Abstract
Recent major findings from studies of SLC6A4 and its corresponding protein, the serotonin (5-HT) transporter (SERT) in humans, rodents and non-human primates indicate that combinations of SLC6A4 non-coding 5′, 3′ UTRs and intronic regions plus coding variants acting together can change 5HT transport as much as 40-fold in vitro. In vivo, SLC6A4 variants in humans and other species lead to marked physiological changes, despite mitigating neurodevelopmental adaptations in 5-HT receptors plus compensatory alterations in 5-HT synthesis and metabolism. Polymorphisms in SLC6A4 are associated with differences in emotional, endocrine, and personality characteristics as well as many diseases. This gene, in combinations with gene x gene (G x G) and gene x environment (G x E) interactions nonetheless remains incompletely understood, with some association findings remaining controversial. Considering its primary importance in the regulation and function of the entire serotonergic system (as evidenced by the consequences of SERT-mediated reuptake inhibition by SRIs like fluoxetine in humans and of genetically-engineered changes in mice and rats), it seems likely that SLC6A4 and SERT will remain areas of high interest in our field’s attempts to better understand and treat 5-HT-related disorders.
Introduction: Identified Variants in SLC6A4
This brief review of an extensive research area (>3000 publications in the time since the first human SLC6A4 polymorphism was reported to be associated with a distinct phenotype [1]) focuses on the most recent reports that have taken into account both this first SLC6A4 polymorphism, the serotonin (5-HT) transporter - linked 5′ promoter region (5HTTLPR) variant [1] and also other more recently-described functional variants present likewise in the 5′ region of SLC6A4 (i.e., rs25531, rs25532) plus an intronic variant (Stin2), and several exonic, rare coding region variants (SERT I425V, I425L and G56A) as well as 3′ SLC6A4 variants not yet as well-studied clinically, as depicted in figure 1A–D [2–5].
Figure 1.
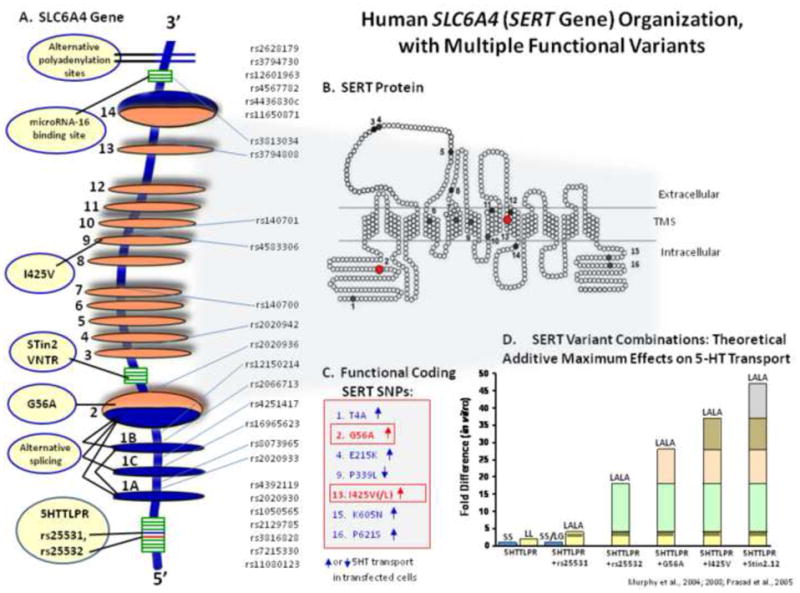
A–D: Human SLC6A4 (SERT Gene) organization with multiple functional variants.
Human SLC6A4 maps to chromosome 17q11.2 and is composed of 14 exons spanning 40 kb (Fig. 1A). The sequence of the transcript predicts a protein comprised of 630 amino acids with 12 transmembrane domains. Alternative promoters, differential splicing involving exons 1A, B, and C, and 3′-untranslated-region (UTR) variability and other SNPs result in multiple mRNA species that regulate gene expression in humans and other species and in cultured cell lines [2–6]. The 5HTTLPR, rs25531 and rs25532 in combination comprise multiple alleles, each with differing effects on gene expression [2]. Ethnicity differences in the proportions of these variants exist across world populations, such that there is a 5HTTLPR 40% difference in Caucasians vs. some Asian groups for the 5HTTLPR S allele and for rs25531 a minor allele frequency of 9–15% in Caucasians and 24% in African-Americans [2, 7] (Figure 2). Several of the less common SLC6A4 coding SNPs are associated with behavioral phenotypes or disorders, including obsessive-compulsive disorder (SERT I425V is “OCD 1” in OMIM) and autism [2, 5, 7].
Figure 2.
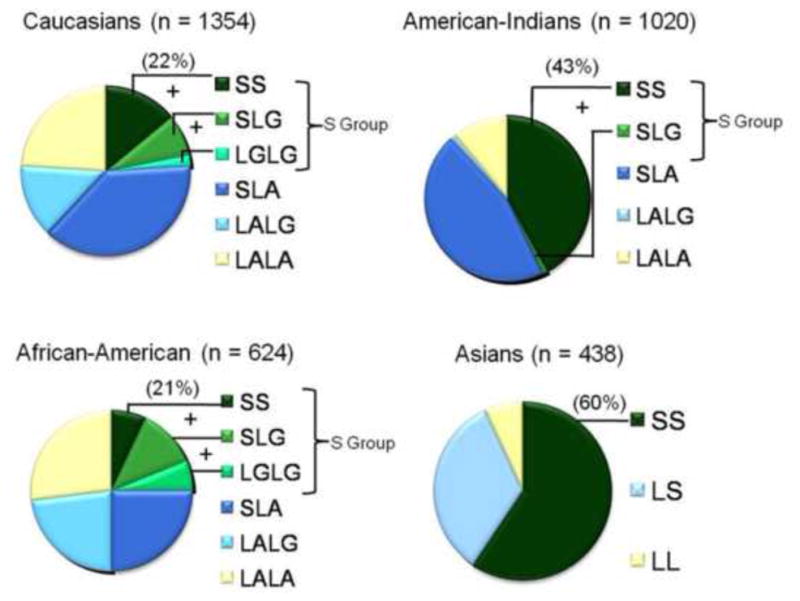
Proportionate distributions (%) of SLC6A4 variants in different global populations.
The 5′ promoter region variants of SLC6A4 affect the expression of SERT, as reflected in reporter gene assays and in numbers of binding sites for radiolabeled SERT ligands measured in lymphoblast and post-mortem brain preparations, consequently affecting the transport of 3H-5-HT in these tissues (as well as in platelets) and altering extracellular 5HT concentrations as evidenced by directly-measured, chronoamperometric assays [1, 7–9]. In addition, a recent review of positron emission tomographic studies in living human brain caudate, putamen and midbrain of SERT binding potentials correlated well with SLC6A4 genotypes when 14C-DASB was used as the ligand, whereas earlier-studied ligands such as 123β-CIT did not [10]. In addition, missense mutations such as SERT I425V and G56A were found to not only produce a gain-of-function as measured by increased 3H 5HT-uptake but, in the case of I425V, also altered the regulation of SERT as measured by responses to nitric oxide synthase precursors plus cyclic GMP and P38 mitogen-activated protein kinase [5, 11].
The SLC6A4 intron 2 (STin2) polymorphic region consists of three alleles: Stin2.9; Stin2.10 and Stin2.12. This variable member of tandem repeats (VNTR) polymorphism yields enhanced expression proportionate to the number of repeat copies of the 16/17 base pair element (12 >10 >9), as determined in embryonic brain and stem cell preparations and in human JAR cells [3]. The alleles of Stin 2 respond differentially to the transcription factors YB-1 and CTCF, which in turn, can be modulated by lithium chloride, an agent useful in the treatment of bipolar affective disorder [3, 12].
Contrary to an extensive and constantly growing literature on the 5HTTLPR and Stin2 variants, clinical genetic research focusing on SLC6A4 3′ UTR variants is sparse. SLC6A4 3′-UTR variants play important roles in mRNA translation, localization and stability. Thus, mutations in the 3′-UTR can affect the termination codon, polyadenylation (polyA) signals, the ratio of multiple polyA signal usage, as well as the secondary structure of the 3′-UTR mRNA, highlighting the multiple ways that polymorphisms in this region may cause a deregulated translational control and thereby disease [12]. The SLC6A4 3′-UTR contains two polyA sites, located at 567 bp and 690 bp downstream of the stop codon. These two sites are also present in mice, with a high degree of sequence similarity, suggesting that both sites have important, evolutionarily-conserved functions. The more distal of the polyA signals contains a common SNP (rs3813034) that alters the balance of the two polyA forms of SERT such that the T>G allele of rs3813034 leads to an increase of the distal polyA signal [12, 13].
In a recent report, SERT expression was shown to be modulated by 3′-UTR microRNAs, with miR-16 found to be a posttranscriptional regulator of SERT levels. Over-expression of miR-16 reduced SERT expression, determined by [125I]-RTI-55 and [3H]-paroxetine binding site changes in a differentiated serotonergic cell line [6]. Correspondingly, miR-16 reductions induced SERT expression. This study showed that fluoxetine reduces SERT via increasing miR-16 in serotonergic neurons, and turns on a serotonergic phenotype in noradrenergic neurons indirectly (via S100β), establishing this microRNA as a central effector regulating the adaptative responses of serotonergic and noradrenergic neurons to SRI treatment [6].
As these multiple SLC6A4 variants appear to have additive or other combinatorial consequences, as suggested by studies in vitro such as those employing transfected cell lines using luciferase and other reporter genes [7, 12, 13] (Figure 1D), this review emphasizes considerations of studies that have examined the SLC6A4 5HTLPR alleles (L, S) and genotypes (LL, LS and SS) in combination with the rs25531 and rs25532 SLC6A4 variants in the 5HTTLPR accomplished in ~30 recent published reports as of February 2011 [7, 12, 13]. As most of these reports can be grouped into three categories, this review considers their contributions thusly: (A) SLC6A4 variants associated with pharmacogenomic consequences; (B) SLC6A4 variants associated with disease/disorder status and pharmacological interventions and (C) SLC6A4 variants associated with gene x environment interactions in relation to physiological, emotional or disorder status, with pharmacological implications.
A. Pharmacogenomic Studies of SLC6A4 Variants
Antidepressant and Anti-Anxiety Disorder 5-HT Reuptake Inhibitors (SRIs)
As the first primary action of the SRIs (e.g., fluoxetine, citalopram) depends upon their binding to SERT and thus reuptake inhibition, the evident, simplest hypothesis regarding the importance of SLC6A4 functional variants is that variants leading to greater SERT expression would be expected to be associated with enhanced responsivity to SRIs, given adequate SRI drug dosage. This hypothesis has been evaluated principally regarding SRI’s efficacy in depression in an increasing number of studies employing large patient groups, as well as meta-analyses and standardized reviews of this question. It has also been evaluated in a smaller number of studies of the efficacy of SRIs in anxiety disorders such as obsessive-compulsive disorder (OCD) plus several other disorders.
Among recent reviews and meta-analyses of the relationship between SLC6A4 regulatory region variants and antidepressant responses, the largest systematic review surveyed 24 studies. When three studies were omitted because of endpoints besides response within 12 weeks, the greater-expressing L allele or LL genotype was associated with a significantly greater response in 12 studies and the S allele or SS genotype in 3 studies, with no difference found in 6 studies, leading to a pooled odds ratio favoring the L/LL variant of 2.57 [14]. The authors re-tabulated the study results after dividing them into 13 studies in Caucasians and 11 studies in Asian populations, noting an ethnicity-based difference in the occurrence of the S allele (42% in Caucasians, 79% in Asians) and that, as in prior studies, equivocal association with antidepressant responses to SRIs were found in Asians unlike the positive results in Caucasians [14].
Recent meta-analyses of SLC6A4 variants associated with SRI antidepressant responses have found varying results. Significant association was found between SLC6A4 variants with antidepressant remission’, in which individuals with the SS genotype had lower rates of full recovery, as evidenced by post-treatment subclinical depression rating levels [15]. Partial response as indicated by reduced depression ratings alone was not associated with SLC6A4 variants. Substantial evidence of heterogeneity complicated the antidepressant response’ analyses. This included an effect of gender and of diagnostic groupings (specifically an inclusion of bipolar subjects in some studies). In other studies, depressed patients with bipolar diagnoses or underlying bipolar proclivities that had S allele or SS genotypes failed to have antidepressant responses or had to stop SRI treatment because of the development of hypomanic or full-blown manic episodes, as exemplified in a recent meta-analysis [16].
Of the >30 studies that examined possible relationships between antidepressant responses to SRIs and SLC6A4 variants, only a few have examined combinatorial relationships among the four common variants (5HTTLPR, rs25531, rs25532, Stin2). In a series of overlapping studies from the “Star*D” cohort of 1347 to 1494 MDD patients evaluated for “remission” (depression ratings of ≤ 5 on the QIDS scale [self- or observer-rated) and genotyped for both 5HTTLPR and rs25531 SLC6A4 variants, two of four studies reported a significant association with L/LL variants [17]. One of two positive studies found an association with 5HTTLPR variants only in non-Hispanic Caucasians (and not in African-Americans) [17]. It should be noted that one of the four studies did not evaluate the “non-Hispanic” Caucasian distinction [17], and one of the studies found that the triallelic (5HTTLPR plus rs25531) combination analysis was associated with citalopram-related adverse side effects (those with the LA genotype had fewer side effects) [16, 18], especially treatment- emergent diarrhea which in combination with other side effects led to SRI intolerance [16, 18]. Subjects who were rated as intolerant or probably intolerant were not included in the non-remission group [19], one obvious interpretation is that the association conclusions may be biased since drop-outs do not receive adequate courses of therapy [18].
One additional Star*D-based study evaluated 5HTTLPR plus Stin2, identifying a positive association between these variants (more 5HTTLPR LL subjects were responders, with an OR of 1.37) in 1074 non-Hispanic Caucasian participants [19]. In this study the approximate percentages of the 5HTTLPR L, Stin2 and the rs25531 A allele were 58, 60 and 93%, respectively, and only the individuals who had the 5HTTLPR LL or the Stin2 12/12 genotype were significantly and positively associated with remission during citalopram treatment. This odds ratio of 1.39 for patients with the HTTLPR LL genotype being associated with remission (observed previously [17]) is in agreement with the odds ratio of 1.37 found in the systematic analytical review discussed above [14]. Of special interest in these pharcogenomic studies are OCD spectrum disorders as prime examples of conditions with exceptional serotonergic interest since specific functional variants have been found to be associated with these disorders in multiple studies. This may be relevant to the fact that SRIs are the only drug group found in replicated studies to be therapeutically useful in OCD, an anxiety disorder that does not respond to other anxiolytic or antidepressant agents such as the tricyclic antidepressants [2, 7].
Of note, it is possible that relatively mild 5-HT syndrome occurrence may contribute to early discontinuation of SRIs and side effects during SRI treatment that are strongly associated with the SS genotype and S allele of the SERT variant [18]. One example among from several studies illustrates this phenomenon. Adverse effects and treatment discontinuation in depressed patients following paroxetine discontinuation were highly related to the SLC6A4 5HTTLPR genotype [20]. Of relevance, the behavioral and physiological serotonin syndrome was initially characterized in rodents given pro-serotonergic agents and subsequently in humans as a consequence of toxic reactions to monoamine oxidase inhibitor (MAOI)-SRI drug interactions, including mild to severe cases of the serotonin syndrome [19]. This conclusion has been partly based on moderate correlations between amounts of drug ingested in suicide attempts relative to outcomes. Genetic vulnerability akin to that observed in humans with the lower-expressing SS SLC6A4 genotype has its parallel in the exaggerated serotonin syndrome following administration of pro-serotonergic agents such as MAOI’s, 5-HTP and tramadol in Slc6a4 +/− and Slc6a4 −/− serotonin transporter deficient mice with a targeted disruption of SERT [21]. This model provides implications for similar SRI side effects in humans with polymorphisms that reduce SERT gene function [20, 21]. Of interest, 5-HTP is promulgated on more than 100 Internet sites and sold over-the-counter as a dietary supplement and as having antidepressant effects and as like prolonging the actions of illicit drugs of abuse such as MDMA. A major question deserving of further study is whether therapeutic effects of SRIs that are related to SRI intolerance might be mitigated by co-administration of 5-HT3 or 5-HT4 receptor antagonists with targeted effects on the gut irritable bowel syndrome (IBS)-like symptoms produced by SRIs that appear to be a major factor in SRI intolerance and that limit treatment efficacy with SRIs [22].
B. SLC6A4 Associations with Neuropsychiatric and Other Medical Disorders: SLC6A4 and its Variants as Biomarkers with Possible Etiologic Contributions and with Pharmacological Treatment Implications
The ubiquity of 5-HT’s neuronal as well as non-neuronal functions has served to stimulate many studies of the 5-HT systems’ genetic components, from the 13-plus 5-HT receptor genes to its synthesis and metabolic pathway genes and especially SLC6A4. In this brief review, it is only possible to note some of the disorders which have been most thoroughly investigated in larger subject number studies, systematic reviews, meta-analyses or some recent instances of findings of special interest in regard to treatment and side effects with SRIs and 5-HT receptor agonists and antagonists.
The bulk of available data regarding SLC6A4 are limited to the first-discovered variant, 5HTTLPR, without its modifying SNPs (rs25531, rs25532) or other SERT variants. However, a few studies in the last decade have extended evaluations to SLC6A4 variant combinations encompassing the additional 5′ variants plus Stin2 or multiple SNPs (in some studies, tag SNPs only) across the gene, with haplotype analyses. Meta-analyses of SLC6A4 variants have found associations with specific 5HTTLPR alleles in several neuropsychiatric disorders: (a) bipolar affective disorder (5HTTLPR, S allele) [23]; (b) autism (5HTTLPR, S allele, in U.S. families only) with a parent of origin effect noted [24]; (c) alcohol and other drug dependencies (5HTTLPR, S allele); (d) schizophrenia (Stin2) (~12 studies, >2000 cases and >2000 controls) [25]; (e) depression, anxiety and related traits in ~1000 cases + ~1000 controls [26]; (f) obsessive-compulsive disorder (5HTTLPR L allele in family-based and case-control studies involving children and Caucasian samples, some including rs25531 and rs25532 and haplotype analyses) [27]; and (g) ADHD (5HTTLPR L allele only, not Stin2 or rs3813034) in a meta-analysis of 20 studies [28] although other studies found positive associations for ADHD only in males or only in combination with early life stress. Other disorders reported to show associations with SLC6A4 alleles or genotypes include the sudden infant death syndrome (SIDS); migraine in European (not Asian) females; musculoskeletal disorders; myocardial infarction (5HTTLPR S allele protective in delaying age of onset) and the LL genotype associated with a greater risk for myocardial infarction in additional studies (OR = 1.4, N > 600); adverse events after coronary artery bypass graft surgery, in combination with depressive symptoms (5HTTLPR L allele) [29]. Mechanisms involved to explain the cardiovascular associations include excess 5-HT accumulation in platelets related to greater SERT expression due to the L allele and hence enhanced releasability of 5-HT, as well as direct effects of 5-HT on vascular proliferation [30].
It is no surprise to find that anxiety and depression-related personality traits and affective disorders [13, 29], IBS-like features [22, 31], the serotonin syndrome [21], some sleep and thermoregulatory disorders, pulmonary hypertension and chronic obstructive pulmonary disease (5HTTLPR L allele frequently implicated in positive studies, but a recent large study was negative and another one was only positive in demonstrating an interaction between SLC6A4 and BMP2, the bone morphogenetic protein 2 gene [30]. Some of the disorders found to be associated with SLC6A4 in humans such as SIDS and myocardial valvulopathy and infarction have not yet been investigated in Slc6a4 −/− and Slc6a4 +/− mice [29, 32]. Likewise, some of the Slc6a4 −/− mouse phenotypes, including obesity and type-2-diabetes, have yet to be studied for SLC6A4 variants in cohorts of human patients. These would seem to be of high priority for investigation.
C. SLC6A4 Variants Studied for Associations via Gene x Environment Models of Diseases and Their Pharmacological Treatment
That diseases becomes manifest when gene (G) variants interact with environmental (E) triggers is a long-recognized truism in medicine: e.g., when an individual with a personal or family history of asthma walks into a hotel room with a moldy smell, that person, but not necessarily her spouse, is likely to begin to wheeze. Nonetheless, while candidate gene variants for asthma are numerous, this disease seems multi-factorial and genetic causes remain undefined. It is in this complex context that high interest was aroused when the S allele or SS genotype of SLC6A4 was identified as a specific variant that, in a prospective, epidemiologic sample, moderated the effect of stressful life events on the subsequent development of depression and suicidality [33]. This finding has been replicated by major groups worldwide and also mirrored in model studies across many species [34, 35]. Some dissenting findings such as that of one meta-analysis have also appeared, with subsequent critiques pointing out that strict meta-analyses may only include a small fraction of positive or partial replications and that the few non-replications, although these had large sample sizes, were most-commonly based on self-reports rather than objective measures and lacked the strengths of prospective investigations [36, 37].
A genetic contribution of SLC6A4 to temperament and behavioral traits including anxiety, excess stress responsiveness, dominance relationships in non-human primate species and alcohol and drug preferences have been established in humans and several other species, probably reflecting selective forces among our ancestral and related animal species. Recent research efforts in this area have therefore been focused on mice, rats and non-human primates, especially rhesus macaques, and are currently proceeding towards the elaboration of an interdisciplinary perspective that will blend behavioral genetics and evolutionary psychology, as well as cognitive and social neuroscience [38, 39]. Special interest has developed regarding models ranging from non-human primates and rodents to C. elegans and D. melanogaster, organisms for which environmental influences might be less complex than in humans and thus less likely to confound associations between behavior and genes [35, 38–40]. Maternal-separation G x E studies in rhesus macaques have demonstrated the occurrence of interactions that affect behavioral traits, including stress reactivity and alcohol preference and dependence, based upon the differential occurrence of a repeat-length SLC6A4 promoter region variant that is orthologous to the human 5HTTLPR and in some cases 3′ UTR variants. This is consistent with data that the 5HTTLPR, and most likely other SLC6A4 variants, influence the risk for anxiety, affective and other behavioral disorders through gene x environment interactions, sometimes reflected in specific brain region alterations such as those found with activation of the amygdale in functional imaging studies [41, 42].
In the formal sense of the G x E concept, we have already considered examples in Section A regarding pharmacogenomics, in which SLC6A4 variants were evaluated for associations with a specific “environmental” influence, i.e., a drug, with most emphasis placed upon the large number of studies of SRIs. Responses to methylphenidate in children with ADHD have also been evaluated and found to be associated with both the triallelic 5HTTLPR and also the Stin2 variant [43]. In one of many experiments employing genetically-engineered SERT-deficient mice or rats, such SERT +/− and/or −/− mice were relatively insensitive to analgesic effects of morphine, tramadol or meperidine, but at the same time had exaggerated serotonin syndrome responses to the latter atypical opioid agents [21, 35, 44, 45]. A few additional, more complex examples in which a drug “environment” was combined with an experimental environment to study a drug response can provide a glimpse of this very extensive literature. Spontaneous use of alcohol in a behavioral dependence model occurred in non-human primates who had a combination of the S allele of the rh5HTTLPR plus six months of experiential peer-rearing (as opposed to parental rearing) [46]. Likewise, in SERT −/− mice, anxiety-like behaviors are intensified after exposure to predator odors or postnatal foot-shock stress compared to SERT −/− mice [47]. Thus, this genetic model has been extremely useful in highlighting how SLC6A4 may predispose to affective disorders though gene x environment interactions [35, 44].
However, the molecular and neural mechanisms that underlie the interplay of genes and environmental adversity that constitute disease risk remain incompletely understood. Finally, gene-gene (G x G) interactions have begun to be studied in combined association studies in humans. More direct studies have been possible in mouse models: for example, Slc6a4 mutant mice have been interbred with mice that lacked either one or both copies of the dopamine transporter gene (Slc6a3), the norepinephrine transporter gene (Slc6a2), the MAOA gene the 5-HT1B receptor gene and the Bdnf gene, with consequent amplified, reduced or qualitatively different new phenotypes. These studies of experimental epistasis’ complement those in which the Slc6a4 −/− variant was placed on congenic C57BL/6J, I29S6 or CD-1 background strains to yield more subtle SERT-related phenotype differences [45].
Of note, evidence for functional effects of these SLC6A4 variants has been mostly obtained indirectly using cultured cells, transfected, or animal model systems or using other in vitro methods; for Stin2, a combined effect of the lesser expressing Stin2.10 plus the 5HTTLPR S allele, and also a three-way interaction incorporating rs25531 on gene expression was observed [1, 2, 48]. Functional consequences for the 5′ 5HTTLPR and rs25531 have been inferred from measured RNA changes but only the 5HTTLPR and rs25531 have been evaluated and found to alter 5-HT transport and other measures in brain, platelet and lymphoblast cells from humans with different genotypes [1, 2, 7, 8]. As suggested above, given the impact that polymorphisms in the 3′-UTR can have effects on mRNA structure, postranscriptional regulation and/or stability, it can be anticipated that all of these polymorphisms may be found to be associated with medical disorders. For example, there exist early reports of associations between SLC6A4 3′-UTR variants and attention deficit disorder (ADHD), sudden infant death syndrome, bipolar disorder, panic disorder and alcoholism, as detailed above.
Conclusions
Gain in knowledge about the influence of variants in SLC6A4, the 5-HT transporter gene, on human disorders and their pharmacological treatment has been immense over the last 15 years since their first discovery and subsequent findings of additional variants and implications of their involvement in additional disorders. However, advances have only partially sorted out whether additive vs. other combinatorial consequences result from the presently identified functional SERT variants (Figure 1), or whether additional variation may exist in reside in newly found functional regions, as most recently exemplified last year by the discovery of miR-16 – mediated SERT expression control. Moreover, important issues in study designs of SLC6A4 variants in phamacogenomic SRI investigations have come to the forefront, such as those at least partially related to inclusion, exclusion or (most commonly, neglect) of different world sub-population differences in frequencies of SLC6A4 variants (Figure 2). Likewise, other issues in study design such as those related to formal meta-analyses vs. broader-based evidential analyses are exemplified in high-impact studies of the contributions of stress of multiple sorts to the occurrence of depression, suicide and ultimately pharmacologic treatment have made headlines in major science journals. It seems clear that although SLC6A4 variants comprise the most intensively studied single gene alterations in human neuropsychiatric genetics, many questions on genotypic, phenotypic, G x G and G x E interactions remain incompletely evaluated and answered—setting, perhaps, an example of the formidable tasks involved in relating individual genes to human disorders and their pharmacological and other treatments.
HIGHLIGHTS.
Recent findings from studies of SLC6A4 and SERT indicate that combinations of variants can affect 5-HT transport and transporter expression up to 40-fold in vitro.
Multiple functional SLC6A4 variants include 5′ region (5HTTLPR, rs25531 and rs25532), 3′-UTR region variants plus intronic (Stin2) and coding variants.
Most of these different SLC6A4 variants have been associated with neuropsychiatric and other medical disorders, from autism and OCD to cardiovascular diseases.
Some of these associations such as those linking stress, amygdala functions and affective disorders appear to depend upon gene-environment (G x E) and gene-gene (G x G) interactions.
Some of the discoveries regarding the functional consequences of SLC6A4 variants have pharmacogenomic relevance for antidepressant SRIs and other drug therapeutics.
Acknowledgments
This research was supported by the Intramural Research Program of the NIMH, NIH. The authors are also grateful to Theresa B. DeGuzman for her editorial and graphical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1**.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. First full report of the consequences of the SLC6A4 5′ region 5HTLPR indel with effects on 3H-5HT uptake, mRNA and reporter gene expression changes plus differential allele associations with neuroticism and related personality traits in >600 individuals. [DOI] [PubMed] [Google Scholar]
- 2**.Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. Discovery of a 5′ SNP modifying the SLC6A4 5HTLPR with combination alleles formed strongly associated with OCD in two large samples, plus explication of strong ethnic influences on allele distributions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3*.MacKenzie A, Quinn J. A serotonin transporter gene intron 2 polymorphic region, correlated with affective disorders, has allele-dependent differential enhancer-like properties in the mouse embryo. Proc Natl Acad Sci U S A. 1999;96:15251–15255. doi: 10.1073/pnas.96.26.15251. First description of a functional Stin2 SLC6A4 variant now associated with multiple disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozaki N, Goldman D, Kaye WH, Plotnicov K, Greenberg BD, Lappalainen J, Rudnick G, Murphy DL. Serotonin transporter missense mutation associated with a complex neuropsychiatric phenotype. Mol Psychiatry. 2003;8:933–936. doi: 10.1038/sj.mp.4001365. [DOI] [PubMed] [Google Scholar]
- 5*.Prasad HC, Zhu CB, McCauley JL, Samuvel DJ, Ramamoorthy S, Shelton RC, Hewlett WA, Sutcliffe JS, Blakely RD. Human serotonin transporter variants display altered sensitivity to protein kinase G and p38 mitogen-activated protein kinase. Proc Natl Acad Sci U S A. 2005;102:11545–11550. doi: 10.1073/pnas.0501432102. Detailed explication of SLC6A4 coding region variants and their regulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6**.Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329:1537–1541. doi: 10.1126/science.1193692. The micro RNA, m-16 was found to be the first 3′ UTR region modulation of SLC6A4 expression with sensitivity to fluoxetine. [DOI] [PubMed] [Google Scholar]
- 7*.Wendland JR, Moya PR, Kruse MR, Ren-Patterson RF, Jensen CL, Timpano KR, Murphy DL. A novel, putative gain-of-function haplotype at SLC6A4 associates with obsessive-compulsive disorder. Hum Mol Genet. 2008;17:717–723. doi: 10.1093/hmg/ddm343. The largest study documenting gain-of-function SLC6A4 expression changes contributing to a haplotypic association with OCD. [DOI] [PubMed] [Google Scholar]
- 8*.Little KY, McLaughlin DP, Zhang L, Livermore CS, Dalack GW, McFinton PR, DelProposto ZS, Hill E, Cassin BJ, Watson SJ, et al. Cocaine, ethanol, and genotype effects on human midbrain serotonin transporter binding sites and mRNA levels. Am J Psychiatry. 1998;155:207–213. doi: 10.1176/ajp.155.2.207. Documentation of SLC6A4 differential allele associations in human post-mortem brain. [DOI] [PubMed] [Google Scholar]
- 9.Singh YS, Sawarynski LE, Michael HM, Ferrell RE, Murphey-Corb MA, Swain GM, Patel BA, Andrews AM. Boron-Doped Diamond Microelectrodes Reveal Reduced Serotonin Uptake Rates in Lymphocytes from Adult Rhesus Monkeys Carrying the Short Allele of the 5-HTTLPR. ACS Chem Neurosci. 2010;1:49–64. doi: 10.1021/cn900012y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.Willeit M, Praschak-Rieder N. Imaging the effects of genetic polymorphisms on radioligand binding in the living human brain: A review on genetic neuroreceptor imaging of monoaminergic systems in psychiatry. Neuroimage. 2010;53:878–892. doi: 10.1016/j.neuroimage.2010.04.030. 14C-DASB shown to be a superior PET ligand for SERT, with demonstration of binding potentials correlating highly in brain with SLC6A4 alleles. [DOI] [PubMed] [Google Scholar]
- 11*.Kilic F, Murphy DL, Rudnick G. A human serotonin transporter mutation causes constitutive activation of transport activity. Mol Pharmacol. 2003;64:440–446. doi: 10.1124/mol.64.2.440. First of now several SLC6A4 coding region variants associated with disease states: SERT I425V is now “OCD1” in OMIM. [DOI] [PubMed] [Google Scholar]
- 12*.Ali FR, Vasiliou SA, Haddley K, Paredes UM, Roberts JC, Miyajima F, Klenova E, Bubb VJ, Quinn JP. Combinatorial interaction between two human serotonin transporter gene variable number tandem repeats and their regulation by CTCF. J Neurochem. 2010;112:296–306. doi: 10.1111/j.1471-4159.2009.06453.x. One of first studies exploring interactions between SLC6A4 5HTTLPR and Stin2 and their regulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13*.Gyawali S, Subaran R, Weissman MM, Hershkowitz D, McKenna MC, Talati A, Fyer AJ, Wickramaratne P, Adams PB, Hodge SE, et al. Association of a polyadenylation polymorphism in the serotonin transporter and panic disorder. Biol Psychiatry. 2010;67:331–338. doi: 10.1016/j.biopsych.2009.10.015. A detailed study of the 3′ poly-A area. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serretti A, Kato M, Kennedy JL. Pharmacogenetic studies in depression: a proposal for methodologic guidelines. Pharmacogenomics J. 2008;8:90–100. doi: 10.1038/sj.tpj.6500477. [DOI] [PubMed] [Google Scholar]
- 15.Taylor MJ, Sen S, Bhagwagar Z. Antidepressant response and the serotonin transporter gene-linked polymorphic region. Biol Psychiatry. 2010;68:536–543. doi: 10.1016/j.biopsych.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Daray FM, Thommi SB, Ghaemi SN. The pharmacogenetics of antidepressant-induced mania: a systematic review and meta-analysis. Bipolar Disord. 2010;12:702–706. doi: 10.1111/j.1399-5618.2010.00864.x. A large study documenting switches to mania during early antidepressant treatment, a contributing side effect that limited SRI tolerance. [DOI] [PubMed] [Google Scholar]
- 17.Mrazek DA, Rush AJ, Biernacka JM, O’Kane DJ, Cunningham JM, Wieben ED, Schaid DJ, Drews MS, Courson VL, Snyder KA, et al. SLC6A4 variation and citalopram response. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:341–351. doi: 10.1002/ajmg.b.30816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraft JB, Peters EJ, Slager SL, Jenkins GD, Reinalda MS, McGrath PJ, Hamilton SP. Analysis of association between the serotonin transporter and antidepressant response in a large clinical sample. Biol Psychiatry. 2007;61:734–742. doi: 10.1016/j.biopsych.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 19.Weinshilboum RM. SLC6A4 variation and citalopram response. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:341–351. doi: 10.1002/ajmg.b.30816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy GM, Jr, Kremer C, Rodrigues HE, Schatzberg AF. Pharmacogenetics of antidepressant medication intolerance. Am J Psychiatry. 2003;160:1830–1835. doi: 10.1176/appi.ajp.160.10.1830. [DOI] [PubMed] [Google Scholar]
- 21.Fox MA, Jensen CL, French HT, Stein AR, Huang SJ, Tolliver TJ, Murphy DL. Neurochemical, behavioral, and physiological effects of pharmacologically enhanced serotonin levels in serotonin transporter (SERT)-deficient mice. Psychopharmacology (Berl) 2008;201:203–218. doi: 10.1007/s00213-008-1268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camilleri M. Genetics and irritable bowel syndrome: from genomics to intermediate phenotype and pharmacogenetics. Dig Dis Sci. 2009;54:2318–2324. doi: 10.1007/s10620-009-0903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho HJ, Meira-Lima I, Cordeiro Q, Michelon L, Sham P, Vallada H, Collier DA. Population-based and family-based studies on the serotonin transporter gene polymorphisms and bipolar disorder: a systematic review and meta-analysis. Mol Psychiatry. 2005;10:771–781. doi: 10.1038/sj.mp.4001663. [DOI] [PubMed] [Google Scholar]
- 24.Kistner-Griffin E, Brune CW, Davis LK, Sutcliffe JS, Cox NJ, Cook EH., Jr Parent-of-origin effects of the serotonin transporter gene associated with autism. Am J Med Genet B Neuropsychiatr Genet. 2010 doi: 10.1002/ajmg.b.31146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan JB, Sklar P. Meta-analysis reveals association between serotonin transporter gene STin2 VNTR polymorphism and schizophrenia. Mol Psychiatry. 2005;10:928–938. 891. doi: 10.1038/sj.mp.4001690. [DOI] [PubMed] [Google Scholar]
- 26.Wray NR, James MR, Gordon SD, Dumenil T, Ryan L, Coventry WL, Statham DJ, Pergadia ML, Madden PA, Heath AC, et al. Accurate, Large-Scale Genotyping of 5HTTLPR and Flanking Single Nucleotide Polymorphisms in an Association Study of Depression, Anxiety, and Personality Measures. Biol Psychiatry. 2009;66:468–476. doi: 10.1016/j.biopsych.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bloch MH, Landeros-Weisenberger A, Sen S, Dombrowski P, Kelmendi B, Coric V, Pittenger C, Leckman JF. Association of the serotonin transporter polymorphism and obsessive-compulsive disorder: systematic review. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:850–858. doi: 10.1002/ajmg.b.30699. [DOI] [PubMed] [Google Scholar]
- 28.Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Hum Genet. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- 29.Phillips-Bute B, Mathew JP, Blumenthal JA, Morris RW, Podgoreanu MV, Smith M, Stafford-Smith M, Grocott HP, Schwinn DA, Newman MF. Relationship of genetic variability and depressive symptoms to adverse events after coronary artery bypass graft surgery. Psychosom Med. 2008;70:953–959. doi: 10.1097/PSY.0b013e318187aee6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ulrich S, Hersberger M, Fischler M, Nussbaumer-Ochsner Y, Treder U, Russi EW, Speich R. Genetic polymorphisms of the serotonin transporter, but not the 2a receptor or nitric oxide synthetase, are associated with pulmonary hypertension in chronic obstructive pulmonary disease. Respiration. 2010;79:288–295. doi: 10.1159/000226243. [DOI] [PubMed] [Google Scholar]
- 31.Colucci R, Blandizzi C, Bellini M, Ghisu N, Tonini M, Del Tacca M. The genetics of the serotonin transporter and irritable bowel syndrome. Trends Mol Med. 2008;14:295–304. doi: 10.1016/j.molmed.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Nakatani D, Sato H, Sakata Y, Shiotani I, Kinjo K, Mizuno H, Shimizu M, Ito H, Koretsune Y, Hirayama A, et al. Influence of serotonin transporter gene polymorphism on depressive symptoms and new cardiac events after acute myocardial infarction. Am Heart J. 2005;150:652–658. doi: 10.1016/j.ahj.2005.03.062. [DOI] [PubMed] [Google Scholar]
- 33**.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. The first documentation in a prospective study showing that interviewed-based prior stress response correlated with SLC6A4 allele distributions in a G x E manner. [DOI] [PubMed] [Google Scholar]
- 34.Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- 35.Murphy DL, Lesch KP. Targeting the murine serotonin transporter: insights into human neurobiology. Nature Reviews Neuroscience. 2008;9:85–94. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- 36.Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karg K, Burmeister M, Shedden K, Sen S. The Serotonin Transporter Promoter Variant (5-HTTLPR), Stress, and Depression Meta-analysis Revisited: Evidence of Genetic Moderation. Arch Gen Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalin NH, Shelton SE, Fox AS, Rogers J, Oakes TR, Davidson RJ. The serotonin transporter genotype is associated with intermediate brain phenotypes that depend on the context of eliciting stressor. Mol Psychiatry. 2008;13:1021–1027. doi: 10.1038/mp.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olivier JD, Van Der Hart MG, Van Swelm RP, Dederen PJ, Homberg JR, Cremers T, Deen PM, Cuppen E, Cools AR, Ellenbroek BA. A study in male and female 5-HT transporter knockout rats: an animal model for anxiety and depression disorders. Neuroscience. 2008;152:573–584. doi: 10.1016/j.neuroscience.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 40.Chang HY, Grygoruk A, Brooks ES, Ackerson LC, Maidment NT, Bainton RJ, Krantz DE. Overexpression of the Drosophila vesicular monoamine transporter increases motor activity and courtship but decreases the behavioral response to cocaine. Mol Psychiatry. 2006;11:99–113. doi: 10.1038/sj.mp.4001742. [DOI] [PubMed] [Google Scholar]
- 41.Mileva-Seitz V, Kennedy J, Atkinson L, Steiner M, Levitan R, Matthews SG, Meaney MJ, Sokolowski MB, Fleming AS. Serotonin transporter allelic variation in mothers predicts maternal sensitivity, behavior and attitudes toward 6-month-old infants. Genes Brain Behav. 2011 doi: 10.1111/j.1601-183X.2010.00671.x. [DOI] [PubMed] [Google Scholar]
- 42.Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGough JJ, McCracken JT, Loo SK, Manganiello M, Leung MC, Tietjens JR, Trinh T, Baweja S, Suddath R, Smalley SL, et al. A Candidate Gene Analysis of Methylphenidate Response in Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry. 2009 doi: 10.1097/CHI.0b013e3181bc72e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44*.Murphy DL, Fox MA, Timpano KR, Moya PR, Ren-Patterson R, Andrews AM, Holmes A, Lesch KP, Wendland JR. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55:932–960. doi: 10.1016/j.neuropharm.2008.08.034. The most thorough review of the entire serotonergic system to date, with a special focus on mouse and human SLC6A4 contributions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy DL, Uhl GR, Holmes A, Ren-Patterson R, Hall FS, Sora I, Detera-Wadleigh S, Lesch KP. Experimental gene interaction studies with SERT mutant mice as models for human polygenic and epistatic traits and disorders. Genes Brain Behav. 2003;2:350–364. doi: 10.1046/j.1601-1848.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 46.Barr CS, Newman TK, Becker ML, Parker CC, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. The utility of the non-human primate; model for studying gene by environment interactions in behavioral research. Genes Brain Behav. 2003;2:336–340. doi: 10.1046/j.1601-1848.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 47.Adamec R, Burton P, Blundell J, Murphy DL, Holmes A. Vulnerability to mild predator stress in serotonin transporter knockout mice. Behav Brain Res. 2006;170:126–140. doi: 10.1016/j.bbr.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 48*.Hranilovic D, Stefulj J, Schwab S, Borrmann-Hassenbach M, Albus M, Jernej B, Wildenauer D. Serotonin transporter promoter and intron 2 polymorphisms: relationship between allelic variants and gene expression. Biol Psychiatry. 2004;55:1090–1094. doi: 10.1016/j.biopsych.2004.01.029. Linkage association was found between higher vs. lower gene expression whrn Stin2 and 5HTTLPR were examined together. [DOI] [PubMed] [Google Scholar]


