Rectification and the Functional Properties of Neostriatal Neurons. In: McKenna T, Davis J, Zornetzer SF, editors. Single Neuron Computation. San Diego: Academic Press; 1992. pp. 141–171. [Google Scholar]
- Wilson CJ. The generation of natural firing patterns in neostriatal neurons. Prog Brain Res. 1993;99:277–297. doi: 10.1016/s0079-6123(08)61352-7. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. Understanding the neostriatal microcircuitry: high-voltage electron microscopy. Microsc Res Tech. 1994;29:368–380. doi: 10.1002/jemt.1070290507. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. Basal ganglia. In: Shepherd GM, editor. The synaptic organization of the brain. Oxford: Oxford UP; 2004. pp. 361–414. [Google Scholar]
- Wilson CJ, Groves PM. Fine structure and synaptic connections of the common spiny neuron of the rat neostriatum: a study employing intracellular inject of horseradish peroxidase. J Comp Neurol. 1980;194:599–615. doi: 10.1002/cne.901940308. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltschko AB, Pettibone JR, Berke JD. Opposite effects of stimulant and antipsychotic drugs on striatal fast-spiking interneurons. Neuropsychopharmacology. 2010;35:1261–1270. doi: 10.1038/npp.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Surmeier DJ. Muscarinic (m2/m4) receptors reduce N- and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast, membrane-delimited, G-protein pathway. J Neurosci. 1996;16:2592–2604. doi: 10.1523/JNEUROSCI.16-08-02592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Surmeier DJ. D5 dopamine receptors enhance Zn2+-sensitive GABA(A) currents in striatal cholinergic interneurons through a PKA/PP1 cascade. Neuron. 1997;19:1115–1126. doi: 10.1016/s0896-6273(00)80402-x. [DOI] [PubMed] [Google Scholar]
- Yan Z, Song WJ, Surmeier J. D2 dopamine receptors reduce N-type Ca2+ currents in rat neostriatal cholinergic interneurons through a membrane-delimited, protein-kinase-C-insensitive pathway. J Neurophysiol. 1997;77:1003–1015. doi: 10.1152/jn.1997.77.2.1003. [DOI] [PubMed] [Google Scholar]
- Yin HH, Lovinger DM. Frequency-specific and D2 receptor-mediated inhibition of glutamate release by retrograde endocannabinoid signaling. Proc Natl Acad Sci U S A. 2006;103:8251–8256. doi: 10.1073/pnas.0510797103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WS, 3rd, Bonner TI, Brann MR. Mesencephalic dopamine neurons regulate the expression of neuropeptide mRNAs in the rat forebrain. Proc Natl Acad Sci U S A. 1986;83:9827–31. doi: 10.1073/pnas.83.24.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]